Abstract
Background
The neuropeptide oxytocin (OT) has been shown to play a role in variety of cognitive and social processes and different hypotheses have been put forth to explain OT’s effects on brain and behavior in humans. However, these previous explanatory accounts do not provide information about OT-related temporal modulation in the brain.
Objectives
This paper systematically reviewed intranasal OT administration studies employing event-related potentials (ERPs) and synthesized the existing evidence into a novel conceptual framework.
Methods
Empirical studies, published until February 2020 and cited in major databases (EBSCOhost, PubMed, and Web of Science), were examined in accordance with PRISMA guidelines. To be included, studies had to: (i) employ intranasal administration of OT, as the chemical modulator; (ii) measure ERPs; (iii) be peer-reviewed journal articles; (iv) be written in English; and (v) examine human participants.
Results
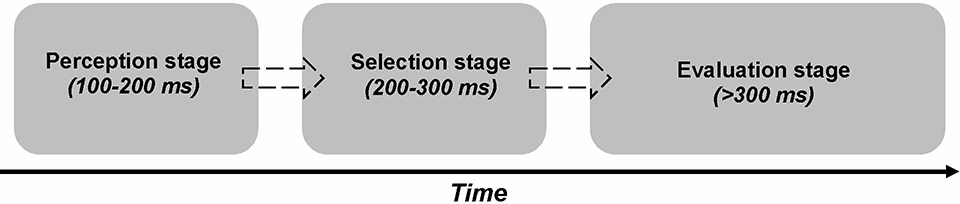
The search criteria yielded 17 empirical studies. The systematic review resulted in conceptualization of the Tri-Phasic Model of Oxytocin (TRIO), which builds on three processing stages: (i) perception, (ii) selection, and (iii) evaluation. While OT increases attention irrespective of stimuli characteristics in the perception stage, in the selection and evaluation stages, OT acts as a filter to guide attention selectively towards social over non-social stimuli and modulates prosociality/approach motivation associated with social stimuli.
Conclusions
TRIO offers an empirically-derived conceptual framework that can guide the study of OT-related modulation on attentional processes, starting very early in the processing stream. This novel account furthers theoretical understanding and informs empirical investigation into OT modulation on the brain.
Keywords: Oxytocin, ERPs, intranasal administration, attention, social salience, prosociality, approach/withdrawal
1. Introduction
The neuropeptide oxytocin (OT) constitutes a crucial chemical modulator of cognition and behavior in both animals and humans. Synthesized mainly in the paraventricular and supraoptic nuclei of the hypothalamus, OT axons project to various areas including the amygdala, hippocampus, striatum, suprachiasmatic nucleus, brainstem, and pituitary gland, leading to modulatory effects both centrally and peripherally (Insel, 2010; Quintana, Alvares, Hickie, & Guastella, 2015a).
While early animal research focused on OT effects in pair bonding, sexual reproduction, and maternal behavior (Young & Wang, 2004), more recent studies investigated OT effects on various aspects of cognition (Hung et al., 2017; Piva & Chang, 2018). In humans, OT has been shown to play a role in basic and more complex processes including modulation of visual attention (i.e., eye gaze modulation, Eckstein et al., 2018; Guastella, Mitchell, & Dadds, 2008), facial expression identification (Shahrestani, Kemp, & Guastella, 2013), trust (Nave, Camerer, & McCullough, 2015), social reward (Groppe et al., 2013), and cooperation (De Dreu & Kret, 2016). OT’s effects also appear to have clinical implications in developmental (autism spectrum disorder; Andari et al., 2010; Domes et al., 2013) and psychiatric (Cochran, Fallon, Hill, & Frazier, 2014) disorders as well as in healthy (Barraza et al., 2013; Campbell, Ruffman, Murray, & Glue, 2014; Horta, Kaylor, Feifel, & Ebner, 2019a) and pathological (Finger et al., 2015) aging.
Different hypotheses have been put forth to explain the effects of OT. One of the earliest is the prosocial hypothesis (MacDonald & Macdonald, 2010; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2007). This hypothesis states that OT increases attention to positive (e.g., happiness) but not to negative (e.g., fear) emotions and thus enhances affiliative prosocial behaviors such as trust, empathy, and altruism. Support for the prosocial hypothesis comes from human research showing an inhibitory effect of OT on the amygdala to negative stimuli (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008; Kirsch et al., 2005). For example, Kirsch et al. (2005) found reduced amygdala activation in response to fearful stimuli following intranasal OT compared to placebo administration, reflecting OT’s anxiolytic properties, possibly by reducing stress and promoting prosocial behavior.
However, contradictory evidence emerged from studies suggesting that OT increased stress (Eckstein et al., 2014) and protective behavior, including aggression (Bosch, Meddle, Beiderbeck, Douglas, & Neumann, 2005). Shamay-Tsoory et al. (2009), for example, found that OT modulated not only positive but also negative social behavior and emotion. They argued that OT enhances prosocial behavior only when the context involves cooperative and positive emotions. In competitive and aggressive contexts, in contrast, OT promotes aggression and negative emotions. This alternative explanatory account was coined the social salience hypothesis, which argues that rather than generally enhancing prosocial behavior, OT increases the perceived salience of social cues in the environment in a context specific manner, regardless of social cue valence (Shamay-Tsoory & Abu-Akel, 2016).
Building on the social salience hypothesis, Kemp and Guastella (2010, 2011) put forward the social approach/withdrawal hypothesis. This account states that OT increases salience of both positive (e.g., joy, trust) and negative (e.g., anger, gloating) emotions that elicit approach-related behavior. At the same time, OT inhibits withdrawal-related behavior which serves moving away from negative stimulation (e.g., anxiety). Evidence for this hypothesis was based on studies showing that OT increased recognition of both positive and negative social stimuli (e.g., happy and angry faces; Tollenaar, Chatzimanoli, van der Wee, & Putman, 2013), decreased the experience of social threat (e.g., attenuation of brain activity for aversive stimuli; Petrovic, Kalisch, Singer, & Dolan, 2008), and encouraged approach-related and prosocial behavior (e.g., trust; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005).
An alternative account put forth by Bartz, Zaki, Bolger, and Ochsner (2011) (see also Olff et al., 2013) proposed that the effects of OT on social cognition are not only influenced by contextual factors but also by interindividual variables. This interactionist model was based on findings that OT can constrain or amplify social cognition and behavior depending on (i) the context (e.g., experimental task features, in- vs out-group status), (ii) interindividual variables (e.g., sex, personality, age, expertise), and (iii) person−context interactions. For example, participants who received intranasal OT behaved more altruistically toward in-group members but more aggressively toward out-group members, when their own group was threatened with financial loss (De Dreu & Kret, 2016). OT was also shown to increase empathy selectively in less compared to more socially proficient individuals (Bartz et al., 2010).
Finally, recently, Piva and Chang (2018) proposed the multistage model. According to this framework OT exerts its effect on multiple stages of the social decision-making process. These stages include sensory perception, valuation, decision formulation, and behavioral output, with possible feedback from each of these stages to one or more previous stage(s) to subsequently impact the next set of computations. Specifically, this model proposes that OT modulates the salience and relative value of stimuli by increasing or decreasing noise at different processing stages, which in turn influences approach motivation and prosocial tendencies. That is, OT simultaneously affects perception and salience of social stimuli as well as the propensity to approach or avoid stimuli as a function of context (e.g., reward present vs. absent; Chang, Barter, Ebitz, Watson, & Platt, 2012) or interindividual differences (e.g., males vs. female rhesus monkeys; Winslow, Noble, Lyons, Sterk, & Insel 2003).
The multistage model provides a unifying framework to consolidate existing OT findings. However, assumptions of the model are currently based on intracellular activity recording in animals and functional magnetic resonance imaging (fMRI) in humans without considering evidence from event-related potential (ERP) studies of OT in humans. Thus, in its current form the multistage model does not integrate fine-grained information about OT-related temporal modulation in the brain.
1.1. Scope and Aims of the Review
In this paper, we systematically reviewed ERP studies using intranasal OT administration to summarize current knowledge on OT’s temporal processes in the human brain and synthesized this literature in the context of a novel conceptual framework, the Tri-Phasic Model of Oxytocin (TRIO). ERPs provide high temporal resolution (on the milliseconds level; see Box 1 for a short description of the ERP technique). To date, the ERP technique has been only rarely used in OT research. There are different methodological approaches to study OT-related effects in humans (see Box 2) and there is an ongoing discussion in the literature as to whether peripheral OT concentrations reliably index central OT levels (Carson et al., 2014; Leng & Ludwig, 2019; Valstad et al., 2017). In addition, there are statistical concerns regarding the power of OT studies leveraging genetic approaches to reliably predict and detect small effects that single nucleotide polymorphisms (i.e., genetic variations) may have on brain functioning and behavior (for a meta-analysis, see Bakermans-Kranenburg & Van Ijzendoorn, 2014). The current systematic review therefore exclusively focused on ERP studies employing intranasal OT administration, which is thought to result in central OT effects (Quintana, Smerud, Andreassen, & Djupesland, 2018).
Box 1: Description of the ERP technique.
ERPs represent the synchronous activation of electrical fields associated with the activity of a large population of neurons in response to sensory, motor, cognitive, affective, etc. events (Fabiani, Gratton, & Federmeier, 2007; Luck, 2005). Specifically, ERPs reflect the summed activation of excitatory postsynaptic potential and inhibitory postsynaptic potential elicited by a stimulus or response. The ERP technique has a poor spatial resolution, but it is very sensitive to real-time neural processes, providing temporal resolution on the scale of milliseconds.
ERPs are characterized in terms of latency (delay of appearance after an event), direction (positive or negative), amplitude (amount of voltage change), and topological (scalp) distribution (frontal, occipital, parietal, etc.) of the components. ERP measurements quantify the amplitude (in microvolts) and latency (in milliseconds) of the waveform associated with a specific stimulus or response. The ERP recording yields several components that provide information about neural processing stages, including both early sensory (i.e., perceptual and attentional operations) and later (i.e., high-level cognitive processing) stages.
Box 2: Measures of OT effects in humans.
OT is released both into the central nervous system (CNS) and into the peripheral circulation from neurosecretory cells in the paraventricular and supraoptical nuclei of the hypothalamus, where most endogenous OT is synthesized. Central and peripheral compartments of the OT system are separated anatomically by the blood-brain barrier (Neumann & Landgraf, 2012). OT level and function cannot be imaged yet, but there are various approaches in humans to measure and/or modulate the OT system centrally.
One approach is to measure endogenous peripheral OT concentrations in blood (Feldman, Weller, Zagoory-Sharon, & Levine, 2007), urine (Seltzer, Ziegler, & Pollak, 2010), or saliva (Carter et al., 2007) and to correlate concentration levels with brain response (Lancaster et al., 2015) and behavior (Feldman, Gordon, & Zagoory-Sharon, 2011).
Another approach is to determine central OT concentrations in the CNS by performing lumbar punctures (Kagerbauer et al., 2013). This approach is highly invasive.
OT can be exogenously administered via nasal spray (Guastella et al., 2013; Quintana et al., 2015a; Striepens et al., 2013), based on evidence that this route of application allows OT to circumvent the blood-brain-barrier (Born et al., 2002; Quintana et al., 2018). There is evidence from animal studies showing increased central OT levels after intranasal administration (Lee et al., 2018; Smith, Korgan, & Young, 2019).
Some studies have focused on genetic variation in the oxytocinergic system, such as related to OT receptor genes (e.g., OXTR reflecting OT receptor, CD38 reflecting OT secretion). For example, GG allele carriers of OXTR rs53576 show higher sensitivity to social cues than A allele carriers (AA/GA) (Kogana et al., 2011). Also, CC carriers of CD38 rs3796863 show improved processing of socially relevant stimuli compared to A allele carriers (AA/AC) (Sauer, Montag, Wörner, Kirsch, & Reuter, 2012).
Finally, OT can be measured and/or modulated via: examining distribution of OT receptors within the human postmortem brain (Mazurek, Beal, Bird, & Martin, 1987); topical gel which affects the area of application locally potentially by increasing cell proliferation (Torky et al., 2018); intravenous (IV) administration, with subsequent action across the blood-brain barrier (Hollander et al., 2003).
Specifically, the current review aimed at: (i) improving understanding of temporal characteristics of OT effects in the brain by providing a systematic review of ERP studies using intranasal OT administration; (ii) synthesizing the existing evidence into a novel conceptual framework that can guide the study of OT-related temporal modulation in the brain; and (iii) identifying current research gaps and promising future research directions for the study of OT-related temporal brain dynamics.
2. Methodology
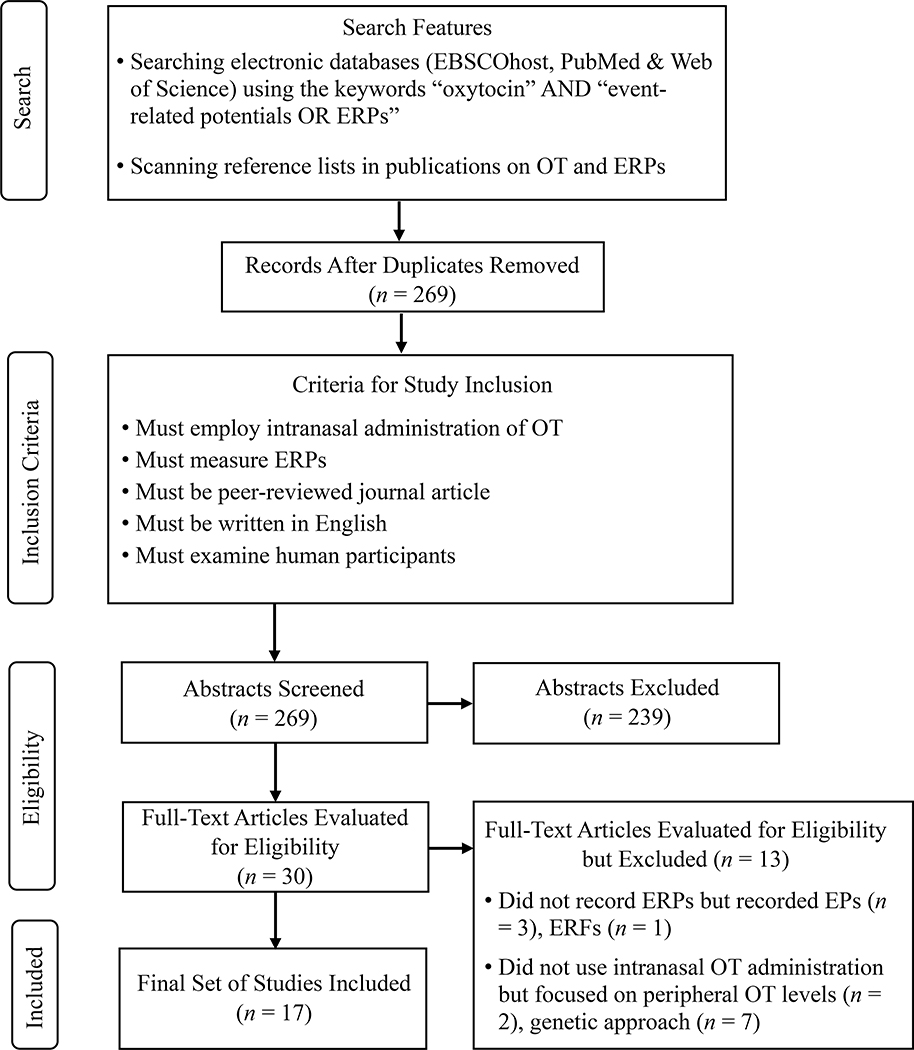
This review was conducted in accordance with PRISMA guidelines (Moher, Liberati, Tetzlaff, Altman, & The PRISMA Group, 2009). The systematic literature search comprised articles published from the first date available to February 2020. We used the keywords “oxytocin” AND “event-related potentials OR ERPs” in EBSCOhost, PubMed, and Web of Science. We also scanned reference lists of the papers found. Details concerning the method of literature search and criteria for inclusion and exclusion of studies are shown in Figure 1. To be included studies had to: (i) employ intranasal administration of OT, as the chemical modulator; (ii) measure ERPs; (iii) be peer-reviewed journal articles; (iv) be written in English; and (v) examine human participants. After removing duplicates and screening abstracts, our search criteria yielded 30 articles. Of the 30 articles, 4 were excluded because they did not record ERPs and 9 were excluded because they did not employ intranasal OT administration (see Table S1 in the supplemental material for an overview of studies pertaining to peripheral OT measures and studies adopting a genetic approach that were identified in our literature search but were not included in our review). The final set included 17 articles using intranasal OT administration (all single dose studies) and ERPs. Table 1 provides an overview of all ERP studies included in this review.
Figure 1.
PRISMA flow chart for the search and inclusion/exclusion criteria for studies in this review. Note: OT = Oxytocin; ERP = Event-related potential; EP = Evoked potential; ERF = Event-related field
Table 1:
ERP research using intranasal OT administration (organized by latency)
| Study (year) | Female/Male (F/M) | Age (range or mean in years) | Sample size | Dosage | Design | Task | Latency in ms (ERP component if applicable) | Main findings |
|---|---|---|---|---|---|---|---|---|
| Peltola et al. (2018) | F | 20–40 | 38 (mothers) | 24 IU | Within | Facial emotion categorization task (happy and sad faces of infants vs. adults) | 120–220 (N170); 300–600(LPP) | N170: OT (vs. P) elicited larger ERPs, regardless facial age and emotion. |
| Huffmeijer et al. (2011) | F | 18–30 | 27 | 24 IU | Within | Eriksen Flanker task with emotional feedback (happy and disgusted faces) | 140–180 (VPP); 300–450 (N400) | No treatment effect on ERPs. |
| Huffmeijer et al. (2013) | F | 18–30 | 48 | 16 IU | Within | Same task as in Huffmeijer et al. (2011) | 140–180 (VPP); 400–800 (LPP) |
VPP: OT (vs. P) elicited larger ERPs for faces in participants reporting lower maternal love withdrawal. LPP: OT (vs. P) elicited larger ERPs for faces. |
| Tillman et al. (2019) | M | 19–32 | 21 | 24 IU | Within | Expt.1: Passive viewing of faces (fearful vs. neutral) Expt.2: Passive viewing of neutral faces |
72–110 (P1); 110–210 (N170); 200–300 (EPN) |
N170: OT (vs. P) elicited shorter latency to fearful than neutral faces in Expt.1. No treatment effect on ERPs in Expt.2. |
| de Bruijn et al. (2017) | M | 18–30 | 24 | 24 IU | Within | Eriksen Flanker task (individual vs social context) | 0–200 (ERN); 150–250 (Early Pe); 300–500 (Late Pe) |
ERN: OT elicited larger ERPs for mistakes occurred in social (vs. individual) contexts. P elicited similar ERPs in both contexts. Late Pe: P elicited decreased ERPs for mistakes occurred in social (vs. individual) contexts. OT elicited similar ERPs in both contexts. |
| Ruissen et al. (2015) | M | 18–35 | 63 (31 OT; 32 P) | 24 IU | Between | Go/No-Go task (individual vs social context) | 225–275 (N2); 300–500 (P3) | N2: OT (vs. P) elicited larger ERPs for Go trials in the social than the individual context. |
| Born et al. (1987) | M | 22–33 | 17 | 40 IU | Within | Auditory Oddball task | 180–360 (N2); 280–460 (P3) | No treatment effect on ERPs |
| Liu et al. (2013) | M | 18–30 | 20 | 32 IU | Within | Trait judgment task | 80–120 (N1); 220–280 (P2); 520–1000 (LPP) |
P2: OT (vs. P) reduced ERPs for self-referential traits. LPP: OT (vs. P) reduced ERPs for self-referential traits but increased ERPs for traits with reference to others. |
| Sheng et al. (2013) | M | 18–26 | 16 | 32 IU | Within | Facial race categorization task (painful or neutral Asian vs. Caucasian faces) | 84–116 (N1); 140–180 (N170); 128–188 (P2); 200–300 (N2); 400–700 (P3) | P2: OT (vs. P) elicited larger ERPs to painful than neutral faces of racial ingroup but not racial out-group faces. OT (vs. P) resulted in positive correlation between P2 amplitudes and implicit attitude toward in-group members. |
| Rutherford et al. (2017) | F | 18–31 | 24 | 24 IU | Within | Passive viewing of houses, infant, and adult faces | 149–225 (N170); 200–400 (P3); 300–600 (LPP) | P3: OT (vs. P) elicited larger ERPs for infant than adults faces. |
| Waller et al. (2015) | M | 30–50 | 24 (fathers) | 24 IU | Within | Passive viewing of child faces (own vs. unfamiliar) | 90–140 (P1); 142–200 (N170); 210–300 (P2); 300–350 (N250); 350–400 (N3) | N3: P elicited more negative ERPs for own-child than familiar-child or unfamiliar-child faces. This own-child effect disappeared under OT in fathers with secure attachment. |
| Althaus et al. (2015) | M | 18-34 | 61 (30 HC; 31 ASD) | 24 IU | Within | Passive viewing of images of humans and scenes | 600–1000 (LPP) | OT (vs. P) elicited larger ERPs to pleasant images with humans than those without human in ASD patients who were oversensitive to stressful situations and in HC who were sensitive to criticism and punishment. |
| Althaus et al. (2016) | M | 18–34 | 61 (30 HC; 31 ASD) | 24 IU | Within | Same task as in Althaus et al. (2015) | 600–1000 (LPP) | OT (vs. P) elicited larger ERPs for images with humans than without humans only in men with higher plasma OT levels in the ASD group. |
| Petereit et al. (2019) | F | 24 | 90 (45 OT; 45 P) | 40 IU | Between | Cyberball game (social vs. nonsocial context) | 800–1400 (LPP) | No treatment effect on ERPs. |
| Herzmann et al. (2013) | F & M | 18–28 | 52 (26 F: 13 OT, 13 P; 26 M: 13 OT, 13 P) | 24 IU | Between | Facial race encoding and recognition task | 100–132 (P1); 140–184 (N170); 188–272 (P2); 300–500; 500–800; 750–1000; 800–1200 |
750–1000 ms: Larger ERPs reflecting encoding advantage for own- (vs. other-) race faces in OT (vs. P) group. 800–1200 ms: Larger ERPs reflecting recognition memory advantage for own-race faces in women in OT (vs. P); in contrast, other-race effect was found for men in OT (vs. P). |
| Wu et al. (2013) | M | 20–25 | 46 (24 OT; 22 P) | 32 IU | Between | Object ownership categorization task (self vs. other) | 300–1000 | OT resulted in larger ERPs for positively than negatively valenced other-owned objects; P resulted in the reversed pattern. |
| Schiller et al. (2020) | M | 18–39 | 86 (43 OT; 43 P) | 24 IU | Between | Decision-making task resulting in gain vs. loss for in-vs. out-group members | 200–500 | OT (vs. P) reduced negative valuation of out-group gains; this pattern was only evident in participants with low (vs. high) empathy. |
ASD=Autism Spectrum Disorder; HC=Healthy controls; ERN=Error related negativity; ERP=Event-related potentials; IU=International unit; Pe=Error positivity; LPP=Late positive potential; P=Placebo; OT=Oxytocin; VPP=Vertex positive potential
Note: Design column indicates whether OT was a within- or between-subjects design factor.
3. Review of OT-related ERP Studies
This section summarizes the results from our systematic literature review regarding ERP studies employing intranasal OT administration (see also Table 1). We have organized major empirical outcomes from short (100–200 ms) over middle (200–300 ms) to long (>300 ms) ERP component latencies towards improved understanding of temporal characteristics of OT effects.
3.1. Short latency (100–200 ms)
Both the N170 and the vertex positive potential (VPP) components are associated with the encoding of structural information from faces based on initial processing of facial features (Rossion & Jacques, 2012). These components are also modulated by valence and/or arousal of stimuli (Carretié, Hinojosa, Martín-Loeches, Mercado, & Tapia, 2004).
In a within-subjects design study, 24 IUs of OT (vs. placebo) enhanced N170 amplitude on occipitotemporal electrode sites in mothers during a facial expression categorization task regardless of the age (infant vs. adult) and expression (happy vs. sad) of the facial stimuli (Peltola, Strathearn, & Puura, 2018). Administration of 16 IUs of OT (vs. placebo) in a within-subjects design study resulted in larger VPP amplitudes over central electrodes in women who reported lower maternal love withdrawal for faces presented as feedback in a flanker task irrespective of facial expression (happy vs. disgusted; Huffmeijer et al., 2013; but see Huffmeijer, Tops, Alink, Bakermans-Kranenburg, and van IJzendoorn, 2011 for non-significant effects in the same task with women who applied 24 IUs of OT vs. placebo). These findings suggest that OT-related attentional facilitation occurs irrespective of stimulus characteristics (e.g., valence, facial age) and under different task settings, ranging from simple categorization to more demanding selective attention/inhibition.
However, there are exceptions to the effect of OT-related facilitation operating irrespective of stimuli and task context in short latency ERP components. In particular, 24 IUs of OT (vs. placebo) administration in a within-subjects design study with men resulted in a shorter N170 latency over posterior electrode sites to fearful than neutral faces, suggesting that OT increased processing efficiency for arousing faces (Tillman et al., 2019). Furthermore, in a within-subjects design study with men who applied 24 IUs of OT vs. placebo, OT elicited larger error related negativity (ERN) at frontal and frontocentral electrodes for mistakes in a flanker task that was performed in dyads (vs. individually). In contrast, the placebo elicited similar ERPs for mistakes regardless of whether the task was performed individually or in dyads (de Bruijn, Ruissen, & Radke, 2017). This finding suggests an OT-related increase in error detection, which could have resulted from distress knowing that one’s actions may negatively affect another person in the dyadic task context.
Taken together, the current evidence supports that OT may enhance information processing irrespective of stimulus characteristics and task demands in short latency ERP components. Exceptions were arousing stimuli or interactive tasks with high social stakes for which OT facilitated recruitment of attentional resources, such as prioritization of arousing over neutral stimuli or social over individual mistakes. There also is evidence that interindividual differences (e.g., childhood experience) moderate OT-related effects in short latency ERPs.
3.2. Middle latency (200–300 ms)
The N2 component reflects processing of emotional arousal (particularly pleasant-going; Olofsson, Nordin, Sequeira, & Polich, 2008) and response conflict (Yeung, Botvinick, & Cohen, 2004). In a between-subjects design study using 24 IUs of OT vs. placebo, men completed a Go/No-Go task either individually or in dyads (Ruissen & de Bruijn, 2015). Relative to placebo, OT resulted in faster response times and larger N2 amplitudes at fronto-central and parietal electrode sites for Go trials in the dyad compared to the individual condition, possibly reflecting OT-related increase in social processing when responding together with a partner. This finding suggests that OT-dependent increase in middle latency ERPs may be selectively associated with social rather than non-social task contexts. This interpretation is also consistent with the finding that N2 amplitudes in a non-social auditory oddball task did not differ between men who self-administered 40 IUs of OT vs. placebo (Born, Fehm-Wolfsdorf, Lutzenberger, Voigt, & Fehm, 1987).
Another mid-latency component is the P2 (Hillyard et al., 1973) which reflects selective attention and feature processing, such as detailed perceptual analysis of faces (Ebner, He, & Johnson, 2011) as well as empathic neural response to other’s pain (Han, 2018). It is particularly sensitive to semantic categorization including self- and other-referential processing (Mu & Han, 2010) and in- and out-group processing (Sheng & Han, 2012). In a within-subjects design study with men, 32 IUs of OT (vs. placebo) reduced the P2 over fronto-central electrodes for self-related personality judgments, but P2 for other-related (well-known celebrity) personality judgments was similar between the drug conditions (Liu, Sheng, Woodcock, & Han, 2013). In addition, this OT effect was stronger in individuals who viewed themselves as being more strongly connected with others (i.e., interconnectedness). These findings suggest that OT may alter the balance between self- and other-referential processing and that an OT-related reduction in self-referential processing may influence understanding of others, with this effect more pronounced in individuals reporting higher levels of interconnectedness. In the context of in- vs. out-group membership, 32 IUs of OT (vs. placebo) resulted in larger P2 at fronto-central electrode sites in response to pain expressions of own-race (Asian) compared to other-race (Caucasian) faces in a within-subjects design study with men (Sheng, Liu, Zhou, Zhou, & Han, 2013). This finding suggests that OT-related facilitation in neural processing related to empathy towards the suffering of in- compared to out-group members.
Taken together, middle latency ERP studies suggest that OT may guide attention by increasing salience of social over non-social task contexts. Importantly, OT may increase attention to self-relevant group membership enhancing empathy towards in- over out-group members. This OT-enhanced neural tuning towards in-group others appears to be moderated by stimulus characteristics (e.g., valence/arousal, facial race), contextual factors (e.g., social vs. individual task setting), and interindividual differences (e.g., sociocultural interconnectedness).
3.3. Long latency (>300 ms)
The later segment of OT effects on ERPs is dominated by the P3 component (also called P3a) which reflects allocation of attention to salient task-relevant stimuli (Polich & Kok, 1995) and higher-order cognitive operations (e.g., updating contents of working memory; Polich, 2007). In a within-subjects design study, non-mothers showed a larger P3 over central electrodes for infant compared to adult faces following 24 IUs of OT (vs. placebo) administration (Rutherford et al., 2017). Focusing on N3, which is another long latency component sensitive to affective processing (Carretié & Iglesias, 1995), in a within-subjects design study with fathers who administered 24 IUs of OT vs. placebo, Waller et al. (2015) found larger N3 for own- than familiar- or unfamiliar-child faces in fathers with insecure attachment following OT administration. This effect was not obtained in fathers with secure attachment who showed similar N3 for all child faces irrespective of familiarity. These findings highlight a role of interindividual differences in OT effects on temporal processing in the context of child-parent attachment during long latency components.
P3 is followed by a positive slow wave denoted as late positive potential (LPP) or P3b which reflects sustained attention toward motivationally significant stimuli (Pehlivanoglu & Verhaeghen, 2019; Schupp, Flaisch, Stockburger, & Junghöfer, 2006) and processes related to semantic evaluation of information (e.g., perspective taking, empathy; Nieuwenhuis, Aston-Jones, & Cohen, 2005). Using a within-subjects design, administration of 24 IUs OT (vs. placebo) elicited greater LPP over parietal electrodes to images containing humans (vs. not containing humans) in healthy men who were sensitive to anticipated punishment and criticism (Althaus et al., 2015). This effect was also evident in men with autism spectrum disorder who were oversensitive to stressful situations (Althaus et al., 2015) and also in those patients who had higher plasma OT concentrations than healthy controls (Althaus et al., 2016). These findings indicate that OT enhances LPP for social over non-social stimuli particularly in some individuals or clinical groups.
The previously mentioned study by Huffmeijer et al. (2013) found larger LPP across centroparietal electrodes in women for both happy and disgusted faces after intranasal OT but not placebo administration. Also, in another previously mentioned study by de Bruijin et al. (2017), placebo elicited decreased ERPs (300–500 ms) for mistakes in social (vs. individual) contexts, while OT elicited similar ERPs in both social and individual contexts. These findings demonstrate that OT effects on LPP are influenced by the arousal level of stimuli and task context (but see Petereit, Rinn, Stemmler, & Mueller, 2019, for a non-significant effect of task context on LPP amplitudes in women who self-administered 40 IUs of OT vs. placebo).
Furthermore, in the previously mentioned study by Liu et al. (2013), OT relative to placebo reduced LPP over fronto-central electrodes for self-referential personality trait judgements, but increased LPP for other-related judgments. This finding suggests that OT effects on LPP are more pronounced for other- than self-related processing. Wu, van Dijk, and, Zhou (2013) used 32 IUs of OT or placebo in a between-subjects design study with men who indicated ownership of objects primed with either negative or positive adjectives. While ERPs during the 300–1000 ms window were larger for positive than negative other-owned objects in the OT group, this pattern was reversed in the placebo group. Thus, OT-related facilitation in other-related processing was linked to positive rather than negative valence.
In the context of in- vs. out-group membership, in a between-subjects design study with men and women, 24 IUs of OT vs. placebo increased ERPs (750–1000 ms) over parietal electrode sites, reflecting familiarity-based encoding of own-race (Caucasian) rather than other-race (Chinese) faces during encoding (Herzmann, Bird, Freeman, & Curran, 2013). During recognition, women in the OT vs. placebo group showed larger ERPs (800–1200 ms) at mid-frontal regions reflecting familiarity-based recognition advantage for own- (vs. other-) race. In contrast, men in the OT (vs. placebo) group showed this memory advantage for other- (vs. own-) race faces. More recently, in a between-subjects design study, a group of men who varied in levels of empathy and group membership (i.e., belonging to rival groups in soccer or politics) administered 24 IUs of OT (vs. placebo). They then completed a decision-making task involving distribution of resources to in- or out-group members which in turn resulted in gain or loss of points/resources (Schiller, Domes, & Heinrichs, 2020). ERP data during 200–500 ms time window over posterior electrode sites showed that negative valuation of out-group gains occurred in the placebo group. The OT group, in contrast, showed positive valuation of out-group gains. Importantly, this pattern was only evident in individuals who reported low (vs. high) levels of empathy. These findings suggest that OT eliminated negative valuation processes towards out-group members especially in individuals with low empathy.
Taken together, OT-related increase in sustained attention during long latency ERP components specifically occurs for socially arousing over non-arousing stimuli, possibly reflecting selective semantic evaluation of motivationally significant information. Importantly, OT tends to modulate attentional processes in this late processing stage toward facilitation of other- compared to self-relevant information or group membership, possibly as a reflection of positive evaluations associated with others. OT effects are further modulated by contextual information and interindividual differences.
4. Tri-Phasic Model of Oxytocin (TRIO)
Based on our systematic review of OT-related ERP studies in humans, we propose the Tri-Phasic Model of Oxytocin (TRIO). The literature demonstrates that OT-related ERP modulation starts early, remains sustained over time, and occurs across different processing stages. In particular, as depicted in Figure 2, TRIO builds on three processing stages: (i) perception, (ii) selection, and (iii) evaluation. Each stage will be introduced in more detail next and discussed in reference to the reviewed literature and in relation to existing hypotheses of OT.
Figure 2.
The Tri-Phasic Model of Oxytocin (TRIO). OT-related modulation on attention allocation starts early, remains sustained over time, occurs across three processing stages: perception, selection, and evaluation. During the short-latency perception stage, OT enhances initial perceived salience, possibly by increasing allocation of attention irrespective of stimuli characteristics and type of cues. During the middle-latency selection stage, OT guides allocation of attention more selectively to prioritize social over non-social stimuli, possibly by increasing allocation of attention towards self-relevant group membership/close others. During the long-latency evaluation stage, OT-induced semantic elaboration takes place through increased sustained attention to motivationally salient information, reflected in a greater focus on other-related higher-order cognitive processing. Interindividual differences impact OT’s temporal aspects in each processing stage. While OT effects during the selection and evaluation stages differ qualitatively depending on stimulus characteristics or contextual factors, such modulatory effects occur only under some circumstances in the perception stage. Dashed arrows depict potential interrelations between processing stages throughout the processing stream. Box length reflects time window associated with the processing stage.
The perception stage is reflected in short-latency OT-related modulation (~100−200 ms). According to TRIO, in this stage, OT enhances initial perceived salience, possibly regardless of stimulus characteristics (e.g., valence, facial age) and in a variety of contexts/tasks (e.g., from passive viewing to selective attention paradigms) (Huffmeijer et al., 2013; Peltola et al., 2018). Although not included in this systematic review, using genotyping and image valence and arousal ratings in males, more negative N1 to images in OXTR rs53576 GG than AA carriers was found irrespective of image content (humans vs. objects) and valence ( Choi, Minote, & Watanuki, 2017; see Table S1). This finding indicates that OT-related enhancement in this early processing stage may occur for both social (e.g., faces, images involving humans) and non-social (e.g., objects) stimuli and is in line with findings from intranasal studies in which OT effects occur irrespective of stimuli characteristics and type of cues. Under some conditions, however, OT prioritizes perception of socially salient information in this stage, namely when the information requires immediate attention (e.g., high arousal stimuli; Tillman et al., 2019; see also Peltola et al., 2014 in Table S1 for shorter N1 latency in response to viewing distressed infant (vs. adult) faces in both mothers (vs. non-mothers) and GG (vs. non-GG) allele carriers of OXTR rs53576) or when social stakes are high (de Bruijn et al., 2017).
The observed modulatory effects of OT in the perception stage are not entirely consistent with predictions from the social salience hypothesis (Shamay-Tsoory & Abu-Akel, 2016). That is, while the social salience hypothesis predicts OT-related enhancement in attention for social over non-social cues, ERP studies suggest that an OT-related boost in attention in this early stage may be independent of stimulus characteristics and may be comparable for social and non-social cues. Moreover, while some studies indicate that OT-related modulation in the perception stage seems to occur mostly based on initial perceived salience, involvement of early processing pertaining to higher-order cognitive processes (e.g., empathy, perspective taking) may factor in under some circumstances (e.g., performing tasks in dyads; de Bruijn et al., 2017) in this early perception stage. Thus, the extent to which ERP effects in this early perception stage can be mapped onto the prosocial (MacDonald & Macdonald, 2010) and the social approach/withdrawal (Kemp & Guastella, 2010) hypotheses is yet to be determined. In contrast, current ERP evidence from the perception stage partially supports the interactionist model (Bartz et al., 2011; Olff et al., 2013), in that interindividual differences (e.g., childhood experiences; Huffmeijer et al., 2013) moderate OT-related attentional modulation in this stage, and those modulatory effects may be conditional on stimulus characteristics and contextual factors (e.g., high arousal, high social stakes; de Bruijn et al., 2017; Tillman et al., 2019).
The selection stage is reflected in middle-latency OT-related modulation. During this stage, OT guides allocation of attention more selectively to prioritize social over non-social cues (Ruissen & de Bruijn, 2015), possibly by increasing allocation of attention towards self-relevant group membership. For example, OT-induced increase in approach and prosocial tendencies, reflected by enhanced empathy-related neural response in P2 for the suffering of members of the in-group compared to the out-group (Sheng et al., 2013) first appears in this stage. Findings from ERP studies employing OT-related genetic approach support these interpretations. Highlighting the selection of social over non-social cues during middle latency ERP components, Choi et al. (2017 in Table S1), for example, found more negative N2 to images of humans relative to objects in male GG allele carriers of OXTR rs53576, while male AA carriers showed the opposite pattern. In the context of inter-group interactions, Luo et al. (2019 in Table S1) reported more inclusive and flexible empathic neural response (P2) toward in-group members in GG compared to AA carriers of OXTR rs53576.
Overall consistent with the social salience hypothesis (Shamay-Tsoory & Abu-Akel, 2016), TRIO proposes that in the selection stage, OT may enhance salience of social over nonsocial stimuli, with stimulus salience modulated by emotional valence and/or arousal. Further, in line with the prosocial hypothesis (MacDonald & Macdonald, 2010) and the social approach/withdrawal hypothesis (Kemp & Guastella, 2010), approach and prosocial motivation become apparent during the selection stage in the form of empathy-related neural response favoring close other in-group members. Finally, consistent with the interactionist model (Bartz et al., 2011; Olff et al., 2013), OT-related attention facilitation for social stimuli in the selection stage may be influenced by interindividual differences (e.g., sociocultural interconnectedness; Liu et al., 2013) in addition to stimulus characteristics (e.g., facial race; Sheng et al., 2013) and contextual factors (e.g., social vs. individual task; Ruissen & de Bruijn, 2015).
The evaluation stage is reflected in long-latency OT-related modulation. During this stage, OT-induced semantic elaboration takes place through increased sustained attention to motivationally salient information. For example, OT effects in this stage become more focused on other-related higher-order cognitive processing (Liu et al., 2013), compared to the selection stage where OT-induced increase in allocation of attention occurs for self-relevant (e.g., in-group) information. Moreover, OT-induced effects favoring other-relevant processing in the evaluation stage were positively rather than negatively valenced (Wu et al., 2013; Schiller et al., 2020). Thus, OT facilitates approach motivation (Kemp & Guastella, 2010) and prosocial tendency (MacDonald & Macdonald, 2010) in this late evaluation stage, possibly enhancing empathy and perspective taking towards motivationally significant stimuli.
Furthermore, consistent with the social salience hypothesis (Shamay-Tsoory & Abu-Akel, 2016), OT-related attention modulation in this late evaluation processing stage occurs particularly for emotionally arousing over non-arousing social stimuli (such as demonstrated in processing of infant (vs. adult) faces in Rutherford et al., 2017). The observed ERP patterns are also in accord with the interactionist model (Bartz et al., 2011; Olff et al., 2013), in that OT-related effects in this late evaluation stage, like in the selection phase, are influenced by interindividual differences (e.g., social skills; Althaus et al., 2015, 2016; attachment patterns; Waller et al., 2015; gender, Herzmann et al., 2013), stimulus characteristics (e.g., valence; Wu et al., 2013; also see Fowler et al., 2018 in Table S1 for larger LPP in GG (vs. A) allele carriers of OXTR rs53576 for socially threatening (vs. neutral or happy) images), as well as contextual factors (e.g., group relations; Schiller et al., 2020).
Taken together, our systematic review of the current intranasal OT-related ERP literature supported a modulatory role of OT on attention allocation that starts early, is maintained over time, and spans over three processing stages. While OT-guided perceptual scanning of stimuli that occurs very early in the processing stream appears to be independent of cue type or stimuli characteristics, upon detection of significant social stimuli, OT may act as gateway to assure that social over non-social stimuli have priority access for higher level processing, possibly by favoring self- relevant group/close other-related information in the selection stage, followed by other-related information in the evaluation stage.
One important advantage of conceptualizing OT effects in the context of TRIO is that it offers a common empirically-derived conceptual framework for OT-modulated attentional effects, ranging from selective to more sustained forms of attention allocation. Although existing hypotheses provide important predictions to explain OT effects on brain and behavior, these predictions do not speak to the temporal processes of OT modulation (e.g., timing and duration of OT effects). TRIO, in contrast, formalizes the temporal dynamics of OT-related modulation in the brain, starting very early in the processing stream and reaching into late processing components. More importantly, the newly proposed TRIO framework builds upon a widely accepted conceptualization of attentional effects in ERP research. As touched on briefly in Box 1, this conceptualization posits that the sequence of components following a stimulus reflects the temporal sequence of neural processes tapping into different cognitive subsystems (e.g., early versus late) triggered by the stimulus, beginning with early sensory processes and proceeding through decision- and response-related processes (Luck, Woodman, & Vogel, 2000). Thus, TRIO is a first attempt to formalize how attentional effects of intranasal OT may operate in different cognitive subsystems under different task conditions by leveraging excellent temporal resolution of the ERP technique. TRIO is empirically derived and theoretically grounded and allows formulating specific research hypotheses that can be tested (confirmed or rejected) in future investigations. Through this process, TRIO can be refined, extended, and revised moving forward.
To exemplify, according to TRIO, processing appears to become more focused on social over non-social stimuli later in the processing stream, while stimuli characteristics are less prominent earlier in the processing. Thus, it is plausible that OT-related modulation may be more automatic in the earlier processing stages and become more controlled later on. To investigate this possibility, future research could employ selective attention or dual-task paradigms and determine (i) whether OT-guided attentional effects occur independent of the availability of attentional resources and (ii) whether these effects are further modulated by processing stages. If the magnitude of OT-related ERP patterns at different processing stages change based on availability of attentional resources as predicted by TRIO, then varying task demands to manipulate required attention allocation could be useful in determining the extent to which OT shifts automatic vs. controlled processes across the processing stream. Depending on the results, TRIO could be further refined and extended to account for variations in OT-related ERP patterns as a function of the availability of attentional resources at different levels of processing. If, however, the magnitude of OT-related ERP patterns proves to be similar throughout processing regardless of the availability of attentional resources, then the stagewise structure of TRIO should be revisited and modified to account for less variation in OT-related processing throughout the processing stream. Thus, TRIO can be advanced in the future via a gradual iterative process involving rigorous hypothesis testing. A selection of important research avenues based on TRIO’s basic assumptions towards uncovering functional significance of OT-related temporal dynamics in the brain are discussed next.
5. Leveraging TRIO in Future Research
Based on our systematic review of OT-related ERP research and our literature synthesis in conceptualizing TRIO, we have identified promising future research directions with potential to advance theory and empirical understanding of OT’s temporal dynamics in the brain. Next, we will briefly discuss select future research suggestions.
5.1. Interrelations Between Processing Stages
Given the scarce literature base, it is not possible at this point to determine whether the three processing stages proposed in TRIO operate in isolation or in tandem throughout the processing stream. In fact, while some studies suggest that OT-related ERP effects may occur sequentially (de Bruijn et al., 2017; Liu et al., 2013), other studies support that the processing stages may overlap and/or interact (Sheng et al., 2013; Ruissen & de Bruijn, 2015). Thus, it is plausible that OT-related modulation in ERPs is sequential, with an initial perceptual scanning of stimuli in the perception stage, followed by more specialized processing operations in the selection and evaluation stages. However, it is also possible that the distinction between the stages become less distinct with OT, resulting in increased overlap and/or interaction between the stages. TRIO-guided research to refine propositions regarding temporal sequencing and interactions among the stages will advance theory and enhance empirical understanding of OT effects. Future ERP studies could, for example, systematically manipulate stimulus aspects (e.g., social nature vs. arousal level) within a task context or vary required processing depth or task complexity (e.g., simple categorization vs. social decision making) to enhance understanding of the interrelations between the processing stages as a function of OT.
5.2. Differentiation of Types of Social-Cognitive Information
According to TRIO, OT boosts processing of information, maybe irrespective of type of cue or stimuli characteristics in the perception stage, while processing appears to become more focused on social over non-social stimuli in the selection and evaluation stages; with a possible prioritization of self-relevant group/close other-relevant information in the selection phase (Sheng et al., 2013), and a prioritization of other-relevant information in the evaluation stage (Liu et al., 2013; Schiller et al., 2020; Wu et al., 2013). A between-subject design fMRI study with men who self-administered either 40 IUs intranasal OT or placebo showed overlap in cortical regions involved in processing of trait judgments describing self and close-others (i.e., one’s mother) in the OT group, but resulted in distinct brain regions for these two conditions in the placebo group (Zhao et al., 2016). This finding further supports that OT may reduce the distinction between self and others, particularly close others. TRIO supports a possible OT-guided shift in attentional focus from self or close-others to others throughout the processing stream. Building on this framework, future studies could benefit from multimodal imaging (e.g., ERP and fMRI) combined with behavioral assessment to follow up on this initial proposal of temporal specifics for different types of social stimuli, with the goal to disentangle self- and other-relevant processing and to identify mechanisms of action underlying OT’s temporal gating of different types of social information.
5.3. Interindividual Differences and Stimulus/Context Variations
Interindividual differences can impact OT’s modulation of temporal aspects in all three processing stages (Althaus et al., 2015, 2016; Huffmeijer et al., 2013; Liu et al., 2013), while variations of OT modulatory effects on temporal dynamics by stimulus characteristics and contextual factors primarily take place during the selection (Ruissen & de Bruijn, 2015) and evaluation (Schiller et al., 2020) stages, and only under some circumstances during the perception stage (high arousing and high social stakes stimuli; de Bruijn et al., 2017; Tillman et al., 2019). Embedding future research in TRIO offers to systematically determine conditions under which OT modulation is affected by interindividual variables in interaction with stimulus/contextual factors along the processing stream. This line of research could be fruitful to identify individual and subgroup responsiveness to OT modulation on temporal aspects under specific circumstances (i.e., stimuli and/or task contexts). For example, there is growing evidence of age- and age-by-sex related changes in the OT system (Campbell et al., 2014; Ebner, Maura, Macdonald, Westberg, & Fischer, 2013; Horta et al., 2019b; Plasencia, Luedicke, Nazarloo, Carter, & Ebner, 2019). However, findings regarding age and sex moderation on OT-related modulation is mixed. For example, while Kirsch et al. (2005) found reduced amygdala activation to fearful faces in men following intranasal OT administration, Domes et al. (2010) found an opposite effect in women. Also, while Campbell et al. (2014; see also Ebner et al., 2015) reported age-differential OT effects pertaining to emotion identification, Grainger et al. (2018) found no age-related benefit of OT on social-cognitive abilities. Of note, only one OT-related ERP study examined effects of sex (Herzmann et al., 2013) and no study to date considered age effects. Thus, future research is needed on OT-modulated attention allocation within TRIO that incorporates age- and sex-heterogenous samples.
5.4. Leveraging OT’s Temporal Dynamics Beyond Social Processes
TRIO predicts that OT increases perceived salience and facilitates processing, possibly irrespective of type of cue (social vs. non-social) or stimuli characteristics (e.g., valence, arousal) earlier in the processing stream (i.e., the perception stage). This prediction is consistent with recent perspectives which conceptualize OT-related modulation in brain and behavior to account for both social and non-social contexts (for reviews, see Hahari-Dahan & Bernstein, 2014; Quintana & Guastella, 2019). Specifically, a recent re-conceptualization of the social-approach/withdrawal hypothesis (aka general approach-avoidance hypothesis of oxytocin; Hahari-Dahan & Bernstein, 2014) posits that OT exerts its effect on a variety of personally relevant and emotionally evocative circumstances that range from social (e.g., interpersonal relationships, bonding) to non-social (e.g., survival and adaptation-related situations, such as sensations of pain and thirst) contexts. Furthermore, this position suggests that the broad range of social and non-social effects of OT may be mediated by its action on approach-avoidance motivation and behavior. This modified conceptualization highlights OT’s modulatory role on behavior beyond social contexts and the influence of motivational processes (i.e., one’s motivational state and the motivational significance of stimuli) on OT effects.
Our systematic review suggests that early OT effects may be independent of type of cue or stimulus characteristics. We furthermore conclude that, only in later processing phases, OT effects may become more specific to socially arousing stimuli or task contexts. In these later stages, higher order-cognitive processes including selective attention (selection stage) and evaluation of motivational significance (evaluation stage) come into play. Of note, in its current form, TRIO does not provide any predictions as to whether non-social but motivationally-relevant stimuli or contexts result in OT-related ERPs in the selection or evaluation stage. Rather it proposes that OT-guided processing favors social over non-social stimuli during later stages of processing. Future OT-related ERP studies will benefit from experimental designs that systematically vary task stimuli and/or context to better capture (and possibly dissociate) social (social vs. non-social) as well as motivational (motivationally significant vs not; stimuli relevant of survival vs. not) aspects of OT-related processing and help to describe their temporal dynamics; with new findings providing the basis for further specification of TRIO.
6. Limitations
Like other field of research fields, the literature on OT-related ERP effects is affected by analytical flexibility across studies and publication bias, which impact comparability across studies as well as conclusions drawn. Concerning the ERP technique, for example, some common practices for quantifying (e.g., using mean vs. peak amplitudes; a-priori vs. post-hoc selection of electrode sites and time windows) and analyzing (e.g., risk of inflating Type-I error rates via multiple comparisons) can lead to very high rates of significant-but-bogus effects (Luck & Gaspelin, 2017). Also, the literature on intranasal OT administration has increasingly highlighted the importance of standardized methodology (Guastella et al., 2013; Quintana et al., 2015b) but agreed-upon-guidelines have not yet been consistently implemented (Horta et al., 2019a; Leng & Ludwig, 2016). There also have been growing concerns about statistical reliability and reproducibility of intranasal OT administration studies (Lane, Luminet, Nave, & Mikolajczak, 2016; Quintana, 2018; Tabak et al., 2019; Walum, Waldman, & Young, 2016). Moving forward research on OT’s temporal dynamics using ERP will benefit from following research standards and recommendations for increasing methodological rigor in OT administration (Horta et al., 2019a; Leng & Ludwig, 2016; Walum et al., 2016) and implementing agreed-upon ERP research and analysis practices (such as suggested by the Society for Psychophysiological Research; Keil et al., 2014). Furthermore, adopting an open science model by pre-registering study procedures and predictions to optimize transparency and counter publication bias (Munafò et al., 2017) has potential to move the field forward in a consistent and methodologically rigorous fashion.
Currently little is known about anatomical sources of the observed OT-related ERP effects in the brain. Only Sheng et al. (2013) and Liu et al. (2013) have identified potential neural generators of ERP activity associated with OT. In particular, focusing on self-relevant processing (Liu et al., 2013) and group membership (Sheng et al., 2013), these studies found that OT-related ERPs in the P2 time window were generated in the anterior cingulate and medial prefrontal cortex. This finding is consistent with both previous source localization studies focusing on neural generators of the P2 component (Carretie et al., 2004) and projection sites of OT in the brain, including prefrontal cortex, hippocampus, amygdala and sensory cortices (e.g., Knobloch et al., 2012; Maejima et al., 2014; Quintana & Guastella, 2019). Recent fMRI studies have also reported OT-related facilitation in connectivity between distinct neural regions (e.g., amygdala-prefrontal connectivity, Ebner et al., 2016; cortico-striatal connectivity, Bethlehem et al., 2017) as well as OT-induced change in large-scale neural resting networks (Xin et al., 2018; Brodmann, Gruber, & Goya-Maldonado, 2017). However, to date, there are no OT studies examining neural generators of ERPs systematically by varying stimuli characteristics (social vs. non-social) throughout the processing stream.
A separate line of research focusing on neural sources of ERPs has consistently identified contribution of sensory cortices (e.g., occipito-parietal and occipito-temporal areas; Herrmann & Knight, 2001) as well as centro-parietal (Keil et al., 2002; Sabatinelli et al., 2007) and frontal (Carretie et al., 2004; Holmes et al., 2003) sites to the generation of both early (e.g., P1, N1) and later (e.g., LPP) ERP components, especially when motivationally significant and/or affective stimuli are present (LeDoux, 2000; Vuilleumier, 2002). Thus, it is plausible that, irrespective of the processing stage, ERPs reflecting non-social effects of OT are generated by involvement of sensory cortices, while ERPs that are more specific to social effects of OT originate from large-scale neural networks including brain sites implicated in non-affective (i.e., sensory cortices) and affective (e.g., amygdala, prefrontal and parietal cortex) processing. These brain response patterns may be further moderated by interindividual differences, as suggested by a recent study showing OT-related increase in resting-state EEG activity of neural networks associated with social processing particularly in individuals with high anxiety levels (Schiller, Koenig, & Heinrichs, 2019). Currently, there is not sufficient evidence to draw connections between ERPs and their anatomical sources in the context of OT effects, so the reflections offered here are speculative. Future source localization studies via dense-array EEG recordings or multimodal integration of ERPs and fMRI, for example, will shed light on spatial characteristics of OT-related ERPs.
7. Conclusions
In this paper, we systematically reviewed and synthesized the existing OT-related ERP literature towards development of a novel conceptual framework, TRIO. Going beyond previous models and hypotheses, TRIO proposes that OT modulation occurs in three processing stages ─ perception, selection, and evaluation ─ and offers a theoretical approach that can guide the study of OT-modulation on attentional processes, starting very early in the processing stream and reaching into late processing components. This newly proposed model has the potential to spur future research on OT’s temporal dynamics in the brain.
Supplementary Material
Table S1: OT-ERP research using peripheral measures or genetic approaches (organized by latency)
Highlights.
Systematic conceptual review of oxytocin-related ERP studies in humans
Novel framework on oxytocin’s temporal dynamics in the brain
Oxytocin modulates attention allocation in three processing stages
Impact of oxytocin on cognition starts early and is maintained over time
Future directions to advance theory and research of OT’s temporal modulation
Acknowledgments
This work was supported by a University of Florida Clinical and Translational Science pilot award (NIH/NCATS; UL1 TR000064), a University of Florida Claude D. Pepper Older Americans Independence Center pilot award (P30 AG028740 to NCE), a National Institute on Aging grant (R01 AG059809), as well as through the Department of Psychology, the Institute on Aging, the Center for Cognitive Aging & Memory, and the McKnight Brain Institute at the University of Florida. The authors would like to thank Sam Smith, Tim Hasty, Matt Crawford, and Marilyn Horta for their contributions. The authors have no conflict of interest to declare. Correspondence concerning this article should be addressed to Didem Pehlivanoglu, Department of Psychology, University of Florida, 945 Center Dr, Gainesville, FL 32603.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althaus M, Groen Y, Wijers AA, Noltes H, Tucha O, & Hoekstra PJ (2015). Neuropsychologia Oxytocin enhances orienting to social information in a selective group of high-functioning male adults with autism spectrum disorder. Neuropsychologia, 79, 53–69. [DOI] [PubMed] [Google Scholar]
- Althaus M, Groen Y, Wijers AA, Noltes H, Tucha O, Sweep FC, … Hoekstra PJ (2016). Do blood plasma levels of oxytocin moderate the effect of nasally administered oxytocin on social orienting in high-functioning male adults with autism spectrum disorder ? Psychopharmacology, 233(14), 2737–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel J, Zalla T, Herbrecht E, Leboyer M, & Sirigu A (2010). Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences, 107, 4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2014). A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatric Genetics, 24(2), 45–51. [DOI] [PubMed] [Google Scholar]
- Barraza JA, Grewal NS, Ropacki S, Perez P, Gonzalez A, & Zak PJ (2013). Effects of a 10-day oxytocin trial in older adults on health and well-being. Experimental and Clinical Psychopharmacology, 21(2), 85–92. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, & Ochsner KN (2010). Oxytocin selectively improves empathic accuracy. Psychological Science, 21(10), 1426–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, & Ochsner KN (2011). Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences, 15(7), 301–309. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, & Fehr E (2008). Oxytocin Shapes the Neural Circuitry of Trust and Trust Adaptation in Humans. Neuron, 58(4), 639–650. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, Lombardo MV, Lai MC, Auyeung B, Crockford SK, Deakin J, ... & Baron-Cohen S (2017). Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Translational Psychiatry, 7(4), e1099–e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Dozier M, Bernard K, Grasso D, & Simons R (2013). Foster Mother-Infant Bonding: Associations Between Foster Mothers’ Oxytocin Production, Electrophysiological Brain Activity, Feelings of Commitment, and Caregiving Quality. Child Development, 84(3), 826–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Fehm-Wolfsdorf G, Lutzenberger W, Voigt KH, & Fehm HL (1987). Vasopressin and electrophysiological signs of attention in man. Peptides, 7(2), 189–193. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, & Fehm HL (2002). Sniffing neuropeptides: A transnasal approach to the human brain. Nature Neuroscience, 5(6), 514–516. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, & Neumann ID (2005). Brain Oxytocin Correlates with Maternal Aggression: Link to Anxiety. The Journal of Neuroscience, 25(29), 6807–6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K, Gruber O, & Goya-Maldonado R (2017). Intranasal oxytocin selectively modulates large-scale brain networks in humans. Brain Connectivity, 7(7), 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Ruffman T, Murray JE, & Glue P (2014). Oxytocin improves emotion recognition for older males. Neurobiology of Aging, 35(10), 2246–2248. [DOI] [PubMed] [Google Scholar]
- Carretié L, Hinojosa JA, Martín-Loeches M, Mercado F, & Tapia M (2004). Automatic attention to emotional stimuli: Neural correlates. Human Brain Mapping, 22(4), 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L, & Iglesias J (1995). An ERP study on the specificity of facial expression processing. International Journal of Psychophysiology, 19(3), 183–192. [DOI] [PubMed] [Google Scholar]
- Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, … Parker KJ (2014). Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Molecular Psychiatry, 20(9), 1085–1090. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, & Schwertz D (2007). Oxytocin: Behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Sciences 1098, 312–322. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, & Platt ML (2012). Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proceedings of the National Academy of Sciences, 109(3), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Minote N, & Watanuki S (2017). Associations between the oxytocin receptor gene ( OXTR ) rs53576 polymorphism and emotional processing of social and nonsocial cues : an event-related potential ( ERP ) study. Journal of Physiological Anthropology, 36, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran D, Fallon D, Hill M, & Frazier JA (2014). The role of oxytocin in psychiatric disorders : A review of biological and therapeutic research findings. Harvard Review Psychiatry, 21(5), 219–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn ERA, Ruissen MI, & Radke S (2017). Electrophysiological correlates of oxytocin-induced enhancement of social performance monitoring. Social Cognitive and Affective Neuroscience, 12(10), 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CKW, & Kret ME (2016). Oxytocin Conditions Intergroup Relations Through Upregulated In-Group Empathy, Cooperation, Conformity, and Defense. Biological Psychiatry, 79(3), 165–173. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, & Herpertz SC (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–1190. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, & Herpertz SC (2013). Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biological Psychiatry, 74(3), 164–171. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, & Herpertz SC (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology, 35(1), 83–93. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, & Cohen RA (2016). Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology, 69, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, He Y, & Johnson MK (2011). Age and emotion affect how we look at a face: Visual scan patterns differ for own-age versus other-age emotional faces. Cognition and Emotion, 25(6), 983–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner N, Horta M, Lin T, Fischer H, Cohen R, & Feifel D (2015). Oxytocin modulates meta-mood as a function of age and sex. Frontiers in Aging Neuroscience, 7, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Maura GM, Macdonald K, Westberg L, & Fischer H (2013). Oxytocin and socioemotional aging: Current knowledge and future trends. Frontiers in Human Neuroscience, 7, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Bamert V, Stephens S, Wallen K, Young LJ, Ehlert U, & Ditzen B (2018). Oxytocin increases eye-gaze towards novel social and non-social stimuli. Social Neuroscience, 14(5), 594–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, & Hurlemann R (2014). Oxytocin facilitates the sensation of social stress. Human Brain Mapping, 35(9), 4741–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, & Federmeier KD (2007). Event-Related Brain Potentials: Methods, Theory, and Applications. Handbook of Psychophysiology, 3, 85–119. [Google Scholar]
- Feldman R, Gordon I, & Zagoory-Sharon O (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Developmental Science, 14(4), 752–761. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, & Levine A (2007). Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science, 18(11), 965–970. [DOI] [PubMed] [Google Scholar]
- Finger EC, MacKinley J, Blair M, Oliver LD, Jesso S, Tartaglia MC, … Boxer A (2015). Oxytocin for frontotemporal dementia: A randomized dose-finding study of safety and tolerability. Neurology, 84(2), 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JJ, Braley KL, Farero RD, Russell ET, Phiel CJ, Kisley MA, & Albeck DS (2018). The late positive potential and subjective arousal ratings evoked by negative images vary as a function of oxytocin receptor genotype SNP rs53576. NeuroReport, 29(14), 1145–1150. [DOI] [PubMed] [Google Scholar]
- Grainger SA, Henry JD, Steinvik HR, Vanman EJ, Rendell PG, & Labuschagne I (2018). Intranasal oxytocin does not reduce age-related difficulties in social cognition. Hormones and Behavior, 99, 25–34. [DOI] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, & Spreckelmeyer KN (2013). Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biological Psychiatry, 74(3), 172–179. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, … Banati RB (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology, 38, 612–625. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin Increases Gaze to the Eye Region of Human Faces. Biological Psychiatry, 63(1), 3–5. [DOI] [PubMed] [Google Scholar]
- Harari-Dahan O, & Bernstein A (2014). A general approach-avoidance hypothesis of oxytocin: accounting for social and non-social effects of oxytocin. Neuroscience & Biobehavioral Reviews, 47, 506–519. [DOI] [PubMed] [Google Scholar]
- Han S (2018). Neurocognitive Basis of Racial Ingroup Bias in Empathy. Trends in Cognitive Sciences, 22(5), 400–421. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Tsuru A, Kishida F, Kim YK, Higuchi S, & Motomura Y (2019). ERP study on the associations of peripheral oxytocin and prolactin with inhibitory processes involving emotional distraction. Journal of Physiological Anthropology, 38(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, & Knight RT (2001). Mechanisms of human attention: event-related potentials and oscillations. Neuroscience & Biobehavioral Reviews, 25(6), 465–476. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Bird CW, Freeman M, & Curran T (2013). Effects of oxytocin on behavioral and ERP measures of recognition memory for own-race and other-race faces in women and men. Psychoneuroendocrinology, 38(10), 2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, & Picton TW (1973). Electrical signs of selective attention in the human brain. Science, 182(4108), 177–180. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria C, Aronowitz B, & Mosovich S (2003). Oxytocin Infusion Reduces Repetitive Behaviors in Adults with Autistic and Asperger’s Disorders. Neuropsychopharmacology (New York, N.Y.), 28(1), 193–198. [DOI] [PubMed] [Google Scholar]
- Holmes A, Vuilleumier P, & Eimer M (2003). The processing of emotional facial expression is gated by spatial attention: evidence from event-related brain potentials. Cognitive Brain Research, 16(2), 174–184. [DOI] [PubMed] [Google Scholar]
- Horta M, Kaylor K, Feifel D, & Ebner NC (2019a). Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neuroscience & Biobehavioral Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta M, Ziaei M, Lin T, Porges EC, Fischer H, Feifel D, ... & Ebner NC (2019b). Oxytocin alters patterns of brain activity and amygdalar connectivity by age during dynamic facial emotion identification. Neurobiology of aging, 78, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffmeijer R, Alink LRA, Tops M, Grewen KM, Light KC, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2013). The impact of oxytocin administration and maternal love withdrawal on event-related potential (ERP) responses to emotional faces with performance feedback. Hormones and Behavior, 63(3), 399–410. [DOI] [PubMed] [Google Scholar]
- Huffmeijer R, Tops M, Alink LRA, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2011). Love withdrawal is related to heightened processing of faces with emotional expressions and incongruent emotional feedback: Evidence from ERPs. Biological Psychology, 86(3), 307–313. [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, … Malenka RC (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science, 357(6358), 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2010). The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron, 65(6), 768–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, & Landgraf R (2013). Plasma Oxytocin and Vasopressin do not Predict Neuropeptide Concentrations in Human Cerebrospinal Fluid. Journal of Neuroendocrinology, 25(7), 668–673. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, & Lang PJ (2002). Large-scale neural correlates of affective picture-processing. Psychophysiology, 39(5), 641–649. [DOI] [PubMed] [Google Scholar]
- Keil A, Debener S, Gratton G, Junghöfer M, Kappenman ES, Luck SJ, ... & Yee CM (2014). Committee report: publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology, 51(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Kemp AH, & Guastella AJ (2010). Oxytocin: Prosocial behavior, social salience, or approach-related behavior? Biological Psychiatry, 67, e33–e34. [DOI] [PubMed] [Google Scholar]
- Kemp AH, & Guastella AJ (2011). The Role of Oxytocin in Human Affect : A Novel Hypothesis. Current Directions in Psychological Science, 20(4), 222–231. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, … Meyer-Lindenberg A (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25(49), 11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, … Grinevich V (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron, 73(3), 553–566. [DOI] [PubMed] [Google Scholar]
- Kogana A, Saslowb LR, Impetta EA, Oveisc C, Keltnerd D, & Saturne SR (2011). Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proceedings of the National Academy of Sciences, 108(48), 19189–19192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, & Fehr E (2005). Oxytocin increases trust in humans. Nature, 435(7042), 673–677. [DOI] [PubMed] [Google Scholar]
- Lancaster K, Carter CS, Pournajafi-Nazarloo H, Karaoli T, Lillard TS, Jack A, … Connelly JJ (2015). Plasma oxytocin explains individual differences in neural substrates of social perception. Frontiers in Human Neuroscience, 9, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane A, Luminet O, Nave G, & Mikolajczak M (2016). Is there a publication bias in behavioural intranasal oxytocin research on humans? Opening the file drawer of one laboratory. Journal of neuroendocrinology, 28(4). [DOI] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, ... & Averbeck BB (2018). Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Molecular Psychiatry, 23(1), 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000). Cognitive- emotional interactions: Listen to the brain In Lane RD & Nadel IL (Eds.), Cognitive neuroscience of emotion (pp. 129–155). New York: Oxford University Press. [Google Scholar]
- Leng G, & Ludwig M (2016). Intranasal oxytocin: myths and delusions. Biological Psychiatry, 79(3), 243–250. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sheng F, Woodcock KA, & Han S (2013). Oxytocin effects on neural correlates of self-referential processing. Biological Psychology, 94(2), 380–387. [DOI] [PubMed] [Google Scholar]
- Luck SJ (2005). Ten simple rules for designing and interpreting ERP experiments. Event-Related Potentials: A Methods Handbook, 17–32. [Google Scholar]
- Luck SJ, & Gaspelin N (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn’t). Psychophysiology, 54(1), 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, & Vogel EK (2000). Event-related potential studies of attention. Trends in Cognitive Sciences, 4(11), 432–440. [DOI] [PubMed] [Google Scholar]
- Luo S, Zhang T, Li W, Yu M, Hein G, & Han S (2019). Interactions between oxytocin receptor gene and intergroup relationship on empathic neural responses to others’ pain. Social Cognitive and Affective Neuroscience, 14(5), 505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Zhu Y, Xu Y, & Kong Q (2017). The oxytocinergic system modulates sadistic context-dependent empathy in humans. Scientific Reports, 7(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, & Macdonald TM (2010). The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry, 18(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Sakuma K, Santoso P, Gantulga D, Katsurada K, Ueta Y, ... & Yada T (2014). Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS letters, 588(23), 4404–4412. [DOI] [PubMed] [Google Scholar]
- Mazurek MF, Beal MF, Bird ED, & Martin JB (1987). Oxytocin in Alzheimer’s disease. Neurology, 37(6), 1001. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12, 524–538. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Mu Y, & Han S (2010). Neural oscillations involved in self-referential processing. NeuroImage, 53(2), 757–768. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Nosek BA, Bishop DV, Button KS, Chambers CD, Du Sert NP, ... & Ioannidis JP (2017). A manifesto for reproducible science. Nature human behaviour, 1(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk AJL, Hermann A, Shazly J. El, Grant P, & Hennig J (2016). The Idea Is Good, but . . . : Failure to Replicate Associations of Oxytocinergic Polymorphisms with Face-Inversion in the N170. PLoS One, 11(3), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave G, Camerer C, & McCullough M (2015). Does oxytocin increase trust in humans? A critical review of research. Perspectives on Psychological Science, 10(6), 772–789. [DOI] [PubMed] [Google Scholar]
- Neumann ID, & Landgraf R (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35(11), 649–659. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, & Cohen JD (2005). Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin, 131(4), 510–532. [DOI] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, … van Zuiden M (2013). The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38(9), 1883–1894. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: an integrative review of ERP findings. Biological psychology, 77(3), 247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehlivanoglu D, & Verhaeghen P (2019). Now you feel it, now you don’t: Motivated attention to emotional content is modulated by age and task demands. Cognitive, Affective, & Behavioral Neuroscience, 19(5), 1299–1316. [DOI] [PubMed] [Google Scholar]
- Peltola MJ, Yrttiaho S, Puura K, Proverbio AM, Mononen N, Lehtimäki T, & Leppänen JM (2014). Motherhood and oxytocin receptor genetic variation are associated with selective changes in electrocortical responses to infant facial expressions. Emotion, 14, 469–477. [DOI] [PubMed] [Google Scholar]
- Peltola MJ, Strathearn L, & Puura K (2018). Oxytocin promotes face-sensitive neural responses to infant and adult faces in mothers. Psychoneuroendocrinology, 91, 261–270. [DOI] [PubMed] [Google Scholar]
- Petereit P, Rinn C, Stemmler G, & Mueller EM (2019). Oxytocin reduces the link between neural and affective responses after social exclusion. Biological Psychology, 145, 224–235. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, & Dolan RJ (2008). Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience, 28(26), 6607–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piva M, & Chang SWC (2018). An integrated framework for the role of oxytocin in multistage social decision-making. American Journal of Primatology, 80(10), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasencia G, Luedicke JM, Nazarloo HP, Carter CS, & Ebner NC (2019). Plasma Oxytocin and Vasopressin Levels in Young and Older Men and Women: Functional Relationships with Attachment and Cognition. Psychoneuroendocrinology, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, & Kok A (1995). Cognitive and biological determinants of P300: an integrative review. Biological Psychology, 41(2), 103–146. [DOI] [PubMed] [Google Scholar]
- Quintana DS (2018). Revisiting non-significant effects of intranasal oxytocin using equivalence testing. Psychoneuroendocrinology, 87, 127–130. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Alvares GA, Hickie IB, & Guastella AJ (2015a). Do delivery routes of intranasally administered oxytocin account for observed effects on social cognition and behavior? A two-level model. Neuroscience and Biobehavioral Reviews, 49, 182–192. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Smerud KT, Andreassen OA, & Djupesland PG (2018). Evidence for intranasal oxytocin delivery to the brain: recent advances and future perspectives. Therapeutic Delivery, 9(7), 515–525. [DOI] [PubMed] [Google Scholar]
- Quintana D, & Guastella A (2019). An integrative allostatic account of oxytocin: maintaining stability through change.
- Quintana DS, Westlye LT, Rustan ØG, Tesli N, Poppy CL, Smevik H, ... & Djupesland PG (2015b). Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: a randomized four-way crossover trial with nasal cavity dimension assessment. Translational psychiatry, 5(7), e602–e602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, & Jacques C (2012). The N170: Understanding the Time Course of Face Perception in the Human Brain. The Oxford Handbook of Event-Related Potential Components, 115–141. [Google Scholar]
- Ruissen MI, & de Bruijn ERA (2015). Is it me or is it you? Behavioral and electrophysiological effects of oxytocin administration on self-other integration during joint task performance. Cortex, 70, 146–154. [DOI] [PubMed] [Google Scholar]
- Rutherford HJV, Guo XM, Graber KM, Hayes NJ, Pelphrey KA, & Mayes LC (2017). Intranasal oxytocin and the neural correlates of infant face processing in non-parent women. Biological Psychology, 129, 45–48. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, & Bradley MM (2007). Emotional perception: Correlation of functional MRI and event-related potentials. Cerebral Cortex, 17,1085–1091. [DOI] [PubMed] [Google Scholar]
- Sauer C, Montag C, Wörner C, Kirsch P, & Reuter M (2012). Effects of a common variant in the CD38 gene on social processing in an oxytocin challenge study: Possible links to autism. Neuropsychopharmacology, 37(6), 1474–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller B, Domes G, & Heinrichs M (2020). Oxytocin changes behavior and spatio-temporal brain dynamics underlying inter-group conflict in humans. European Neuropsychopharmacology, 31, 119–130. [DOI] [PubMed] [Google Scholar]
- Schiller B, Koenig T, & Heinrichs M (2019). Oxytocin modulates the temporal dynamics of resting EEG networks. Scientific Reports, 9(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, & Junghöfer M (2006). Emotion and attention: event-related brain potential studies. Progress in Brain Research, 156, 31–51. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, & Pollak SD (2010). Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences, 277(1694), 2661–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, & Guastella AJ (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: A meta-analysis. Neuropsychopharmacology, 38(10), 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, & Levkovitz Y (2009). Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biological Psychiatry, 66, 864–870. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202. [DOI] [PubMed] [Google Scholar]
- Sheng F, & Han S (2012). Manipulations of cognitive strategies and intergroup relationships reduce the racial bias in empathic neural responses. NeuroImage, 61(4), 786–797. [DOI] [PubMed] [Google Scholar]
- Sheng F, Liu Y, Zhou B, Zhou W, & Han S (2013). Oxytocin modulates the racial bias in neural responses to others’ suffering. Biological Psychology, 92(2), 380–386. [DOI] [PubMed] [Google Scholar]
- Slane MM, Lusk LG, Boomer KB, Hare AE, King MK, & Evans DW (2014). Social cognition, face processing, and oxytocin receptor single nucleotide polymorphisms in typically developing children. Developmental Cognitive Neuroscience, 9, 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Korgan AC, & Young WS (2019). Oxytocin delivered nasally or intraperitoneally reaches the brain and plasma of normal and oxytocin knockout mice. Pharmacological Research, 146, 104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, & Hurlemann R (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports, 3, 3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, Teed AR, Castle E, Dutcher JM, Meyer ML, Bryan R, ... & Eisenberger NI (2019). Null results of oxytocin and vasopressin administration across a range of social cognitive and behavioral paradigms: Evidence from a randomized controlled trial. Psychoneuroendocrinology, 107, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman R, Gordon I, Naples A, Rolison M, Leckman JF, Feldman R, … McPartland JC (2019). Oxytocin enhances the neural efficiency of social perception. Frontiers in Human Neuroscience, 13, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar MS, Chatzimanoli M, van der Wee NJA, & Putman P (2013). Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology, 38(9), 1797–1802. [DOI] [PubMed] [Google Scholar]
- Torky HA, Taha A, Marie H, El-Desouky E, Raslan O, Moussa AA, … Eesa A (2018). Role of topical oxytocin in improving vaginal atrophy in postmenopausal women: a randomized, controlled trial. Climacteric, 21(2), 174–178. [DOI] [PubMed] [Google Scholar]
- Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT, & Quintana DS (2017). The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 78, 117–124. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2002). Facial expression and selective attention. Current Opinion in Psychiatry, 15(3), 291–300. [Google Scholar]
- Waller C, Wittfoth M, Fritzsche K, Timm L, Wittfoth-Schardt D, Rottler E, … Gündel H (2015). Attachment representation modulates oxytocin effects on the processing of own-child faces in fathers. Psychoneuroendocrinology, 62, 27–35. [DOI] [PubMed] [Google Scholar]
- Walum H, Waldman ID, & Young LJ (2016). Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biological psychiatry, 79(3), 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, & Insel TR (2003). Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology, 28(5), 910–918. [DOI] [PubMed] [Google Scholar]
- Wu Y, van Dijk E, & Zhou X (2013). Evaluating self- vs. other-owned objects: The modulatory role of oxytocin. Biological Psychology, 92(2), 179–184. [DOI] [PubMed] [Google Scholar]
- Xin F, Zhou F, Zhou X, Ma X, Geng Y, Zhao W, ... & Becker B (2018). Oxytocin modulates the intrinsic dynamics between attention-related large scale networks. BioRxiv, 371971. [DOI] [PubMed] [Google Scholar]
- Young LJ, & Wang Z (2004). The neurobiology of pair bonding. Nature Neuroscience, 7(10), 1048–1054. [DOI] [PubMed] [Google Scholar]
- Zhao W, Yao S, Li Q, Geng Y, Ma X, Luo L, … Kendrick KM (2016). Oxytocin blurs the self-other distinction during trait judgments and reduces medial prefrontal cortex responses. Human Brain Mapping, 37(7), 2512–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: OT-ERP research using peripheral measures or genetic approaches (organized by latency)