In patients with acute myeloid leukemia (AML), leukemia-initiating cells (LIC) are believed to be responsible for disease initiation, maintenance, and relapse. While the highest frequency of LIC exists in the CD34+/CD38– bone marrow (BM) compartment,1 over the past years it has become evident that a subset of the CD34– and/or CD38+ population may also harbor AML stem cell potential.2,3 LIC phenotypes also show interindividual heterogeneity dependent on genetic subtypes,4 and new potential LIC markers might better define this population. The G protein-coupled adhesion molecule GPR56 regulates survival, migration, and adhesion in various cell types.5 GPR56 was shown to be upregulated in healthy hematopoietic stem cells as well as LIC, especially when residing in a quiescent state, and to be downregulated during hematopoietic maturation.6 In AML, GPR56 may be important for the LIC - stem cell niche interaction.7 With the ability to serially transplant leukemia in NOD/SCID mice, high GPR56 expressing AML cells were functionally validated as LIC.8,9 This ability was also found in LIC with low or absent CD34 expression, supporting GPR56 as a marker able to identify LIC independent of the CD34 expression status.8
High GPR56 expression has also been linked to a dismal prognosis in AML patients consolidated with chemotherapy.8-11
Recently, we demonstrated that a high CD34+/CD38- BM cell burden (≥6%) at diagnosis is associated with shorter survival in AML patients receiving allogeneic hematopoietic stem cell transplantation (HSCT).12 While the outcome was dismal in most patients with a high CD34+/CD38– cell burden, outcome remained heterogeneous in the large patient group (85%) with a low CD34+/CD38– cell burden at diagnosis, ranging from relapse 1 month after HSCT to 10 years disease-free follow up.
Here, we retrospectively evaluated the prognostic impact of a differential GPR56 expression additionally to the CD34+/CD38– cell burden in 213 AML patients for whom BM aspirate material at diagnosis was available. All patients were treated with cytarabine-based chemotherapy and consolidated with non-myeloablative (NMA; 72%) or myeloablative (MAC; 28%) HSCT in complete remission (CR; 87%) or CR with incomplete peripheral recovery (CRi; 13%). For further details see the Online Supplementary Materials and Methods. Patients’ characteristics are shown in Table 1 and in the Online Supplementary Tables S1-2. Median follow up after HSCT for patients alive was 5.2 years.
At diagnosis, GPR56 expression and the expression of an internal control gene (ABL1) were determined using quantitative real time PCR in all patients and a median cut-off of the normalized gene expression was used to define high and low GPR56 expressers. In a patient subset GPR56 expression assessed by flow cytometry correlated well with GPR56 expression (R2=0.80; Online Supplementary Figure S1). Flow cytometry determined the CD34+/CD38– cell burden in patients with data available (n=180) and the previously evaluated 6% cut12 defined patients with a high or low CD34+/CD38– cell burden. Karyotypes, the mutation status of 52 recurrently mutated genes in AML, and expression levels of BAALC, MN1, and EVI1 were evaluated at diagnosis in patients with available material (see the Online Supplementary Material and Methods).
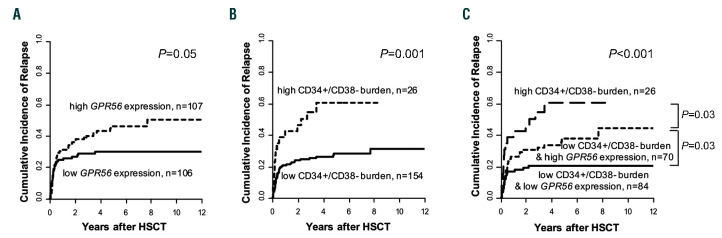
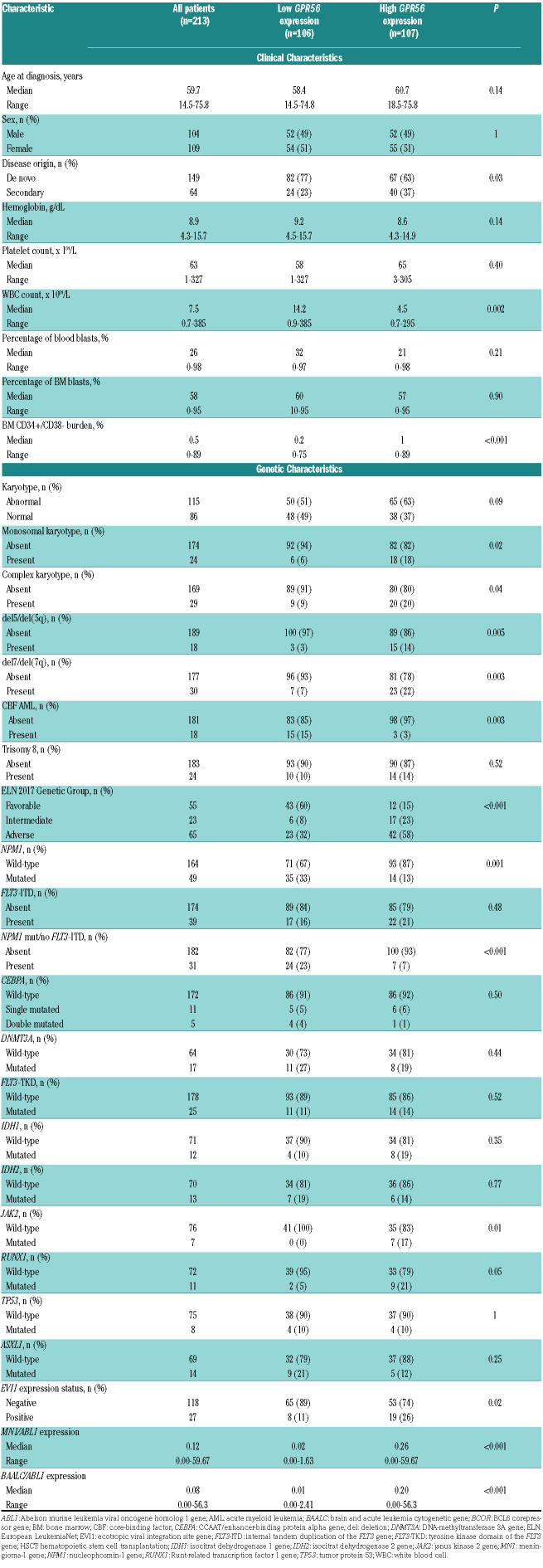
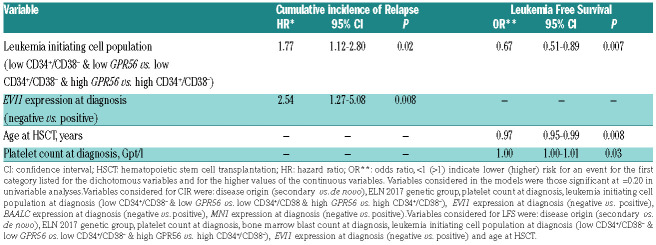
Previously, a high GPR56 expression has been linked to AML with adverse cytogenetic or molecular risk, especially to anomalies of chromosomes 5 or 7, high EVI1 expression or the presence of RUNX1 mutations.7,8 EVI17 and RUNX16 were also described to directly target GPR56. In line with these findings, we observed a higher incidence of deletion (del) 7/7q (P=0.003), del5/5q (P=0.005), complex (P=0.04) and monosomal (P=0.02) karyotypes, higher frequency of RUNX1 mutations (P=0.05) and more EVI1 positive cases (P=0.02) in high GPR56 expressing patients. In contrast, a low GPR56 expression was previously described in core-binding factor (CBF) AML, AML with NPM1 mutations without the presence of a FLT3-ITD and AML with monocytic/monoblastic morphology.8,9 Consistently, we observed a lower incidence of French American British M5 (P=0.003) cases, CBF AML (P=0.003) as well as NPM1 mutations (P=0.001) and NPM1 mutations without FLT3-ITD (P<0.001) in patients with high GPR56 expression. We could not confirm the previously described high GPR56 expression in patients with mutated NPM1 and FLT3-ITD (GPR56 as continuous variable, P=0.37), but only 18 NPM1 mutated patients in our set harbored a FLT3-ITD. Additionally, we observed an association of high GPR56 expression with lower white blood count (P=0.002), secondary AML (P=0.03), and an adverse ELN 201713 genetic risk (P<0.001), more JAK2 mutated AML (P=0.01), and higher BAALC and MN1 expression levels (P<0.001 and P<0.001, respectively) at diagnosis. High GPR56 expressing patients also showed a higher CD34+/CD38– cell burden at diagnosis (P<0.001) and a distinct immunophenotype (see the Online Supplementary Materials and Methods). Similar to patients consolidated with chemotherapy,8-10 a high GPR56 expression associated with a higher cumulative incidence of relapse (CIR; P=0.05, Figure 1A) in patients receiving HSCT. As expected, a high CD34+/CD38– cell burden also associated with a higher CIR in the here presented patient population (P=0.001, Figure 1B). As described above, outcome in the low CD34+/CD38– cell burden group remained heterogeneous, but no further prognostic impact was observed introducing an additional 1% or 2% cut for the CD34+/CD38– cell burden (Online Supplementary Figure S2). We hypothesized that this may be because of a LIC population not defined by the CD34+/CD38– phenotype and that GPR56 – described to define LIC independently of the CD34+/CD38– phenotype8 - might identify individuals at higher relapse risk in patients with a low CD34+/CD38– cell burden (n=154). Interestingly, in the low CD34+/CD38– cell burden group, a high GPR56 expression identified AML patients with a higher CIR than those with low GPR56 expression (P=0.03), but a lower CIR than those with a high CD34+/CD38– cell burden (P=0.03, Figure 1C). Thus, the GPR56 expression may add further prognostic information to the established CD34+/CD38– LIC phenotype. In multivariable analysis, a higher LIC burden defined by the CD34+/CD38– cell burden and GPR56 expression at diagnosis significantly associated with a higher CIR after adjustment for EVI1 expression status and shorter leukemia free survival (LFS) after adjustment for platelet count at diagnosis and age at HSCT (Table 2). The prognostic significance of the CD34+/CD38– cell burden and the GPR56 expression levels were also confirmed in an independent patient cohort receiving HSCT, as well as a second validation cohort consolidated with chemotherapy (see Online Supplementary Materials and Methods and Online Supplementary Figure S3-4), and a third validation cohort using mRNA data within the TCGA dataset (Online Supplementary Figure S5).
Figure 1.
Cumulative incidence of relapse according to leukemia initiating cell burden. (A) according to the GPR56 expression at diagnosis, high vs. low, median cut; (B) according to the CD34+/CD38– cell burden at diagnosis, high vs. low, 6% cut; (C) according to the CD34+/CD38–cell burden and the GPR56 expression.
Table 1.
Clinical and genetic characteristics of acute myeloid leukemia patients treated with hematopoietic stem cell transplantation according to the GPR56 expression at diagnosis, median cut, n=213.
Table 2.
Multivariable outcome analysis of 180 acute myeloid leukemia patients treated with hematopoietic stem cell transplantation according to the GPR56 expression and the CD34+/CD38– cell burden at diagnosis.
In previous studies, the dismal prognostic impact of a high GPR56 expression was shown in patients consolidated with chemotherapy8-10 and as part of a 17-gene stemness score for outcome also in patients consolidated with HSCT.14 To our knowledge, we are the first to analyze GPR56 expression levels as a single marker and in context of the CD34+/CD38– cell burden at diagnosis and its impact on the outcome after HSCT, a procedure that relies on immunological graft-versus-leukemia (GvL) effects. In line with previously published data, we observed associations with adverse-risk and immature genetic and immunophenotypic markers as well as a higher CIR in patients with high GPR56 expression. Despite a correlation between the CD34+/CD38– cell burden and GPR56 expression levels, a high GPR56 expression identified individuals with a higher CIR within the low CD34+/CD38– cell burden cohort, subsequently adding valuable prognostic information to the CD34+/CD38– phenotype.
Our findings underline the reduced immunogenicity of LIC15 and indicate that the GvL effects after HSCT may not be sufficient to prevent high GPR56 expressing patients from relapse after HSCT. Despite the lack of difference in non-relapse mortality (Online Supplementary Figure S6A) and a separation of the LFS curves, we observed no significant impact of high GPR56 expression on LFS (P=0.14, Online Supplementary Figure S6B) in univariable analysis. The GvL effects after HSCT may contribute to an improved success of relapse therapy consisting of cytotoxic treatment, reduction of immunosuppressive drugs and/or donor lymphocyte infusion. Therefore, GvL effects might not fully overcome the inferior prognosis associated with a high CD34+/CD38– cell burden,12 but may have this potential in patients with low CD34+/CD38– cell burden but high GPR56 expression. Prospective clinical studies are needed to further address this point before implementation into clinical practice. Finally, beyond their role as a prognostic factor in AML, targeted therapies against the LIC population, including GPR56, might improve patients’ outcome.
Supplementary Material
Acknowledgments
The authors would like to thank Christel Müller, Daniela Bretschneider, Evelin Hennig, Sabine Leiblein, Martina Pleß, Ulrike Bergmann, Janet Bogardt, Annette Jilo and Dagmar Cron for their help in performing cytogenetic, morphologic, and immunological analyses, and Christine Günther, Scarlett Schwabe, Ines Kovacs and Kathrin Wildenberger for their help in sample processing. Presented in part at the Annual Meeting 2016 of The European Hematology Association (EHA, Abstract S820) in Copenhagen and published in abstract form.
Funding Statement
Funding: this study was supported by the Verein zusammen gegen den Krebs e.V and the Deutsche Jose-Carreras-Stiftung (04R/2016 and PS15/05).
References
- 1.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645-648. [DOI] [PubMed] [Google Scholar]
- 2.Taussig DC, Vargaftig J, Miraki-Moud F, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(−) fraction. Blood. 2010; 115(10):1976-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eppert K, Takenaka K, Lechman ER, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17(9):1086-1093. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki M, Liedtke M, Gentles AJ, Cleary ML. CD93 marks a non-quiescent human leukemia stem cell population and is required for development of MLL-rearranged acute myeloid leukemia. Stem Cell. 2015;17(4):412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway. J Biol Chem. 2008; 283(21):14469-14478. [DOI] [PubMed] [Google Scholar]
- 6.Rao TN, Marks-Bluth J, Sullivan J, et al. High-level Gpr56 expression is dispensable for the maintenance and function of hematopoietic stem and progenitor cells in mice. Stem Cell Res. 2015;14(3):307-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito Y, Kaneda K, Suekane A, et al. Maintenance of the hematopoietic stem cell pool in bone marrow niches by EVI1-regulated GPR56. Leukemia. 2013;27(8):1637-1649. [DOI] [PubMed] [Google Scholar]
- 8.Pabst C, Bergeron A, Lavallée VP, et al. GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo. Blood. 2016;127(16):2018-2027. [DOI] [PubMed] [Google Scholar]
- 9.Daria D, Kirsten N, Muranyi A, et al. GPR56 contributes to the development of acute myeloid leukemia in mice. Leukemia. 2016; 30(8):1734-1741. [DOI] [PubMed] [Google Scholar]
- 10.Daga S, Rosenberger A, Quehenberger F, et al. High GPR56 surface expression correlates with a leukemic stem cell gene signature in CD34-positive AML. Cancer Med. 2019;8(4):1771-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulze I, Rohde C, Scheller-Wendorff M, et al. Increased DNA methylation of Dnmt3b targets impairs leukemogenesis. Blood. 2016;127(12):1575-1586. [DOI] [PubMed] [Google Scholar]
- 12.Jentzsch M, Bill M, Nicolet D, et al. Prognostic impact of the bone marrow CD34+/CD38- cell burden at diagnosis in acute myeloid leukemia patients undergoing allogeneic stem cell transplantation. Am J Hematol. 2017;92(4):388-396. [DOI] [PubMed] [Google Scholar]
- 13.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng SW, Mitchell A, Kennedy JA, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016; 540(7633):433-437. [DOI] [PubMed] [Google Scholar]
- 15.Costello RT, Mallet F, Gaugler B, et al. Human acute myeloid leukemia CD34+/CD38- progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60(16):4403-4411. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.