Abstract
With advances in multimodality therapy, childhood cancer cure rates approach 80%. However, both radiotherapy and chemotherapy can cause debilitating or even fatal late adverse events that are critical to understand, mitigate, or prevent. QUANTEC (Quantitative Analysis of Normal Tissue Effects in the Clinic) both identified radiation dose constraints for normal tissues in adults and pointed out the uncertainties in those constraints. The range of adverse events seen in children is different from that in adults, in part due to the vulnerability/characteristics of radiation damage to developing tissues, and in part due to the typical body sites affected by childhood cancer that lead to collateral irradiation of somewhat different normal tissues and organs compared with adults. Many childhood cancer survivors have long life expectancy and may develop treatment-induced secondary cancers and severe organ/tissue injury 10, 20, or more years after treatment. Collaborative long-term observational studies and clinical research programs for survivors of pediatric and adolescent cancer provide adverse event data for follow-up periods exceeding 40 years. Data analysis is challenging due to the interaction between therapeutic and developmental variables, the lack of radiation dose-volume data, and the fact that most childhood malignancies are managed with combined modality therapy.
PENTEC (Pediatric Normal Tissue Effects in the Clinic) is a volunteer research collaboration of more than 150 physicians, medical physicists, mathematical modelers, and epidemiologists organized into 18 organ-specific working groups conducting a critical review and synthesis of quantitative data from existing studies aiming to (1) establish quantitative, evidence-based dose/volume/risk guidelines to inform radiation treatment planning and, in turn, improve outcomes after radiation therapy for childhood cancers; (2) explore the most relevant risk factors for toxicity including developmental status; (3) describe specific physics and dosimetric issues relevant to pediatric radiotherapy; and (4) propose dose-volume-outcome reporting standards for publications on childhood cancer therapy outcomes. The impact of other critical modifiers of normal tissue radiation damage, including chemotherapy, surgery, stem cell transplantation and underlying genetic predispositions are also considered. The aims of the PENTEC reports are to provide clinicians with an analysis of the best available data to make informed decisions regarding radiation therapy normal organ dose constraints for planning childhood cancer treatment, and to define future research priorities.
Keywords: PENTEC, pediatric cancer, radiation therapy, late effects, treatment guidelines, survivorship
“Every child begins the world again.” Henry David Thoreau
Introduction
While the cellular and sub-cellular effects of ionizing radiation is identical in adults and children, the tissue remodeling and the functional consequences of radiation injury differ markedly. The fibrotic-atrophic pathway dominates the radiation pathogenesis of late normal tissue effects across the age range [1]; what is unique to children, however, is that this pathway interferes with growth and tissue maturation and may result in hypoplasia and hypofunction [2]. This gives rise to a marked dependence for the risk and severity of these effects as a function of the child’s age (a surrogate for development) at the time of irradiation [3]. Additionally, the long latent period for many of these effects also creates a dependency of risk as a function of attained age. In a report from the Childhood Cancer Survivor Study (CCSS), the 30-year cumulative incidence for severe (grade 3) or disabling/life-threatening (grade 4) conditions or death (grade 5) due to a chronic condition was 42% [4]. In another report from this same cohort study, the 30-year cumulative mortality was 18% among long-term survivors; RT increased the risk 2.2-fold [5]. Overall, 60%-90% of childhood cancer survivors will develop one or more chronic health conditions, and 20%-80% will experience severe or life-threatening complications [6–8].
The challenge for clinicians is to devise therapy that can simultaneously optimize health-related quality of life and maximize the child’s life expectancy. In fact, it is the late adverse effects of treatment that have driven the evolution of therapies for many childhood curable cancers. Unfortunately, many pediatric malignancies require an aggressive treatment approach that often causes a substantial risk of late adverse effects. Thus, the potential to ameliorate or prevent such normal tissue damage, or to manage and rehabilitate affected patients, requires an understanding of normal tissue tolerances to radiation and systemic therapy across the age spectrum. This is affected by the total and fractional dose of radiation, the dose rate, overall treatment time, radiation modality, and radiation dose distribution [9]. Cytotoxic or molecular targeted agents may have a direct effect on normal tissue injury, but in addition may interfere with radiation damage induction and processing. PENTEC seeks to unravel this conundrum by exploring and defining normal tissue tolerance in developing children as a function of radiation dose/volume, type and scheduling of chemotherapy, and surgery. This information can ideally be used to inform radiation oncologists, patients, and parents on the risk-benefit of multimodality therapy involving radiation therapy, define treatment planning radiation dose constraints, and propose new research directions. PENTEC has three main objectives:
To establish quantitative evidence-based dose/volume guidelines to inform treatment planning and potentially improve outcomes for survivors of radiation therapy for childhood cancers;
To appreciate clinical, dosimetric, and modeling complexities that are important to pediatric radiation therapy;
To recognize the special considerations for normal tissue radiation response of children/adolescents, e.g. the interplay between development and RT effects, and the impact of other risks factors such as systemic therapies.
Physics Considerations
Most of the published literature reviewed by the PENTEC work groups reports outcomes in children treated before the CT treatment planning era. In these studies, it is common for reported doses to be those prescribed to the tumor, rather than doses to adjacent critical normal tissues. When normal-tissue doses are reported, uncertainties resulting from lack of 3D planning need to be considered such as hand calculation-based planning often to a single reference point. Correction for tissue homogeneity was only performed in the recent two decades; therefore, previously reported doses might be somewhat under- or over-estimated. Much higher levels of uncertainty should be expected when organs are located out of field or in the penumbral regions, such as the gonads or the lens. Even when the treatment fields were designed to include the entire structure such as vertebral bodies, this target may not have received a uniform dose simply because of the rapid dose fall-off of electron and orthovoltage beams commonly used before the 1980s. In a minority of cases, the organ dose has been retrospectively reconstructed based on computational phantoms of varying complexity aided by a review of patient treatment records [10, 11]. These tend to be the basis for large–scale epidemiology studies that group organ doses into wide bins. Nonetheless, a number of quantitative late effect studies have analyzed continuous dose metrics to assess the shape of the dose-response curve, taking advantage of rich treatment and outcome data collection [12]. When organ doses were not reported, and treatment methods were not sufficiently described, then it is necessary to assume common field arrangements for the era in which the patient was treated, and this may lead to dosimetric inconsistencies that are difficult to quantify. The physicist members of each working group were tasked to participate in the review of the papers used in the modelling of the dose-response data to provide guidance on interpretation of the stated doses. PENTEC task forces and future late effect studies are encouraged to clearly state the methodology and assumptions made in estimating the organ doses as well as how this affects derived dose-volume constraints.
With the advent of 3D CT-based treatment planning, prospective archival registration of patients Dose Volume Histograms and centralized storage of DICOM-based treatment plans is increasingly advocated. The advantages of the latter include the possibility to re-contour organs at risk and/or to add contours of tissues that previously were not considered of prime interest in treatment planning. Wide-spread use of 3D conformal and other multibeam techniques did not occur until after 1990; therefore, large-scale studies addressing side effects after 30 or 40 years of follow-up will not be available soon. Extrapolations from risk measures derived from older cohorts treated with other techniques resulting in different dose distributions across an organ will remain important for at least another two decades while the “modern RT” survivor cohorts mature [13].
Epidemiologic Considerations
Epidemiologic studies are a cornerstone to increase our knowledge on the impact of ionizing radiation on human health [14, 15]. The late Dr. G. D’Angio, a pediatric radiation oncologist, introduced such study designs to investigate the side effects among children treated for cancer [16]. Several existing large-scale cohort studies provide knowledge on radiation-related side effects among children, adolescents, and young adults spanning over four decades of follow-up for those treated in the 1970s and 1980s. Comparatively few clinical trials randomize radiotherapy dose schedules, let alone trials comparing old and newly developed technology for radiotherapy. Nonetheless, opportunities to study side effects of radiotherapy beyond 90 days after initial treatment based on clinical trials are increasingly available.
Large-scale retrospective cohort studies, including hundreds or even several thousands of 1-yr, 2-yr- or 5-yr childhood cancer survivors (CCS) typically involve retrospective registration of treatment characteristics to a varying degree of detail (and completeness) while smaller-scale efforts typically allow for a more in-depth characterization of treatment per individual; short-term side effects cannot be studied here. For the pre-millennium era, in-depth studies rely on retrospective radiation dose reconstruction, using 2D simulation films and radiotherapy treatment charts as a basis to derive mean organ doses, or, in nested case-control studies, to estimate the radiation dose received by the tissue that later developed a second tumor or adverse normal tissue effects [17, 18].
Modeling Considerations
Normal tissue complication probability (NTCP) modeling applies a relatively well-defined set of statistical tools. What is modeled typically is the probability (or incidence in a group of patients) of a dichotomous (binary or yes/no) endpoint as a function of dose, volume and possibly adjusting for patient-level risk factors or other treatment modalities. Central to this is the mathematical formulation of a sigmoidal dose response curve, frequently assumed to follow a logistic regression relationship or, in case of (for example) a probit dose-response curve [19]. Analysis of late endpoints require an analytic method that takes latent time and censored observations into account and this is often accomplished using the Cox proportional hazards model or in some studies a mixture model [20, 21]. In general, it is necessary to model both the effect of follow-up time since treatment as well as attained age. In some situations, attained age has been used as the underlying time scale, rather than follow-up time [22]. In terms of reporting the outcome of a Cox model analysis, it is desirable to report the baseline hazard function in addition to the estimated hazard ratios. This will allow assessment of the progression of the dose response of toxicity over time. Note though, that the proportional hazards assumption may or may not be fulfilled for a given endpoint. This assumption should be tested to evaluate whether this model is an adequate description of risk-time relationships.
This data analytical framework is not always a good fit for the endpoints of relevance in childhood cancer survivors, largely for two reasons. First, because of continuous growth and development in childhood cancer survivors, modeling the probability of exceeding a threshold effect size (i.e. dichotomizing the outcome) represents a loss of information when the endpoint is naturally represented as a continuous (scale) variable, e.g. retardation of bone growth [23], intelligence quotient decline [24], or endocrine function [25]. In these cases, a more adequate description may be obtained from considering the actual value at a given time point as a function of dose, age, or other variables. In case of serial observations, the measurements at different time points will not be independent and this must be taken into account in the modeling approach. Second, many of the late morbidities associated with childhood cancer therapy are not specific to treatment. Examples could be cardiovascular events evaluated as valvular dysfunction or acute coronary events, or second solid malignancies. These events will also occur with a given probability in external (non-cancer survivor) controls. In this case it is more informative to consider the excess absolute risk or the excess relative risk of developing the morbidity at a given attained age. Again, multivariable models may be used that allows consideration of covariates.
When individual level dose assessment is available the linear-quadratic model is often applied to adjust for dose-time-fractionation [12, 14]. Adjustment for patient characteristics or other treatment modalities in multivariable models and, where relevant, stratified analyses, is key owing to the importance of these variables for risk estimation. This relates to the increasing number of side effects from chemotherapeutic agents and their additive or synergistic effects with radiation that are being recognized and the fact that demographic and treatment details are far from being uniformly distributed in a childhood cancer survivorship cohort [26].
Reports of pediatric late effects often have a relatively small sample size, may have heavy censoring i.e. relatively short follow-up in many patients, or may not have accurate individual organ doses. PENTEC researchers face the dilemma whether to exclude those data that likely underestimated the incidence of morbidity or have a significant uncertainty in estimated doses.
Infants and young children may require specific toxicity evaluations to capture effects on cognitive ability and cooperation. An example is the use of auditory brainstem response and optoacoustic emissions for evaluating hearing of younger children and pure-tone air conduction testing for older children. When test methods and toxicity scales change during long-term follow-up, care should be taken to minimize the influence of such changes in model fitting. Another source of variability arises from variation in selection of endpoints and toxicity grading instruments adopted by different institutions. Judgement should be exercised in determining if models built based on synthesized data are reliable given the heterogeneity, and if they contradict empirical experience. When modeling toxicity for paired organs, such as ovaries, cochlea, and kidneys, one should consider if the radiation effect can be assessed per organ, and measures of organ function in the literature reports to be synthesized are consistent with each other. Glomerular filtration rate for kidney function is an example of a test where it is difficult to tease out the effect of differential irradiation to paired organs.
Data Identification and Abstraction Methodology
Like its predecessor QUANTEC [27], the PENTEC collaboration aims to provide a comprehensive overview of the published literature on long-term side effects of radiation therapy for cancer in children and adolescents and to derive guidance for current and future clinical practice on the dose-volume-risk relationship for several organs at risk (OAR). Given the potential impact of these findings on care for individual patients, adequate methodology and critical appraisal of available data is required.
The PENTEC Core Group thus proposed a standardized methodology to identify, select, and evaluate eligible evidence. The methods were based on the experience with systematic review development within the Cochrane Childhood Cancer group [28] and with clinical guideline development [29]. A systematic review summarizes the evidence on a specific subject in a transparent and reproducible manner.
PENTEC uses a four-step approach for identification and extraction of data for each Organ Specific Report (OSR). Each working group (task force) prepared a protocol with support from Core Group members with experience in systematic reviews, guideline development, and epidemiology (LK, CMR). First, relevant clinical questions (CQ) were formulated, e.g.: “What is the association between radiation dose/volume and impaired spermatogenesis, defined as azoospermia and oligospermia, in male childhood cancer survivors treated with radiotherapy involving the testes?“(Figure 1). For each CQ, a so-called PICO was established, unequivocally defining four elements of eligible research findings: P=patient, I=intervention; C=comparison; O=outcome. Second, based on the PICOs, the Cochrane Childhood Cancer group together with the individual task force leaders developed a literature search in an iterative process. Then each of the four elements of the PICO was translated into standardized terms that could be entered in a PubMed search combined by AND or OR. (Figure 2) Most searches were of the form: #Radiotherapy AND #Childhood Cancer (4) AND #<specific outcome>. The exceptions were the CQ’s for total body irradiation (TBI) as a conditioning for hematopoietic stem cell transplantation (HSCT) where the Intervention (I) was defined more specifically compared to the standardized Radiotherapy search term developed for other chapters. The expert task force leader was consulted at least once during the building of the final search definition, to allow for further adaptations. Generally, a balance was necessary between overly specific search terms, resulting in a few hundred potentially eligible titles, but missing several relevant reports, versus a rather general search that captures thousands or tens of thousands of reports. Third, a set of initial inclusion and exclusion criteria for eligible research studies were defined, based on Study Design (e.g. no case reports), Patient Groups (e.g. maximum proportion of adults in a study), radiation assessment definition (depending on preferences of the respective task force and availability of evidence that greatly varies across health outcomes); and Outcomes. Also, some groups chose to limit eligible reports to English only, depending on foreign language proficiency of their respective group members, and most groups limited the eligibility to reports published in recent decades only (Figure 3). Fourth, the data were abstracted and general aspects of the quality of studies such as selection bias or follow-up bias were assessed by one or two task force members, using model data extraction and coding forms where needed with organ-specific extensions or modifications to suit the goals of the respective task force. Finally, data analysis, including descriptive synopsis of the most relevant reports and, where possible, meta-analysis, will be undertaken. The analyses are currently in progress. The final compendium of reports will include a series of introductory reviews that discuss relevant methodologic issues (Table 1), and “visionary” reviews that look to the future (Table 2). The organ specific reports will discuss a spectrum of critical elements inherent to fulfilling the objectives of PENTEC (Table 2). The organ-specific teams and leaders are denoted in Table 3, and the PENTEC core committee members in Table 4.
Figure 1:

Example research question
Figure 2:

Example PubMed search strategy
Figure 3:
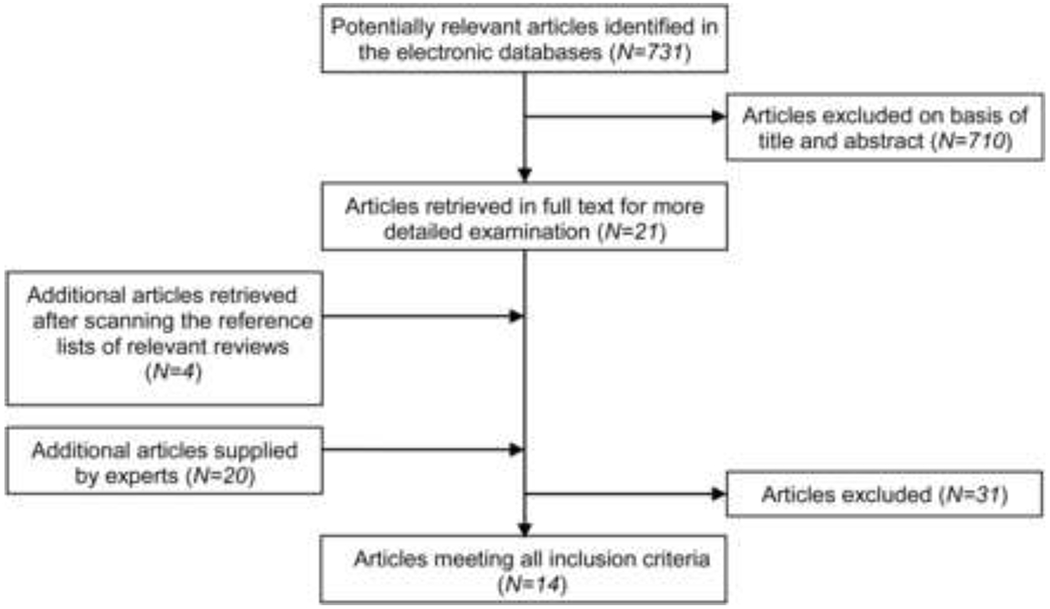
Example flow diagram to show included and excluded studies
Table 1:
Introductory and Visionary Reports
| Introductory Reports | Visionary Reports |
|---|---|
| Introduction to scientific issues | Methodology for accurate data acquisition of radiation dose data |
| Summary of Pediatrics NTCP data and models | Biomarkers and surrogate endpoints |
| Pediatric bio-developmental considerations | Pediatric imaging issues |
| Pediatric physics aspects | Secondary malignancy as impacted by evolution of technology |
| Epidemiologic considerations | Recommendations for reporting and gathering data—to cooperative groups |
| Improving NTCP and modeling in pediatrics | Future directions |
| Contrasting PENTEC vs. QUANTEC |
Table 2:
Sections within each organ-specific report (OSR)
| Required Sections | Content |
|---|---|
| Anatomy & developmental dynamics | •Define anatomy as it impacts normal tissue damage •Describe anatomic and physiologic development according to age as it impacts organ sensitivity to damage and repair |
| Clinical significance | •Describe the clinical situations where the organ is irradiated •Describe the frequency, characteristics, significance of injury |
| Endpoints & toxicity scoring | •Address strengths and limitations of existing systems •Recommend how to score organ injury •Describe the different endpoints often considered when assessing injury •Describe the time course of organ injury |
| Challenges defining volumes: pediatric imaging issues | •Describe recommended imaging modality and acquisition methods •Discuss the impact of intra-/inter-fractional organ movement or volume change during the course of treatment •Discuss the need for contouring planning organ-at risk volumes (PRV) •Note normal organ contouring atlases or reference existing publically available atlases. |
| Review of dose volume response data/risk factors | •Review of dose-volume data: For each organ (tissue), published data on toxicity risks as correlated with dosimetric parameters and other relevant variables (i.e. age, developmental status), are reviewed. From the available data, meaningful dose/volume limits with associated risk rates are presented. Include data on various dose fractionations, adequacy, quality, and bias. •Dose Volume Endpoints: --Organ Function: Lab/subclinical endpoints, imaging endpoints, physiologic/functional issues --Organ Development: Impact of age --Second malignant neoplasms: volume, dose •Risk Factors: genetic predispositions, gender, race, age, co-medical conditions •Chemotherapy/Combined modality: Relevant chemotherapy data impacting radiation sensitivity •Mathematical/Biologic models + Epidemiologic issues: For each organ, models that have been used to relate dose/volume data to normal tissue complications and second malignancies in the organ are summarized, along with associated model parameters, limitations and uncertainties. |
| •Recommended Dose/Volume Limits- The available information is condensed into meaningful dose/volume limits, with associated risk rates, for clinical application. Limits are according to endpoints and age. •Level of evidence: specify for each report •Special situations: situations where the presented data/models may not apply (e.g. hypofractionation) |
|
| Toxicity scoring recommendations | •Recommendations on how to score organ injury and toxicities. |
| Interventions and Management | |
| Contrast Pediatric & Adult NTCP data | |
| Future Investigations | •Describes areas in need of future study |
Table 3:
PENTEC Working Groups
| Organ | PI |
|---|---|
| Central Nervous System | Anita Mahajan, MD |
| Head and Neck | Arnold Paulino, MD |
| Cerebrovascular | Shannon MacDonald, MD |
| Endocrine | Gregory Wheeler, MBBS, FRANZCR |
| Hearing | Torunn Yock, MD |
| Ocular | Stephanie Terezakis, MD |
| Thyroid | Michael Milano, MD, PhD |
| Pulmonary | Mary Frances McAleer, MD, PhD |
| Breast | Karen Marcus, MD |
| Cardiovascular | David Hodgson, MD, MPH, FRCPC |
| GI and Hepatic | Julie Bradley, MD |
| Genitourinary | Matthew Poppe, MD |
| Male reproductive system | Bradford Hoppe, MD, MPH |
| Female reproductive system | Christine Hill-Kayser, MD |
| Musculoskeletal/Integument | Natia Esiashvili, MD |
| Spinal cord | Nadia Laack, MD |
| Second malignancies | Kenneth Roberts, MD |
| Stem cell transplantation (TBI) | Kathryn Dusenbery, MD |
| Re-irradiation | Thankamma Ajithkumar, MD |
Table 4:
PENTEC Core Committee
| Name | Affiliation |
|---|---|
| Louis S. Constine, MD, FASTRO, FACR | University of Rochester |
| Soren Bentzen, DSc, PhD, FASTRO | University of Maryland School of Medicine |
| David Hodgson, MD, MPH | University of Toronto |
| Rebecca Howell, PhD | M. D. Anderson |
| Chia-Ho Hua, PhD | St. Jude Children’s Research Hospital |
| Melissa Hudson, MD | St. Jude Children’s Research Hospital |
| Andrew Jackson, PhD | Memorial Sloan-Kettering Cancer Center |
| Leontien Kremer, MD, PhD | Emma Children’s Hospital/AMC, Amsterdam |
| Karen Marcus, MD, FACR | Dana Farber Cancer Institute |
| Lawrence Marks, MD | University of North Carolina |
| Michael Milano, MD, PhD | University of Rochester |
| Arthur Olch, PhD, FAAPM | University of Southern California Keck School of Medicine and Children’s Hospital Los Angeles |
| Cecile Ronckers, PhD | Emma Children’s Hospital/AMC, Amsterdam |
| Jackie Williams, PhD | University of Rochester |
| Ellen Yorke, PhD | Memorial Sloan-Kettering Cancer Center |
Summary: Challenges, Needs, Hurdles, Knowledge Gaps
PENTEC has revealed a multiplicity of challenges in exploring and defining normal tissue tolerances in developing children as a function of radiation dose/volume, chemotherapy, and surgery. This information could inform individual radiation oncologists regarding tolerance doses, inform protocols regarding radiation dose constraints, and envision research directions. However, PENTEC has identified several hurdles to surmount:
Inadequate data on the organ developmental changes that alter tissue radiation effects, and shortcomings of existing models
Inadequate data on the association of radiation dose/volume/fraction size and other parameters that impact radiation sensitivity to injury
Inadequate consideration of confounding factors (surgery, chemotherapy, pre-existing conditions) in decision-making on radiation dose-volume-fractionation choices
Inadequate data analytical methods and insufficient reporting of modeling results
Inadequate patient-level dosimetry for relevant organs at risk
Selected examples of more specific knowledge gaps are:
Age dependence of dose tolerances for most organs; for some we have excellent data but for most we have huge data deficiencies
The influence of chemotherapy (agents, doses) on RT dose tolerance for many organs
For many pediatric organ systems, we lack data on plasticity/compensatory potentials following injury
Role of known behavioral risk factors (e.g. smoking, obesity, nutrition, etc.) on radiation-related adverse events (e.g. cardiac, pulmonary, some SMN, etc.)
Dose-response associations for long-term (>10 yr .. >20 yr ... >30 yr) risk of almost all of the PENTEC outcomes
Retreatment dose tolerances
- For most organs, substructures exist and for these we lack data on dose tolerance much less the impact of volume (i.e. the entirety of the substructure vs. a portion of that substructure). Examples include:
- Normal brain substructure tolerances such as the hippocampus, corpus collosum
- Specific cardiac substructures (chambers, valves) and coronary arteries
- Individual and joint roles of cardiac, carotid artery, and cerebral vessel radiation exposure on cerebrovascular accident risk
Role of hormonal exposures (either intrinsic deficiencies or external exposures) on the probability of developing organ-specific SMN (in particular hormone-receptor positive tumors).
The timing of exposures during the years of development and puberty on bone hypoplasia
Relevance of low dose exposures versus peak or mean doses on SMNs
Normal tissue tolerances after particle therapy, acknowledging the immaturity of the follow-up data available to address this
Besides exposure assessment for radiotherapy, there is a great need for clear, clinically-relevant definitions of health outcomes, and well-described methods to ascertain health outcomes in the reality of clinical practice, insurance programs, funding for research, and data protection laws [13].
While PENTEC will address some of these (and multiple other) knowledge gaps, many of these clinically and scientifically important issues will await future investigations.
Conclusions
Advances in radiation therapy and chemotherapy have markedly increased the survival rate for almost all pediatric malignancies. Unfortunately, these treatment modalities on their own or in combination may result in long term adverse outcomes that may affect the individual’s quality of life. Therefore, it is essential to balance the benefits against risks. Both length of survival and the impact of late normal-tissue effects on the quality of that survival must be considered in therapy decision-making. While the range of adverse effects is well described, the quantitative dose-volume-effect estimates for their generation must be better defined, as well as the impact of risk/patient factors such as developmental status and genetic susceptibility. Other therapeutic factors are also critical to explore; chemotherapy may enhance radiation effects, and very little is known about the evolving use of immunotherapy and how these agents interact with other treatments including radiation. Thus, the optimal use of radiation therapy in multimodality therapy that can cure children with cancer requires a better understanding of the adverse consequences of all treatment modalities. Finally, new patterns of late morbidity and mortality may emerge as survivors continue to age, and it is only through continued study that such patterns will be identified and interventions for treatment and prevention of adverse effects can be designed. These are the gaps that PENTEC seeks to elucidate and potentially fill.
The PENTEC investigators are gratified to play a role in refining and enhancing the role of radiation therapy in safely and effectively treating pediatric malignancy, and we dedicate our efforts to all future children who will be affected by this malady.
Highlights.
RT for pediatric cancer can cause long-term adverse normal tissue effects
Radiation damage depends on the radiation dose and volume, and developmental status
For some organs, chemotherapy can exacerbate the effects of radiation
PENTEC seeks to increase knowledge about pediatric RT dose constraints for organs
Radiation dosimetric data should be precisely reported in pediatric RT studies
Acknowledgements
The authors thank Mrs. Laura Finger of the University of Rochester for editorial assistance, and the American Association of Physicists in Medicine (AAPM) for its logistical support.
AJ was supported in part by NIH Grant P30 CA008748. SMB was supported in part by NIH grant P30 CA134274. CMR was supported by the Dutch Cancer Society grant UvA 2012-5517. LK and Cochrane Childhood Cancer received support from Children Cancer Free Foundation (KiKa), The Netherlands
References
- [1].Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–13. [DOI] [PubMed] [Google Scholar]
- [2].Krasin MJ, Constine LS, Friedman DL, Marks LB. Radiation-related treatment effects across the age spectrum: differences and similarities or what the old and young can learn from each other. Semin Radiat Oncol. 2010;20:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Paulino AC, Constine LS, Rubin P, Williams JP. Normal tissue development, homeostasis, senescence, and the sensitivity to radiation injury across the age spectrum. Semin Radiat Oncol 2010;20:12–20. [DOI] [PubMed] [Google Scholar]
- [4].Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. [DOI] [PubMed] [Google Scholar]
- [5].Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Geenen MM, Cardous-Ubbink MC, Kremer LC, van den Bos C, van der Pal HJ, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–15. [DOI] [PubMed] [Google Scholar]
- [7].Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390:2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cohen L, Creditor M. Iso-effect tables for tolerance of irradiated normal human tissues. Int J Radiat Oncol Biol Phys. 1983;9:233–41. [DOI] [PubMed] [Google Scholar]
- [10].Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–57. [DOI] [PubMed] [Google Scholar]
- [11].Lee C, Jung JW, Pelletier C, Pyakuryal A, Lamart S, Kim JO, et al. Reconstruction of organ dose for external radiotherapy patients in retrospective epidemiologic studies. Phys Med Biol. 2015;60:2309–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–23. [DOI] [PubMed] [Google Scholar]
- [13].Newhauser WD, Berrington de Gonzalez A, Schulte R, Lee C. A Review of Radiotherapy-Induced Late Effects Research after Advanced Technology Treatments. Front Oncol 2016;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hawkins MM, Wilson LM, Burton HS, Potok MH, Winter DL, Marsden HB, et al. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst. 1996;88:270–8. [DOI] [PubMed] [Google Scholar]
- [15].Boice JD Jr, Blettner M, Kleinerman RA, Stovall M, Moloney WC, Engholm G, et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst. 1987;79:1295–311. [PubMed] [Google Scholar]
- [16].Breslow NE, Norkool PA, Olshan A, Evans A, D’Angio GJ. Second malignant neoplasms in survivors of Wilms’ tumor: a report from the National Wilms’ Tumor Study. J Natl Cancer Inst. 1988;80:592–5. [DOI] [PubMed] [Google Scholar]
- [17].Stovall M, Donaldson SS, Weathers RE, Robison LL, Mertens AC, Winther JF, et al. Genetic effects of radiotherapy for childhood cancer: gonadal dose reconstruction. Int J Radiat Oncol Biol Phys. 2004;60:542–52. [DOI] [PubMed] [Google Scholar]
- [18].Alziar I, Bonniaud G, Couanet D, Ruaud JB, Vicente C, Giordana G, et al. Individual radiation therapy patient whole-body phantoms for peripheral dose evaluations: method and specific software. Phys Med Biol. 2009;54:N375–83. [DOI] [PubMed] [Google Scholar]
- [19].Bentzen SM, Tucker SL. Quantifying the position and steepness of radiation dose-response curves. Int J Radiat Biol. 1997;71:531–42. [DOI] [PubMed] [Google Scholar]
- [20].Bentzen SM, Thames HD, Travis EL, Ang KK, Van der Schueren E, Dewit L, et al. Direct estimation of latent time for radiation injury in late-responding normal tissues: gut, lung, and spinal cord. Int J Radiat Biol. 1989;55:27–43. [DOI] [PubMed] [Google Scholar]
- [21].Tucker SL, Dong L, Bosch WR, Michalski J, Winter K, Mohan R, et al. Late rectal toxicity on RTOG 94–06: analysis using a mixture Lyman model. Int J Radiat Oncol Biol Phys. 2010;78:1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yasui Y, Liu Y, Neglia JP, Friedman DL, Bhatia S, Meadows AT, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–13. [DOI] [PubMed] [Google Scholar]
- [23].Silber JH, Littman PS, Meadows AT. Stature loss following skeletal irradiation for childhood cancer. J Clin Oncol. 1990;8:304–12. [DOI] [PubMed] [Google Scholar]
- [24].Merchant TE, Kiehna EN, Li C, Shukla H, Sengupta S, Xiong X, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;65:210–21. [DOI] [PubMed] [Google Scholar]
- [25].Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738–44. [DOI] [PubMed] [Google Scholar]
- [26].Teepen JC, van Leeuwen FE, Tissing WJ, van Dulmen-den Broeder E, van den Heuvel-Eibrink MM, van der Pal HJ, et al. Long-Term Risk of Subsequent Malignant Neoplasms After Treatment of Childhood Cancer in the DCOG LATER Study Cohort: Role of Chemotherapy. J Clin Oncol. 2017;35:2288–98. [DOI] [PubMed] [Google Scholar]
- [27].Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cochrane Childhood Cancer. https://childhoodcancer.cochrane.org/
- [29].Kremer LC, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


