Transcription factors play an essential role in hematopoiesis. Aberrations in transcription factors may contribute to the development and maintenance of leukemia. Runt related transcription factor 1 (RUNX1, a.k.a. AML1, CBFa2 or PEBP2aB) is a transcription factor which belongs to the core-binding factor (CBF) family. RUNX1 directly contacts DNA, but the binding affinity for DNA significantly increases after dimerizing with its cofactor, core binding factor-β (CBFβ). RUNX1 plays a key role in definitive hematopoiesis, and disruption of RUNX1 contributes to malignant transformation.1 In acute myeloid leukemia (AML), RUNX1 mutations (RUNX1mut) are frequently found and are associated with a poor prognosis.1-3 Furthermore, more than a dozen different chromosomal translocations have been described that involve either RUNX1 or its partner CBFβ. Of these, the most common translocations are the t(8;21)(q22;q22), leading to a fusion protein RUNX1- RUNX1T1, and the inv(16)(p13;q22)) leading to a CBF-MYH11 fusion protein. These aberrations are found in approximately 12-15% and 8-10% of adult AML respectively.1 Interestingly, CBF translocations are associated with a favorable prognosis.1 For almost a decade, t(8;21) (RUNX1-RUNX1T1) and inv(16) (CBFβ-MYH11) have been incorporated as a separate AML entity with favorable prognosis in the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. In addition, in the European Leukemia Net (ELN) recommendations of diagnosis and management of AML they are incorporated as important markers of good prognosis. In the recent WHO classification update in 2016, AML with a RUNX1mut has been added as a provisional entity and this category of AML has also been classified in the ELN recommendations as a poor prognostic group. The exact working mechanism via which RUNX1mut operates has not yet been elucidated. Previously, we found that the expression level of transcription factor 4 (TCF4, also known as E2-2 and ITF2) is an independent prognostic factor in AML, after correction for ELN risk group (2010), age and white blood cell count. Patients with high TCF4 mRNA expression (highest quartile) have a worse survival and benefit more from a more aggressive treatment approach compared to patients with low TCF4 expression.4 In accordance, TCF4 expression significantly contributes to expression signatures linked with poor AML outcome.5 TCF4 is implicated in erythroid-megakaryocytic differentiation, B- and T-cell development and is crucial for plasmacytoid DC (pDC) development.6,7 In blastic plasmacytoid dendritic cell neoplasms (BPDCN) TCF4 has been shown to be the master regulator of the oncogenic program.7 A role for TCF4 in malignant hematopoiesis is further indicated by the finding that mutations in TCF4 are found in AML and myelodysplastic syndromes (MDS), albeit at low frequencies. 4 Furthermore, high TCF4 expression is associated with self-renewal properties8 and is downregulated during differentiation unless progenitors obtain transformed properties.8-10
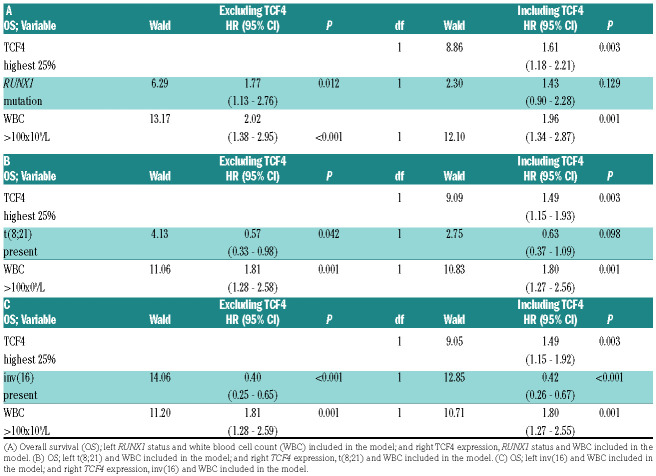
Table 1.
Multivariate Cox regression analysis.
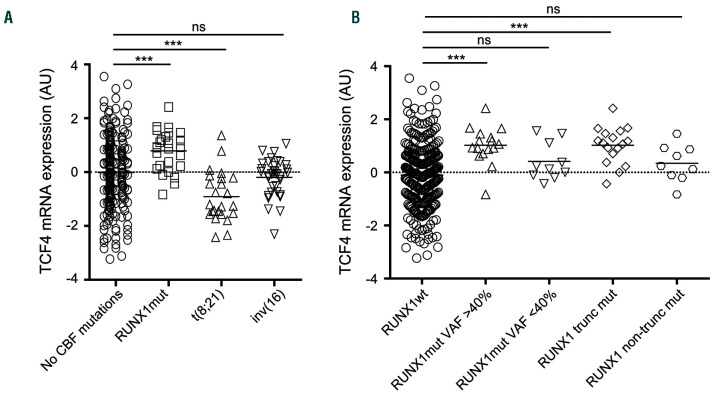
Figure 1.
TCF4 expression in acute myeloid leukemia (AML) patients with (A) no core binding factor (CBF) mutations (n=245), RUNX1 mutations (n=26), t(8;21) (n=24) and inv(16) (n=35). (B) RUNX1 wild-type (n=306), RUNX1mut variant allele frequency (VAF) ≥40 (n=16), RUNX1mut VAF <40% (n=10), truncated RUNX1mut (n=17), non-truncated RUNX1mut (n=9). AU: arbitrary unit; ***: P<0.0001; ns: not significant.
We analyzed the TCF4 expression in an independent cohort of 436 AML patients, of which 330 cases with a known RUNX1 status. Patients with a RUNX1mut (n=26 [7.9%]), had a significantly higher TCF4 expression while t(8;21) patients had a significantly lower TCF4 expression compared to patients without a CBF aberration (Figure 1A; mean 0.79 vs. -0.02, P<0.001 and -0.91 vs. -0.02, P<0.001, respectively). Patients with an inv(16) (affecting the CBFβ gene, which serves as a dimerization partner for RUNX1), had a TCF4 expression comparable to patients without a CBF aberration (Figure 1A, mean -0.20 vs. -0.02, P=0.374). Patients with RUNX1mut with a variant allele frequency (VAF) ≥40% (n=16) showed a higher TCF4 expression compared to patients with a RUNX1 mutation with a VAF <40% (n=10; Figure 1B; mean 1.02 vs. 0.42, P=0.061). Furthermore, RUNX1mut mutations that were more likely to alter protein function (truncating mutations (n=17)) showed a significantly higher impact on TCF4 expression than non-truncated RUNX1mut mutations (n=9; Figure 1B; mean 1.02 vs. 0.35, P=0.024). To corroborate these findings, we analyzed the publicly available Vizome dataset (http://www.vizome.org/aml/). In 484 AML patients, there were significantly more RUNX1mut patients in the high TCF4 (highest 25%) expressing group compared to the low TCF4 expressing group (lowest 25%) (27.3% vs. 2.5%, P<0.0001). Furthermore, others have shown a correlation between RUNX1 mutations and TCF4 expression.2,3,11
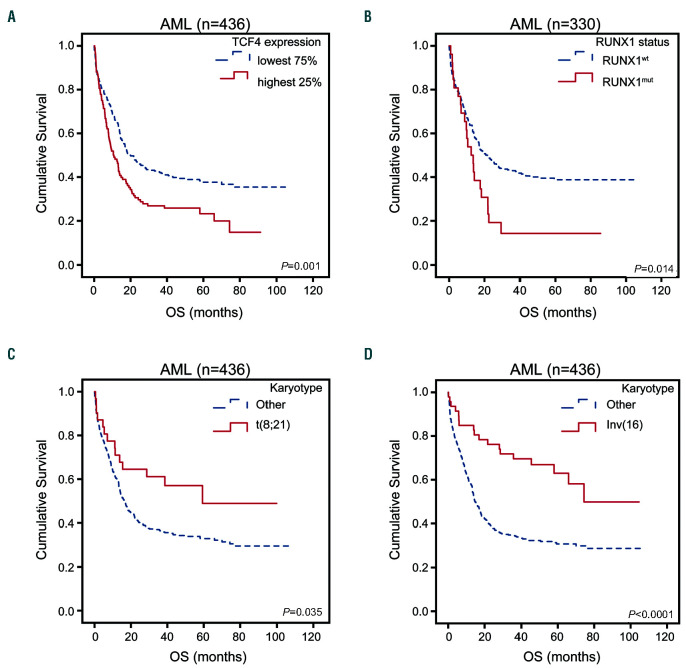
In line with previous work,4 in univariate analyses, patients in the present cohort with a high TCF4 expression (highest 25%) showed an inferior overall survival (OS) and event free survival (EFS) compared to patients with a low TCF4 expression (lowest 75%) (Figure 2A, Online Supplementary Figure S1A and Online Supplementary Table S1; 5-year OS 23% vs. 38%, P=0.001; 5-year EFS 13% vs. 31%, P<0.0001). As expected, the presence of a RUNX1mut mutation correlated with inferior survival, whereas a t(8;21) or inv(16) correlated with better survival (Figure 2B-D, Online Supplementary Figure S1B-D and Online Supplementary Table S1; 5-year OS 14% vs. 38%, P=0.014, 5-year OS 49% vs. 33%, P=0.035; 5-year OS 63% vs. 31%, P<0.0001; respectively). In addition, we analyzed TCF4 expression and RUNX1 status is the context of well-known prognostic factors, including white blood cell count (WBC), cytogenetics, age and presence of CEBPa, NPM1 or FLT3-ITD mutations in a multivariate Cox regression model. Also here we found that TCF4 expression is an independent prognostic factor, either divided in highest 25% and 75% lowest expression or as continues variable, in and out of the context of RUNX1 status (Online Supplementary Table S2A-D). We did not consider t(8;21) in this context, since it has already been incorporated in the cytogenetic risk groups. Therefore, we performed another multivariate analyses merely to test if the predictive effect of RUNX1mut or t(8;21) is dependent on TCF4 expression, since both these RUNX1 aberrations and TCF4 expression are correlated and have an impact on univariate survival. A multivariate Cox regression analysis including WBC, RUNX1 mutational status and/or TCF4 expression (highest quartile/ lowest 75%) was performed. Cytogenetics, molecular markers and age were not included in this multivariate model, because of their correlation with TCF4 expression or RUNX1 status (Online Supplementary Table S3A). When TCF4 expression was not taken into account, RUNX1 status and WBC were both independent prognostic variables for OS (Table 1A; RUNX1mut hazard ratio [HR] 1.77, 95% confidence interval (CI): 1.13-2.76, P=0.012) and EFS (Online Supplementary Table S3B). However, when TCF4 expression was added to this model, WBC and TCF4 expression showed a significant effect on OS, while the RUNX1 mutational status lost its significance and had a diminished HR of more than 10% (indicating there is a direct correlation between these variables) in both OS (Table 1A; RUNX1mut HR 1.43, 95% CI: 0.90-2.28, P=0.129) and EFS (Online Supplementary Table S3B). This indicates that the significance of RUNX1mut is dependent on TCF4 expression. In contrast, when adding RUNX1mut to an equation with TCF4 expression and WBC, the addition of RUNX1 status had no noteworthy impact on the significance or HR of TCF4 expression (Online Supplementary Table S4A-B). Analyzing RUNX1 translocation in the multivariate model showed that also the prognostic impact of t(8;21) diminished strongly when TCF4 expression was included (Table 1B and Online Supplementary Table S3C), however less pronounced than RUNX1mut. Again, the addition of t(8;21) had no noteworthy impact on the significance or HR of TCF4 expression (Online Supplementary Table S4C-D). In contrast, the effect of the inv(16) remained significant on OS and EFS independently of the presence of TCF4 expression (Table 1C, Online Supplementary Table S3D and Online Supplementary Table S4E-F). This is in accordance with the independency of inv(16) for TCF4 expression.
Figure 2.
Overall survival (OS) curves for acute myeloid leukemia patients with available data stratified on (A) TCF4 expression, lowest 75% (n=324), highest 25% (n=108); (B) RUNX1 mutational status, RUNX1 wild-type (n=304), RUNX1 mutation (n=26); (C) presence (n=31) or absence of t(8;21) (n=405); (D) presence (n=47) or absence of inv(16) (n=389).
Aberrations of RUNX1 are widely used in the clinic for the biological and prognostic classification of AML. The pathomechanisms explaining the different outcome of the different RUNX1 aberrations, however, is not yet elucidated. We and others show that TCF4 is highly expressed in RUNX1mut AML,2,3,11 while there is a low expression in t(8;21) AML patients.4,12 This corresponds with the poor prognosis of high TCF4 (highest 25%) expression and RUNX1mut and the favorable prognosis of low TCF4 expression and t(8;21) AML patients.4 Interestingly, we show in a multivariate analysis that TCF4 expression prevails over RUNX1 status in predicting outcome in AML. Possibly, RUNX1, being a transcription factor, has a direct effect on the TCF4 promoter, which is different between RUNX1 wild-type, RUNX1mut and RUNX1-RUNX1T1. In primary AML, RUNX1 has been shown to bind to the TCF4 promoter in RUNX1 wild-type and RUNX1mut cells, however there was no AML-ETO binding on the TCF4 promoter in AML-ETO primary cells (Online Supplementary Figure S2).13 In contrast, in Kasumi cells, RUNX1 did bind to the TCF4 promoter. 14 TCF4 has been described as one of the most dominant factors involved in self-renewal.8-10,15 In granulocyte- monocyte progenitors (GMP), transduced with various other oncogenes, TCF4 was up-regulated and proposed to be part of the 'leukemia initiation signature.8 In addition, RUNX1 is not the only factor influencing TCF4 expression, since only 5-10% RUNX1 mutations are found in AML patients, while 25% of AML patients have a high TCF4 expression. For example, in AML and mouse models with translocations of the mixed lineage leukemia gene (MLL) and its most common partner AF9 (MLL-AF9), TCF4 was identified as a direct transcriptional target of MLL-AF9.15 In MLL-AF9 positive cells, TCF4 was strongly up-regulated9,10,15 and identified to be part of the "self-renewing signature".9 Moreover, in MLL-rearranged AML cells, down-regulation of TCF4 or upregulation of its natural inhibitor, prolonged the survival in transplantation experiments.15 The TCF4-induced enhanced stemness may contribute to the poor performance of AML patients with high TCF4 expression. Various oncogenes like BCL2, MYC, TCL1A and TCL1B have been reported as downstream targets of TCF4.7 To what extent these downstream targets are moderators in survival and the effects of RUNX1 mutations remains to be studied.
In conclusion, we found a correlation between TCF4 expression and RUNX1 aberrations, TCF4 being upregulated in RUNX1mut, and downregulated in RUNX1 translocated AML (t(8;21)). TCF4 expression appeared to mediate the prognostic outcome of RUNX1 aberrations. This indicates that TCF4 might be an important downstream target of RUNX1. Studies to identify the biological mechanism are warranted.
Acknowledgments
we thank dr. Priya Vart from the Department of Health Evidence, Radboud Institute of Health Sciences, Nijmegen, the Netherlands for his advice on statistical analysis.
References
- 1.Speck NA, Gilliland DG. Core-binding factors in haematopoiesis and leukaemia. Nature reviews Cancer. 2002;2(7):502-513. [DOI] [PubMed] [Google Scholar]
- 2.Greif PA, Konstandin NP, Metzeler KH, et al. RUNX1 mutations in cytogenetically normal acute myeloid leukemia are associated with a poor prognosis and up-regulation of lymphoid genes. Haematologica. 2012;97(12):1909-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendler JH, Maharry K, Radmacher MD, et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and MicroRNA expression signatures. J Clin Oncol. 2012; 30(25):3109-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.in 't Hout FE, van der Reijden BA, Monteferrario D, Jansen JH, Huls G. High expression of transcription factor 4 (TCF4) is an independent adverse prognostic factor in acute myeloid leukemia that could guide treatment decisions. Haematologica. 2014;99(12):257-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullinger L, Dohner K, Bair E, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350(16):1605-1616. [DOI] [PubMed] [Google Scholar]
- 6.In 't Hout FEM, van Duren J, Monteferrario D, et al. TCF4 promotes erythroid development. Exp Hematol. 2019;69:17-21. [DOI] [PubMed] [Google Scholar]
- 7.Ceribelli M, Hou ZE, Kelly PN, et al. A druggable TCF4- and BRD4- dependent transcriptional network sustains malignancy in blastic plasmacytoid dendritic cell neoplasm. Cancer Cell. 2016;30(5):764-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kvinlaug BT, Chan WI, Bullinger L, et al. Common and overlapping oncogenic pathways contribute to the evolution of acute myeloid leukemias. Cancer Res. 2011;71(12):4117-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442(7104):818-822. [DOI] [PubMed] [Google Scholar]
- 10.Horton SJ, Jaques J, Woolthuis C, et al. MLL-AF9-mediated immortalization of human hematopoietic cells along different lineages changes during ontogeny. Leukemia. 2013;27(5):1116-1126. [DOI] [PubMed] [Google Scholar]
- 11.Silva FP, Swagemakers SM, Erpelinck-Verschueren C, et al. Gene expression profiling of minimally differentiated acute myeloid leukemia: M0 is a distinct entity subdivided by RUNX1 mutation status. Blood. 2009;114(14):3001-3007. [DOI] [PubMed] [Google Scholar]
- 12.Liu N, Song J, Xie Y, et al. Different roles of E proteins in t(8;21) leukemia: E2-2 compromises the function of AETFC and negatively regulates leukemogenesis. Proc Natl Acad Sci U S A. 2019;116(3):890-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerritsen M, Yi G, Tijchon E, et al. RUNX1 mutations enhance selfrenewal and block granulocytic differentiation in human in vitro models and primary AMLs. Blood Adv. 2019;3(3):320-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens JH, Mandoli A, Simmer F, et al. ERG and FLI1 binding sites demarcate targets for aberrant epigenetic regulation by AML1-ETO in acute myeloid leukemia. Blood. 2012;120(19):4038-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghisi M, Kats L, Masson F, et al. Id2 and E proteins orchestrate the initiation and maintenance of MLL-rearranged acute myeloid leukemia. Cancer Cell. 2016;30(1):59-74. [DOI] [PubMed] [Google Scholar]