Abstract
We report the final 2-year end-of-study results from the first clinical trial investigating combination treatment with ruxolitinib and low-dose pegylated interferon-α2 (PEG-IFNα2). The study included 32 patients with polycythemia vera and 18 with primary or secondary myelofibrosis; 46 patients were previously intolerant of or refractory to PEGIFNα2. The primary outcome was efficacy, based on hematologic parameters, quality of life measurements, and JAK2 V617F allele burden. We used the 2013 European LeukemiaNet and International Working Group- Myeloproliferative Neoplasms Research and Treatment response criteria, including response in symptoms, splenomegaly, peripheral blood counts, and bone marrow. Of 32 patients with polycythemia vera, ten (31%) achieved a remission which was a complete remission in three (9%) cases. Of 18 patients with myelofibrosis, eight (44%) achieved a remission; five (28%) were complete remissions. The cumulative incidence of peripheral blood count remission was 0.85 and 0.75 for patients with polycythemia vera and myelofibrosis, respectively. The Myeloproliferative Neoplasm Symptom Assessment Form total symptom score decreased from 22 [95% confidence interval (95% CI):, 16-29] at baseline to 15 (95% CI: 10-22) after 2 years. The median JAK2 V617F allele burden decreased from 47% (95% CI: 33-61%) to 12% (95% CI: 6-22%), and 41% of patients achieved a molecular response. The drop-out rate was 6% among patients with polycythemia vera and 32% among those with myelofibrosis. Of 36 patients previously intolerant of PEG-IFNα2, 31 (86%) completed the study, and 24 (67%) of these received PEG-IFNα2 throughout the study. In conclusion, combination treatment improved cell counts, reduced bone marrow cellularity and fibrosis, decreased JAK2 V617F burden, and reduced symptom burden with acceptable toxicity in several patients with polycythemia vera or myelofibrosis. #EudraCT2013-003295-12.
Introduction
This is the first clinical trial investigating combination treatment with ruxolitinib and pegylated interferon-a2 (PEG-IFNa2) for patients with chronic Philadelphia-negative myeloproliferative neoplasms. Hydroxyurea is the most frequently used cytoreductive agent, but some patients are intolerant of or resistant to this drug.1-3 PEGIFNa2 is another first-line treatment option, but its clinical use is limited by toxicity.3-12 Ruxolitinib reduces symptom burden in patients with polycythemia vera (PV) and primary or secondary myelofibrosis (MF), but the clinical benefit in patients with PV is still controversial.3,13-19 Ruxolitinib may increase the efficacy and tolerability of PEG-IFNa2,20,21 and we have reported promising results from a 1-year interim analysis, with an overall remission rate of 9% in PV patients and 39% in MF patients.22
PEG-IFNa2 effectively normalizes blood cell counts and may prevent disease-related thromboembolic complications in patients with essential thrombocythemia, PV, and MF.4-8,10,11,23-26 Furthermore, an anti-clonal activity of PEGIFNa2 has been shown by reductions of the Januskinase-2 (JAK2) V617F allele burden and sustained deep molecular, hematologic, and histological remission in some patients, even several years after cessation of treatment.5,6,23,27-29 However, some patients do not respond adequately to PEG-IFNa2, and treatment is limited by frequent toxicities and high discontinuation rates, of 10–50%, due to adverse events.5-8,10-12 In particular, inflammatory side effects such as fever, flu-like symptoms, fatigue, and autoimmune thyroiditis are troublesome.5-7,10,11
JAK1-2 inhibitor treatment with ruxolitinib reduces disease- related symptoms and splenomegaly, and it may prolong survival in patients with MF.13,14,16,18,19 Furthermore, ruxolitinib reduces elevated blood cell counts and symptom burden in patients with PV.15,17 Inflammation is a critical element of myeloproliferative neoplasms, and the effect of ruxolitinib seems to be mediated mainly by antiinflammatory mechanisms.20 Moreover, a reduction in the JAK2 V617F allele burden has been observed.30 Adding ruxolitinib to PEG-IFNa2 treatment may increase the efficacy and tolerability of PEG-IFNa2 by reducing inflammation. 21,22 Combination treatment may also have a synergistic effect on the malignant clone, and reduced dosage of both drugs may lead to fewer side-effects compared with monotherapies.20 The rationales for the combination treatment have previously been described in detail.21
We report the 2-year end-of-study results from the phase II COMBI study assessing efficacy and safety of combination treatment with ruxolitinib and PEG-IFNa2.
Methods
Study design
The COMBI study (#EudraCT2013-003295-12) was an investigator- initiated, multicenter, open-label, single-arm phase II study; we included 50 patients: 32 with PV and 18 with MF. The study was conducted between 2014 and 2018 at three sites in Denmark and approved by the Danish Regional Science Ethics Committee and the Danish Medicines Agency. It was done under the principles of the Declaration of Helsinki. Patients gave written informed consent to their participation. For further details on the methods, see the Online Supplementary Methods section.
Patients aged ≥18 years with a diagnosis of PV or MF according to the 2008 World Health Organization criteria were considered eligible, if they had evidence of active disease, defined as one or more of the following: need for phlebotomy, white blood cell count ≥10x09/L, platelet count ≥400x109/L, constitutional symptoms, pruritus, symptomatic splenomegaly, and previous thrombosis. Key exclusion criteria were: Eastern Cooperative Oncology Group performance status ≥3, severe comorbidity, white blood cell count <1.5x109/L, and platelet count <100x109/L.
Patients were initially treated with PEG-IFNa2a [Pegasys®; Genentech (Roche), South San Francis-co, CA, USA] 45 μg/week or PEG-IFNa2b (PegIntron®; Merck Sharp & Dohme, Hertfordshire, UK) 35 μg/week subcutaneously and ruxolitinib (Jakavi®; Novartis, Basel, Switzerland) 5-20 mg BID orally depending on platelet count.
Study visits included documentation of adverse events, fullscale hematology, blood biochemistry investigations, determination of the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) score,31 and assessment of compliance by research staff. Bone marrow biopsies were done at baseline and after 1 and 2 years of treatment. Spleen size was measured as the longest diameter by sonography. The proportions of JAK2 V617F and CALR-mutated alleles were quantified using a high-sensitivity real-time quantitative polymerase chain reaction method on whole blood.32,33
Endpoints
The primary outcome was efficacy, based on hematologic parameters, quality of life measurements, and the JAK2 V617F burden. The 2013 European LeukemiaNet and International Working Group-Myeloproliferative Neoplasms Research and Treatment response criteria were used to assess efficacy.34,35
In brief, for patients with PV, a complete remission (CR) required resolution of disease-related symptoms and hepatosplenomegaly, peripheral blood count remission (PBCR), no progression of the disease, no hemorrhagic or thrombotic events, and bone marrow histological remission (BMHR). A partial remission (PR) required all the above except BMHR.
For patients with MF, a CR required resolution of disease-related symptoms and hepatosplenomegaly, and PBCR. A PR required resolution of disease-related symptoms and hepatosplenomegaly, and either PBCR or BMHR. Clinical improvement was defined as an anemia response or symptoms response. Progressive disease was defined as significantly increased splenomegaly or leukemic transformation.34,35
Molecular response (MR) was classified as either complete (CMR), defined as eradication of a pre-existing abnormality, or partial (PMR), defined as a ≥50% decrease in JAK2 V617F allele burden from baseline in patients with a baseline allele burden ≥20%.
Statistical analyses
We did the statistical analyses using R.3.2.3. Response rates are presented with descriptive statistics. All patients initiating the study treatment were evaluated for response, similar to the modified intention-to-treat principle used for randomized, controlled trials. Statistical analyses are described in the Online Supplementary Methods section. P values <0.05 were considered statistically significant.
Results
Patients’ characteristics
In total, we included 51 patients in the study, and 50 patients initiated combination treatment; 32 had PV, and 19 had MF (Table 1). One patient with MF developed acute myeloid leukemia and died shortly after inclusion, before initiation of the combination treatment. The patient was excluded from further analysis. The MF patients were in an early stage of disease, with three (17%) having intermediate-2 risk and none having highrisk disease based on the Dynamic International Prognostic Scoring System-Plus (DIPSS+) score. Notably, 47 (94%) of the patients had previously been treated with PEG-IFNa2; 31 (66%) were intolerant, ten (21%) were refractory, and six (13%) were both. Moreover, 27 (54%) had been treated with hydroxyurea, and 25 (50%) had been treated with both hydroxyurea and PEG-IFNa2.
Response evaluations
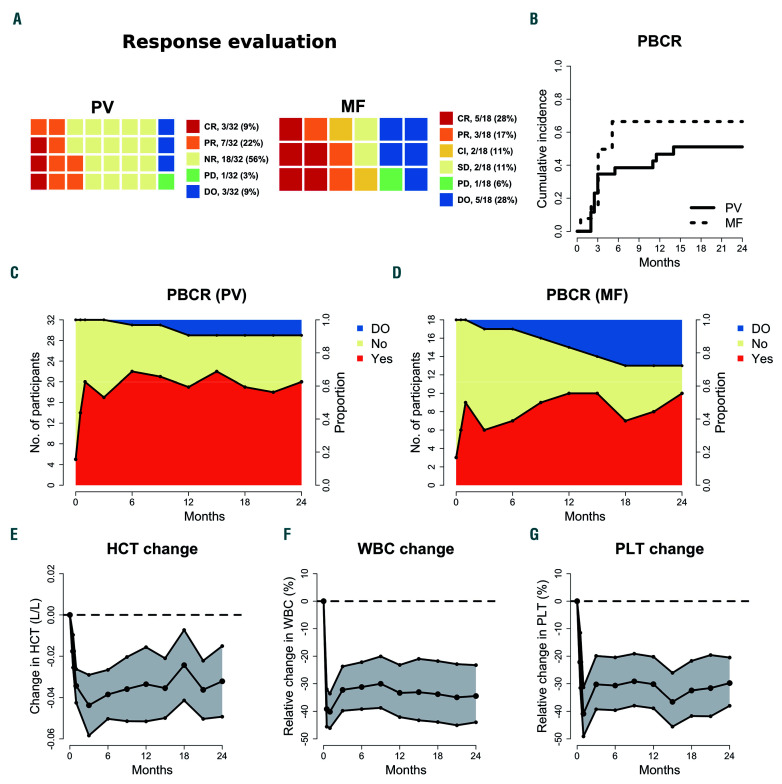
Of 32 patients with PV, ten (31%) achieved a remission; three (9%) achieved CR, and seven (22%) achieved PR (Figure 1A). Three additional patients achieved BMHR but did not fulfill the criteria for remission; one patient had an increase in total symptom score (TSS); one patient had elevated platelet count, and one patient had both. One patient had progressive disease due to the development of post-PV MF. Three PV patients dropped out and were classified as having no response. One patient chose not to have a bone marrow biopsy done after 2 years, and five patients had an unsuccessful biopsy. Two of these were in PR.
Of 18 patients with MF, eight (44%) achieved remission; five (28%) achieved CR, and three (17%) achieved PR. Moreover, two (12%) had clinical improvement, both having symptoms response with a ≥50% reduction in TSS (Figure 1A). One additional patient achieved BMHR and PBCR but suffered from grade 1 fatigue and was defined as having stable disease. Two patients had stable disease. Five patients dropped out but did not have progressive disease.
Peripheral blood cell count remission
Of 32 PV patients, five fulfilled the criteria for PBCR at baseline. For the 27 patients who did not have PBCR at baseline, the median time to PBCR was 1 month, and the cumulative incidence of PBCR after 2 years was 0.85 (Figure 1B). Of the five patients with PBCR at baseline, four had PBCR at 2 years. The median duration of the first PBCR was 14 months (Online Supplementary Figure S1), but 12 of the 13 patients who lost PBCR achieved it again during the study period. The proportion of PV patients in PBCR during the study is shown in Figure 1C. Of 14 PV patients in need of phlebotomies within 3 months before inclusion, four needed phlebotomies during the trial, three of whom required just one phlebotomy. Two additional patients required phlebotomies during the trial.
Of 18 MF patients, three fulfilled the criteria for PBCR at baseline. For the 15 patients who did not have PBCR at baseline, the median time to PBCR was 3 months, and the cumulative incidence of PBCR after 2 years was 0.73 (Figure 1B). Of the three patients with PBCR at baseline, two had PBCR after 2 years. The median duration until the first PBCR was 5 months (Online Supplementary Figure S1), but seven of the eight patients who lost PBCR achieved it again during the study period. The proportion of MF patients in PBCR during the study is shown in Figure 1D.
Hematocrit, white blood cell count, and platelet count were all significantly reduced after 2 weeks, and throughout the study period (Figure 1E-G); there were no significant differences between patients with PV and those with MF.
Patient-reported quality-of-life outcomes
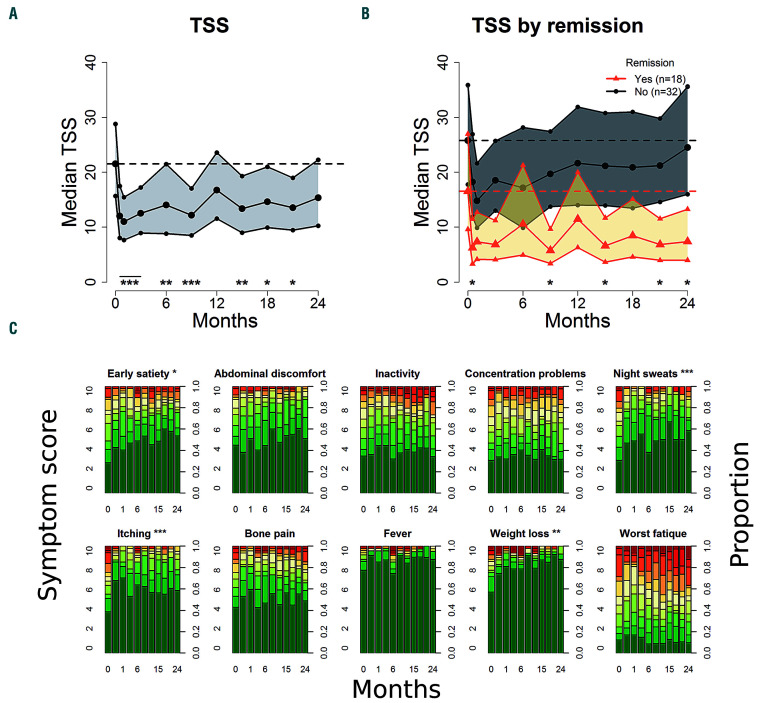
During the 2 years of treatment, we observed a statistically significant reduction in MPN-SAF TSS from baseline at all time points except at 1 and 2 years (Figure 2A). The median TSS was reduced from 22 [95% confidence interval (95% CI): 16-29] at baseline to 15 (95% CI: 10-22) after 2 years. Compared with patients not achieving remission, patients in remission after 2 years had significantly (P<0.05) larger reductions in TSS at several time points (figure 2B). The median TSS in patients achieving remission was reduced from 17 (95% CI: 10-27) at baseline to 7 (95% CI: 4-13) after 2 years. The median TSS in patients not achieving remission was 26 (95% CI: 18-37) at baseline and 25 (95% CI: 16-36) after 2 years. The following items of the TSS were significantly reduced at more than half of the time points, compared with baseline: early satiety (P<0.05), night sweats (P<0.01), itching (P<0.01) and weight loss (P<0.001) (Figure 2C). We found no significant difference in TSS change between patients with MF and patients with PV.
Table 1.
Patients’ characteristics at baseline.
Spleen size
In nine patients, five with PV and four with MF, palpable splenomegaly was present at baseline. After 2 years, four of these patients no longer had palpable splenomegaly, two had reduced palpable splenomegaly, one had increased palpable splenomegaly, and two had discontinued treatment. In one of the two patients who dropped out, palpable splenomegaly was reduced before drop-out; in the other, it increased. Spleen size, measured by sonography, was reduced during treatment (P<0.001) (Online Supplementary Figure S2), and after 2 years of therapy, spleen size was reduced by 10% (95% CI: 6-15%). The reduction was statistically significant for PV patients at all time points, but not for MF patients at 6 months and subsequently (Online Supplementary Figure S2).
Molecular response
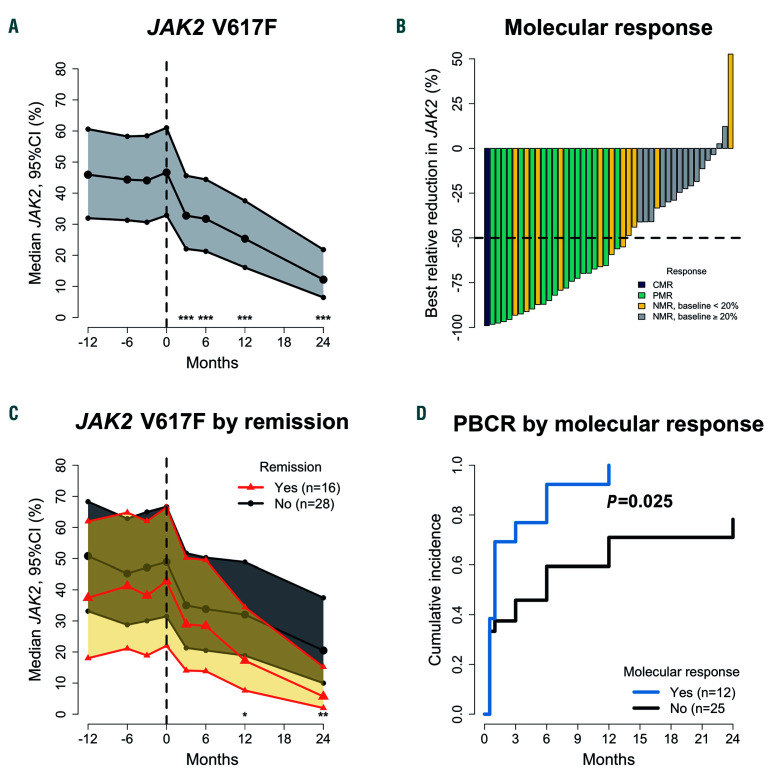
We observed statistically significant reductions in the JAK2 V617F allele burden at all time points (P<0.001) (Figure 3A). The median JAK2 V617F allele burden after 2 years was 12% (95% CI: 6-22%) compared with 47% (95% CI: 33-61%) at baseline. In 33 of 44 JAK2 V617Fmutated patients, measurements of the JAK2 V617F allele burden were available from within the year before inclusion. There were no significant differences in the JAK2 V617F allele burden before inclusion compared with baseline. Of the 44 patients with the JAK2 V617F mutation, one (2%) achieved CMR, and 17 (39%) achieved PMR within 2 years of treatment (Figure 2B). Setting the limit of CMR at 1%, four (9%) patients achieved CMR, and 16 (36%) achieved PMR within 2 years of treatment (Online Supplementary Figure S3). Further analyses were done with a limit of CMR 1-5% (Online Supplementary Table S1). We observed no difference in the JAK2 V617F allele burden change between patients with PV and patients with MF (Online Supplementary Figure S4). The JAK2 V617F allele burden of patients who did not complete 2 years of treatment (n=5) is shown in Online Supplementary Figure S5; two of these patients had reductions in JAK2 V617F allele burden.
Figure 1.
Response evaluations and peripheral blood cell remission. (A) Response evaluations after 2 years of treatment in patients with polycythemia vera (PV) or myelofibrosis (MF). Responses are classified as complete remission (CR), partial remission (PR), no response (NR), progressive disease (PD), drop-outs (DO), and, for MF patients, clinical improvement (CI) and stable disease (SD) (B) Kaplan-Meier plot depicting the cumulative incidence of peripheral blood count remission (PBCR) in patients not in PBCR at baseline (n=42). (C, D) Distribution of participants in PBCR and the number of patients dropping out of the protocol, patients with PV, and patients with MF. (E-G) Estimated change, with 95% confidence interval, in hematocrit (HCT) (E), white blood cell count (WBC) (F), and platelet count (PLT) (G), using generalized linear mixed models.
Of four patients with a CALR mutation, one had a decrease in allele burden during treatment, two had an increase, and one dropped out of the study before a second measurement could be done (Online Supplementary Figure S6).
Patients achieving remission after 2 years had a statistically significant greater reduction in JAK2 V617F allele burden compared with patients not achieving remission (Figure 2C). Of the 39 patients with the JAK2 V617F mutation who completed the study, 15 had a MR and 24 did not; 11 (73%) patients with a MR achieved remission, while five (21%) without a MR achieved remission (P=0.003). Likewise, 14 (93%) patients with a MR were in PBCR, while 12 (50%) without MR were in PBCR (P=0.014). Moreover, patients with a MR at 2 years had a higher rate of PBCR (P=0.025) with a median time to PBCR of 1 month, compared with 6 months for patients without a MR (Figure 2D).
Figure 2.
Change in Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) total symptom score and individual symptoms. (A) Median total symptom score (TSS) with 95% confidence interval (95% CI). (B) The median TSS with 95% CI in participants with remission after 2 years and participants without remission. (C) Individual symptom scores during treatment. Significance is defined as a statistically significant reduction compared with baseline at more than half of the time points. All analyses were done using generalized linear mixed models comparing baseline values with measurements during treatment. *P<0.05; **P<0.01; ***P<0.001
Drop-out and toxicity
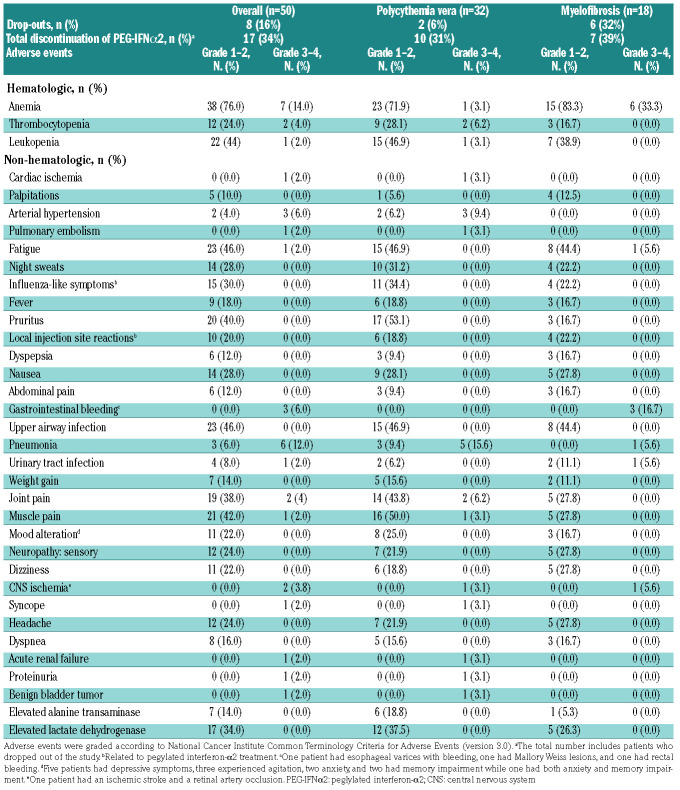
Of the 32 PV patients who initiated treatment, two (6%) dropped out within 2 years. Of 18 MF patients, six (33%) dropped out. In total, the drop-out rate was 16%. Three patients had an inadequate response; four had adverse events; among these, two had neuropsychiatric adverse events, one had multiple infections and gastrointestinal bleeding and requested to be taken off protocol (Online Supplementary Table S2). Eight (25%) PV patients and one (6%) MF patient discontinued PEG-IFNa2 treatment while continuing treatment with ruxolitinib, and one PV (3%) patient discontinued ruxolitinib treatment. In total, 17 (34%) patients, ten (31%) with PV and seven (39%) with MF, discontinued PEG-IFNa2; ten (59%) of these due to side-effects likely related to PEG-IFNa2 (Table 2). Of the 50 patients, 32 (64%) had the dosage of ruxolitinib reduced, and 35 (70%) had the dosage of PEG-IFNa2 reduced.
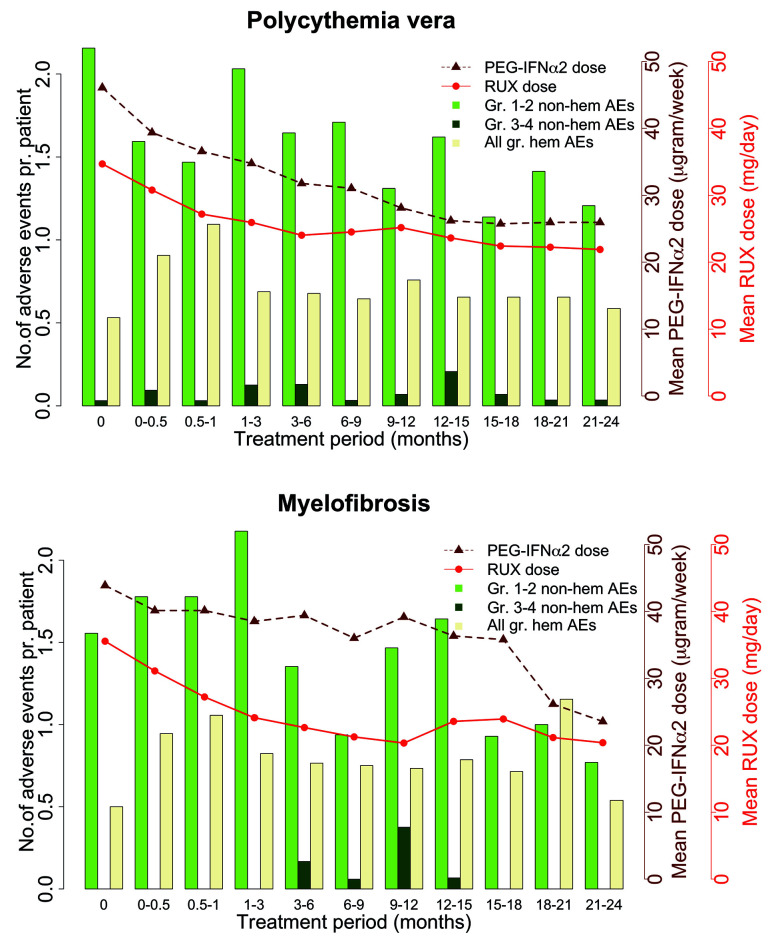
The prevalence of adverse events, including diseaserelated and study drug-unrelated events, observed in ≥10% of the patients and all grade 3 or 4 adverse events, are presented in Table 2. Four thromboembolic events occurred in three patients, two patients with PV and one with MF: the events were myocardial infarction in one patient, ischemic stroke in one patient, and both retinal artery occlusion and multiple small pulmonary emboli in one PV patient. Grade 1 or 2 hematologic adverse events, and grade 3 anemia in patients with MF, were frequent. We only recorded a few other grade 3 or 4 hematologic adverse events. With regards to non-hematologic adverse events, fatigue, influenza-like symptoms, pruritus, upper airway infection, joint pain, muscle pain, and elevated lactate dehydrogenase were observed in ≥30% of patients. Six (12%) patients had grade 3 or 4 pneumonia, and one (2%) had a grade 3 or 4 urinary tract infection. Two (4%) patients had herpes zoster infection grade 1 or 2. In 11 (22%) patients, mood alterations, including depressive symptoms, agitation, anxiety, and memory impairment, were observed. No autoimmune thyroiditis occurred in our study, but one patient was diagnosed with Sjögren syndrome. All the drug-related adverse events are known toxicities of either ruxolitinib or PEG-IFNa2. The drug doses and adverse events over time are shown in Figure 4. The highest number of adverse events was observed within the first 3 months of treatment.
Figure 3.
Change in JAK2-V617F allele burden and molecular response. (A) Median JAK2 V617F allele burden (JAK2) with 95% confidence interval (95% CI), using a generalized linear mixed model to compare baseline with time points during treatment. (B) Waterfall plot over the best relative reduction in JAK2 with an indication of complete molecular response (CMR), partial molecular response (PMR), and no molecular response (NMR) with baseline value below or above 20%. (C) Median JAK2 and 95% CI in patients in partial or complete remission after 24 months and patients not in remission with stars representing a statistically significant difference in change from baseline between groups. (D) Cumulative incidence of peripheral blood cell remission (PBCR) in patients achieving a molecular response after 2 years and patients not achieving a molecular response. *P<0.05; **P<0.01; ***P<0.001
Table 2.
Adverse events reported in ≥ 10% of patients and all grade 3 or 4 adverse events.
Subgroup analyses
Of 32 PV patients who initiated treatment, 20 completed the study per protocol. Of these 20 patients, ten (50%) achieved remission; three (15%) had CR, and seven (35%) had PR. Eight of the 20 patients (40%) had a MR. At 2 years, 16 (80%) were in PBCR. Of the 18 MF patients who initiated treatment, 12 completed the study per protocol. Of these 12 patients, eight (67%) achieved remission; five (42%) had CR, three (25%) had PR. Two (13%) had a clinical improvement. Six patients (50%) had a MR, and nine (75%) were in PBCR.
Eight patients with the JAK2 V617F mutation discontinued PEG-IFNa2 and continued ruxolitinib as monotherapy. Their JAK2 V617F allele burdens are shown in Online Supplementary Figure S7. Of these patients, three had PBCR, and none had remission at 24 months. Three achieved MR; however, two of these continued PEGIFNa2 until 15 and 21 months.
We stratified the main results based on the reason for the prior discontinuation of PEG-IFNa2 (Online Supplementary Table S3). Patients who were previously intolerant of PEG-IFNa2 or naïve to this treatment had more substantial reductions in the JAK2 V617F allele burden compared with patients previously refractory to PEGIFNa2 treatment (mean reductions 61%, 65%, and 34%, respectively). None of the three PEG-IFNa2-naïve patients dropped out or discontinued PEG-IFNa2 treatment, and two achieved remission and MR. We observed no other notable differences.
Discussion
In this study, we showed that a novel combination of ruxolitinib and low-dose PEG-IFNa2 is an effective treatment with acceptable toxicity for patients with PV or MF. We observed remission rates of 31% for patients with PV and 44% for patients with MF. Moreover, both groups had relatively high rates of sustained PBCR. Furthermore, we found statistically significant reductions in the MPN-SAF TSS, spleen size, and JAK2 V617F allele burden. The dropout rate was comparable with that in previous studies of PEG-IFNa2 in interferon-naïve patients.4-8,10,11 The preliminary results from the phase I/II RUXOPEG study (ClinicalTrials.gov NCT02742324) in patients with more advanced MF support our findings.36 In that study, a decrease in spleen size, improvement in blood counts, and a reduction in JAK2 V617F allele burden were observed in ten evaluable patients.
Figure 4.
Adverse events and drug doses in patients with polycythemia vera (left) and myelofibrosis (right). The number of adverse events per patient during the study period with mean doses of pegylated interferona2 (PEG-IFNa2) and ruxolitinib (RUX). Gr. 1-2 non-hem AEs: grade 1-2 non-hematologic adverse events; gr. 3-4 non-hem AEs: grade 3-4 nonhematologic adverse events; All gr. hem AEs: All grade hematologic adverse events.
In total, 19% of PV patients and 33% of MF patients fulfilled the criteria for BMHR. These findings suggest a disease- modifying effect of the combination treatment.34,35 Profound effects of PEG-IFNa2 on bone marrow histology have been observed before, but usually after longer periods of treatment.28,29,37,38 Long-term treatment with ruxolitinib in patients with MF seems to decrease bone marrow fibrosis in only a small subset of patients.39 In a study investigating PEG-IFNa2 treatment in 30 patients with low- or intermediate-1 risk MF, two (7%) had CR, nine (30%) had PR, and four (13%) had clinical improvement after a median of 80.3 months.37 In our study, 28%, 17%, and 12% of MF patients achieved CR, PR, and clinical improvement, respectively, within 2 years.
Normalization of blood cell counts may be critical in reducing the risk of thrombosis and improving survival. 1,40,41 Therefore, a key finding of this study is the fast and sustained normalization of elevated blood cell counts. Similar high rates of hematologic response were observed previously with a starting dose of PEG-IFNa2 90 μg/week in patients with PV.5-7 In two phase II studies on PV patients, the median time to complete hematologic response was 2 and 3 months, respectively.5,6 In our study, the median time to PBCR was 1 month for PV patients. Similar to our study, most patients in one of those studies had received previous cytoreductive treatment.6 In the phase II study of ropeginterferon-a2b for PV patients, the median time to complete hematologic resposne was more than 1 year.42 Notably, baseline cell counts were higher in these studies than in ours, and fewer patients had received cytoreductive treatment before inclusion in two of the studies, which may explain the shorter time to response for PV patients in our study. Importantly, the response criteria are not identical in the studies, and comparisons between the studies should, therefore, be interpreted with caution. Ropeginterferon-a2b induced higher rates of blood cell count and spleen responses than did hydroxy - urea after 2 years in an ongoing randomized controlled trial.43 Ruxolitinib monotherapy in patients with PV previously treated with hydroxyurea, in the RESPONSE I and II studies, resulted in PBCR rates of only 24% and 17% after 6 months.15,17 In comparison, 80% of PV patients in our study achieved PBCR within 6 months.
The MPN-SAF TSS decreased significantly during combination treatment. Ruxolitinib has previously been shown to reduce symptom burden in patients with MF or PV with relatively few side-effects.14,15,17,19 In contrast, in a study comparing hydroxyurea with PEG-IFNa2, no reduction in TSS was observed in either group.44
The marked reduction in the JAK2 V617F allele burden and a 41% MR rate add further evidence of a selective effect of combination treatment on the malignant clone. Similar findings were made in patients treated with PEGIFNa2, and in the RESPONSE I study in which 34% achieved MR after a median of 25 months of ruxolitinib treatment.5,6,23,30 Notably, the baseline median JAK2 V617F allele burden was 83% in the RESPONSE I study compared with 47% in our study. It is likely that a higher proportion of patients in RESPONSE I was evaluable for PMR than in our study. Ruxolitinib treatment in patients with MF has resulted in markedly lower rates of MR.45 Importantly, most previous studies assessing the JAK2 V617F allele burden have a limit of detection of ≥1%.5,6.30,42,45 The assay used in our study has a sensitivity of ≤0.1% mutated alleles; this is important for comparisons of CMR and the total rate of MR.
We found an association between MR and both remission and PBCR. Similarly, during ropeginterferon-a2b treatment, an association between MR and hematologic response was observed.42,43 Likewise, in a phase II study of PEG-IFNa2, 94% of patients with MR also had a hematologic response, and ten of 13 patients with complete bone marrow response, also achieved MR; seven had CMR.11,29 The clinical benefit of MR is still unclear, but higher JAK2 V617F allele burden may be associated with a higher risk of thrombotic events and progression to MF.41,46
The drop-out rate in our study was 16%; 8% stopped due to adverse events. Notably, the drop-out rate was 6% in PV patients and 32% in MF patients, respectively. In total, 31% of PV patients and 37% of MF patients discontinued PEG-IFNa2 when including patients who continued ruxolitinib monotherapy and drop-outs. This proportion was similar in the two groups. Similar rates were reported in previous studies on PEG-IFNa2 treatment.4-8,10,11 However, in our study, 94% had previously been treated with PEG-IFNa2; we therefore expected a high rate of PEG-IFNa2 discontinuation. Moreover, in an ongoing Danish study, the rate of PEG-IFNa2 discontinuation in treatment-naïve patients was approximately 50%.12 Consequently, our findings are still very encouraging. Ruxolitinib has been shown to increase the risk of infections, particularly in patients with MF.13,15,17,19,47 We observed a relatively high infection rate, with six patients (12%) developing grade 3 pneumonia. Importantly, patients with fever were hospitalized and treated with intravenous antibiotics, immediately for safety precautions, and thereby fulfilled the criteria for grade 3 or 4 adverse events. This may have led to an overestimation of grade 3 or 4 infectious adverse events. Indeed, only three of the six grade 3 or 4 cases of pneumonia registered were radiologically or microbiologically verified. Grade 1 or 2 anemia was recorded in 71.9% of PV patients. A high initial dose of ruxolitinib may account for this. The initial ruxolitinib dose used in this study was 20 mg BID for most patients compared with 10 mg BID in the RESPONSE studies, since the phase II study dose-finding study was published after preparation of the protocol.15,17 Accordingly, dosage reduction was frequent.
The inclusion criteria used in this trial do not directly reflect the European LeukemiaNet guidelines for the initiation of cytoreductive treatment.3 Indeed, both high- and low-risk PV patients were included. This is similar to other clinical trials investigating PEG-IFNa2 treatment.5,6,42 The indications for administering PEG-IFNa2 are wider in Denmark than internationally, and a strategy of an early intervention targeting the malignant clone has previously been described.48 Indeed, combination treatment as an early intervention may induce deep clinical, histological and molecular remission more frequently than PEG-IFNa2 monotherapy.5,6,23,27-29 Achieving deep remission could lead to periods of observation without treatment or maintenance treatment with a low dose of PEG-IFNa2, which could greatly improve the quality of life of patients with myeloproliferative neoplasms. Moreover, the risk of thrombosis is greatest shortly after diagnosis, and aggressive initial treatment may reduce the risk of early thrombosis. 49 To this end, a new phase II study investigating combination therapy in newly diagnosed PV patients has been initiated (#EudraCT2018-0041-50).
One strength of our study is the investigation of bone marrow remissions, which is essential in assessing a possible disease-modifying effect of new treatments.34,35 Additionally, we used the validated MPN-SAF questionnaire to assess the symptom burden during the treatment. In patients with myeloproliferative neoplasms, in whom it is essential to balance the efficacy of a treatment with its toxicity, quality of life is a clinically relevant endpoint. 34,35 Furthermore, we used a highly sensitive method to assess the JAK2 V617F allele burden.32 A limitation of our study is the relatively small population of MF patients. Accordingly, results in this group should be interpreted with caution. Moreover, the study lacked a dose-finding phase I part, which would have been relevant. Furthermore, the study was planned for 2 years of treatment, but hematologic and histological responses have been observed after several years of PEG-IFNa treatment, and the response after 2 years may underestimate the long-term clinical effect.28,29,37,38 Most patients on combination treatment at the end of the study continued treatment and post-hoc analyses with longer follow-up are being planned to assess the long-term response to combination treatment.
In conclusion, combination treatment with ruxolitinib and low-dose PEG-IFNa2 improved peripheral blood cell counts, bone marrow cellularity and fibrosis along with symptom burden with acceptable toxicity in some patients with PV and proliferative MF. Most patients in the study were intolerant of or refractory to standard PEGIFNa2 treatment, and more than half had discontinued previous treatment with hydroxyurea, highlighting that this combination treatment is a viable choice for patients with few treatment options left.
Acknowledgments
The authors thank the patients and their families and the research staff. Danish Telemedicine A/S developed the software system for the online MPN-SAF questionnaire. We thank Associate Professor Julie Lyng Formann from the Section of Biostatistics, University of Copenhagen, for guidance in repeated measurements statistics.
References
- 1.Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119(6):1363-1369. [DOI] [PubMed] [Google Scholar]
- 2.Parasuraman S, DiBonaventura M, Reith K, et al. Patterns of hydroxyurea use and clinical outcomes among patients with polycythemia vera in real-world clinical practice: a chart review. Exp Hematol Oncol. 2015;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbui T, Tefferi A, Vannucchi AM, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuelsson J, Hasselbalch H, Bruserud O, et al. A phase II trial of pegylated interferon alpha-2b therapy for polycythemia vera and essential thrombocythemia: feasibility, clinical and biologic effects, and impact on quality of life. Cancer. 2006;106(11):2397-2405. [DOI] [PubMed] [Google Scholar]
- 5.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065-3072. [DOI] [PubMed] [Google Scholar]
- 6.Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa- 2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27(32):5418-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowin K, Thapaliya P, Samuelson J, et al. Experience with pegylated interferon alpha- 2a in advanced myeloproliferative neoplasms in an international cohort of 118 patients. Haematologica. 2012;97(10):1570-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ianotto JC, Boyer-Perrard F, Gyan E, et al. Efficacy and safety of pegylated-interferon alpha-2a in myelofibrosis: a study by the FIM and GEM French cooperative groups. Br J Haematol. 2013;162(6):783-791. [DOI] [PubMed] [Google Scholar]
- 9.Kiladjian JJ, Giraudier S, Cassinat B. Interferon-alpha for the therapy of myeloproliferative neoplasms: targeting the malignant clone. Leukemia. 2016;30(4):776-781. [DOI] [PubMed] [Google Scholar]
- 10.Gowin K, Jain T, Kosiorek H, et al. Pegylated interferon alpha - 2a is clinically effective and tolerable in myeloproliferative neoplasm patients treated off clinical trial. Leuk Res. 2017;54:73-77. [DOI] [PubMed] [Google Scholar]
- 11.Masarova L, Patel KP, Newberry KJ, et al. Pegylated interferon alfa-2a in patients with essential thrombocythaemia or polycythaemia vera: a post-hoc, median 83 month follow-up of an open-label, phase 2 trial. Lancet Haematol. 2017;4(4):e165-e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudsen TA, Hansen DL, Ocias LF, et al. Long-term efficacy and safety of recombinant interferon alpha-2 vs. hydroxyurea in polycythemia vera: preliminary results from the three-year analysis of the daliah trial - a randomized controlled phase III clinical trial. Blood. 2018;132(Suppl 1):580-580. [Google Scholar]
- 13.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. [DOI] [PubMed] [Google Scholar]
- 14.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ali HK, Griesshammer M, le Coutre P, et al. Safety and efficacy of ruxolitinib in an open-label, multicenter, single-arm phase 3b expanded-access study in patients with myelofibrosis: a snapshot of 1144 patients in the JUMP trial. Haematologica. 2016;101(9):1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18(1):88-99. [DOI] [PubMed] [Google Scholar]
- 18.Verstovsek S, Gotlib J, Mesa RA, et al. Longterm survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017;10(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palandri F, Tiribelli M, Benevolo G, et al. Efficacy and safety of ruxolitinib in intermediate- 1 IPSS risk myelofibrosis patients: results from an independent study. Hematol Oncol. 2018;36(1):285-290. [DOI] [PubMed] [Google Scholar]
- 20.Koschmieder S, Mughal TI, Hasselbalch HC, et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia. 2016;30(5):1018-1024. [DOI] [PubMed] [Google Scholar]
- 21.Bjorn ME, Hasselbalch HC. Minimal residual disease or cure in MPNs? Rationales and perspectives on combination therapy with interferon-alpha2 and ruxolitinib. Expert Rev Hematol. 2017;10(5):393-404. [DOI] [PubMed] [Google Scholar]
- 22.Mikkelsen SU, Kjaer L, Bjorn ME, et al. Safety and efficacy of combination therapy of interferon-alpha2 and ruxolitinib in polycythemia vera and myelofibrosis. Cancer Med. 2018;7(8):3571-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stauffer Larsen T, Iversen KF, Hansen E, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res. 2013;37(9):1041-1045. [DOI] [PubMed] [Google Scholar]
- 24.Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107(3): 451-458. [DOI] [PubMed] [Google Scholar]
- 25.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224-2232. [DOI] [PubMed] [Google Scholar]
- 26.Barbui T, Carobbio A, Rumi E, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021-3023. [DOI] [PubMed] [Google Scholar]
- 27.Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood. 2013;122(6):893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Utke Rank C, Weis Bjerrum O, Larsen TS, et al. Minimal residual disease after long-term interferon-alpha2 treatment: a report on hematological, molecular and histomorphological response patterns in 10 patients with essential thrombocythemia and polycythemia vera. Leuk Lymphoma. 2016;57(2):348-354. [DOI] [PubMed] [Google Scholar]
- 29.Masarova L, Yin CC, Cortes JE, et al. Histomorphological responses after therapy with pegylated interferon alpha-2a in patients with essential thrombocythemia (ET) and polycythemia vera (PV). Exp Hematol Oncol. 2017;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vannucchi AM, Verstovsek S, Guglielmelli P, et al. Ruxolitinib reduces JAK2 p.V617F allele burden in patients with polycythemia vera enrolled in the RESPONSE study. Ann Hematol. 2017;96(7):1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen TS, Pallisgaard N, Moller MB, et al. Quantitative assessment of the JAK2 V617F allele burden: equivalent levels in peripheral blood and bone marrow. Leukemia. 2008;22(1):194-195. [DOI] [PubMed] [Google Scholar]
- 33.Kjaer L, Cordua S, Holmstrom MO, et al. Differential dynamics of CALR mutant allele burden in myeloproliferative neoplasms during interferon alfa treatment. PLoS One. 2016;11(10): e0165336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group- Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiladjian J-J, Soret-Dulphy J, Resche-Rigon M, et al. Ruxopeg, a multi-center Bayesian phase 1/2 adaptive randomized trial of the combination of ruxolitinib and pegylated interferon alpha 2a in patients with myeloproliferative neoplasm (MPN)-associated myelofibrosis. Blood. 2018;132 (Suppl 1):581-581. [Google Scholar]
- 37.Silver RT, Barel AC, Lascu E, et al. The effect of initial molecular profile on response to recombinant interferon-alpha (rIFNalpha) treatment in early myelofibrosis. Cancer. 2017;123(14):2680-2687. [DOI] [PubMed] [Google Scholar]
- 38.Silver RT, Vandris K, Goldman JJ. Recombinant interferon-alpha may retard progression of early primary myelofibrosis: a preliminary report. Blood. 2011;117(24): 6669-6672. [DOI] [PubMed] [Google Scholar]
- 39.Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. Long-term effects of ruxolitinib versus best available therapy on bone marrow fibrosis in patients with myelofibrosis. J Hematol Oncol. 2018;11(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22-33. [DOI] [PubMed] [Google Scholar]
- 41.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176-2184. [DOI] [PubMed] [Google Scholar]
- 42.Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126(15): 1762-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gisslinger H, Klade C, Georgiev P, et al. Evidence for superior efficacy and disease modification after three years of prospective randomized controlled treatment of polycythemia vera patients with ropeginterferon alfa-2b Vs. HU/BAT. Blood. 2018;132(Suppl 1):579-579. [Google Scholar]
- 44.Mesa RA, Kosiorek HE, Mascarenhas J, et al. Impact on MPN symptoms and quality of life of front line pegylated interferon alpha- 2a vs. hydroxyurea in high risk polycythemia vera and essential thrombocythemia: results of myeloproliferative disorders research consortium (MPD-RC) 112 global phase III trial. Blood. 2018;132(Suppl 1):3032-3032. [Google Scholar]
- 45.Deininger M, Radich J, Burn TC, et al. The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood. 2015;126(13):1551-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vannucchi AM, Pieri L, Guglielmelli P. JAK2 allele burden in the myeloproliferative neoplasms: effects on phenotype, prognosis and change with treatment. Ther Adv Hematol. 2011;2(1):21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer. 2014;120(4):513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasselbalch HC, Holmstrom MO. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: minimal residual disease and cure? Semin Immunopathol. 2019;41(1):5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019;98(5):1071-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]