Abstract
The aims of this article are to review the evidence regarding the use of non-vitamin K oral anticoagulants (NOACs) for secondary stroke prevention as compared to vitamin K antagonists in patients with atrial fibrillation (AF) and in patients with embolic strokes of uncertain source (ESUS), and when to initiate or resume anticoagulation after an ischaemic stroke or intracranial haemorrhage.
Four large trials compared NOACs with warfarin in patients with AF. In our meta-analyses, the rate of all stroke or systemic embolism (SE) was 4.94% with NOACs vs. 5.73% with warfarin. Among the patients with AF and previous transient ischaemic attack or ischaemic stroke, the rate of haemorrhagic stroke was halved with a NOAC vs. warfarin, and the rate of major bleeding was 5.7% with a NOAC vs. 6.4% with warfarin. There was no significant difference in mortality. In a trial comparing apixaban with aspirin in patients with AF, the rate of stroke or SE was 2.4% at 1 year with apixaban vs. 9.2% at 1 year with aspirin and the rates of major bleeding were 4.1% with apixaban vs. 2.9% with aspirin. Data from registries confirmed the results from the randomized trials. Initiation or resumption of anticoagulation after ischaemic stroke or cerebral haemorrhage depends on the size and severity of stroke and the risk of recurrent bleeding. Two large trials tested the hypothesis that NOACs are more effective than 100 mg aspirin in patients with ESUS. Neither trial showed a significant benefit of the NOAC over aspirin.
In the meta-analysis, the rate all stroke or SE was 4.94% with NOACs vs. 5.73% with warfarin and the rate of haemorrhagic stroke was halved with a NOAC. The four NOACs had broadly similar efficacy for the major outcomes in secondary stroke prevention.
Keywords: Non-vitamin K oral anticoagulants (NOACs), Atrial fibrillation, TIA, Stroke, Secondary stroke prevention, Warfarin, Embolic stroke of undetermined source (ESUS)
Introduction
Patients with atrial fibrillation (AF) have a high risk of stroke. The risk of stroke is particularly high in patients with AF who have a history of transient ischaemic attack (TIA) or stroke.1
Oral anticoagulation with vitamin K antagonists such as warfarin is highly effective in preventing recurrence of stroke with a relative risk reduction of 60–70%.2 However, stroke prevention with warfarin has a number of practical limitations such as a narrow therapeutic window, interaction with food and other drugs, and the need to monitor coagulation levels regularly. Non-vitamin K oral anticoagulants (NOACs) such as apixaban, dabigatran, edoxaban, or rivaroxaban do not require regular monitoring of coagulation parameters. The NOACs have no interaction with food and relatively minor interactions with other drugs.
Non-vitamin K oral anticoagulants have been compared with warfarin in four large randomized trials in patients with AF.3–6 All trials included subgroups of patients with previous TIA or an ischaemic stroke.7–10 The results of these subgroups are summarized and evaluated below. The primary endpoint in all studies comparing NOACs with warfarin in patients with AF was stroke and systemic embolism (SE). However, this endpoint includes an efficacy endpoint of the prevention of ischaemic stroke and a complication of anticoagulation, cerebral haemorrhage.
Non-vitamin K oral anticoagulants for secondary prevention in patients with atrial fibrillation and prior stroke or transient ischaemic attack
The efficacy and safety of dabigatran, rivaroxaban, apixaban, and edoxaban have been compared with warfarin for stroke prevention in AF in four large phase 3 clinical trials: ARISTOTLE,3 RE-LY,4 ROCKET-AF,5 and ENGAGE-AF.6 All trials included a substantial number of AF patients with a history of prior stroke or TIA (Table 1).
Table 1.
Patients with atrial fibrillation and prior TIA or stroke in randomized trials: baseline data
| ARISTOTLE |
AVERROES |
RE-LY |
ROCKET AF |
ENGAGEa |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Apixaban | Warfarin | Apixaban | Aspirin | Dabigatran 110 | Dabigatran 150 | Warfarin | Rivaroxaban | Warfarin | Edoxabanb | Warfarin | |
|
|
|||||||||||
| N | 1694 | 1742 | 390 | 374 | 1195 | 1233 | 1195 | 3754 | 3714 | 1976 | 1991 |
| Age (years) | 70.1 | 71.7 | 70.2 | 70.8 | 70.4 | 71 | 71 | 70 | |||
| Females | 37% | 44% | 36% | 38% | 42% | 39% | 39% | 38% | |||
| Hypertension | 83% | 81% | 77% | 77% | 76% | 85% | 85% | 86% | |||
| Diabetes | 26% | 20% | 22% | 24% | 21% | 25% | 24% | 27% | |||
| CHADS ≥3 | 92% | 93% | 90% | 90% | 89% | NA | NA | 67% | |||
| Aspirinc | 31% | 28% | 40% | 40% | 42% | 38% | 38% | 28% | |||
Adapted from Diener et al.11
Dabigatran; 110 = 110 mg twice daily; 150 = 150 mg twice daily.
Data not reported by treatment group.
Edoxaban 60 mg or 30 mg in patients fulfilling the dose reduction criteria.
Aspirin intake at baseline.
Apixaban vs. warfarin
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial compared apixaban 5 mg twice daily with warfarin [target Internatiiona Normalized Ratio (INR) 2.0–3.0].3 Patients with impaired renal function [serum creatinine >2.5 mg/dL (221 μmol/L) or creatinine clearance <25 mL/min], previous intracranial haemorrhage (ICH), or any stroke within 7 days before randomization were excluded. Patients who fulfilled two out of three criteria received a lower dose of apixaban of 2.5 mg twice a day: elderly (80 years or older), or low bodyweight (60 kg or lighter), or a serum creatinine of 133 μmol/L (1.5 mg/dL) or greater.3
Among the 3436 patients with AF and a previous ischaemic stroke or TIA who were enrolled in the ARISTOTLE trial (Table 1), the relative effects of apixaban vs. warfarin were consistent with the relative effects of apixaban vs. warfarin in the 14 765 AF patients without previous stroke or TIA for major outcomes.8 Among the 3436 patients with prior stroke or TIA, the rate of stroke or SE was 2.46%/year with apixaban vs. 3.24%/year with warfarin [hazard ratio (HR) 0.76, 95% confidence interval (CI) 0.56–1.03].8 Among patients with prior stroke or TIA, the rate of major bleeding was 2.84%/year with apixaban vs. 3.91%/year warfarin (HR 0.73, 0.55–0.98).8
Dabigatran vs. warfarin
The RE-LY (Randomized Evaluation of Long-term anticoagulant therapY) trial was a three-armed trial that compared two doses of dabigatran (110 mg twice a day or 150 mg twice a day) to standard dose-adjusted warfarin (target INR 2.0–3.0) in 18 113 patients with AF.4 Patients were excluded if they had poor renal function (creatinine clearance of <30 mL/min) or a stroke within 14 days of randomization. A total of 3623 (20%) patients with AF had a history of ischaemic stroke or TIA more than 14 days before randomization, of whom 1233 were randomized to dabigatran 150 mg b.i.d., 1195 to dabigatran 110 mg b.i.d., and 1195 to warfarin (Table 1).
The relative effects of dabigatran vs. warfarin in the 3623 patients with previous stroke or TIA were consistent with the effects of dabigatran vs. warfarin in the 14 490 patients without previous stroke or TIA for all major efficacy and safety outcomes.7 Among the 3623 patients with AF and prior stroke or TIA, the rate of stroke or SE was 2.32%/year with dabigatran 110 mg vs. 2.78%/year warfarin [risk ratio (RR) 0.84, 0.58–1.20], and 2.07%/year with dabigatran 150 mg vs. 2.78%/year warfarin (RR 0.75, 0.52–1.08).7 Among patients with prior stroke or TIA, the rate of major bleeding was 2.74%/year with dabigatran 110 mg vs. 4.15%/year warfarin (RR 0.66, 0.48–0.90) and 4.15%/year with dabigatran 150 mg vs. 4.15%/year warfarin (RR 1.01, 0.77–1.34).7
Rivaroxaban vs. warfarin
The oral factor Xa inhibitor rivaroxaban was compared with warfarin in ROCKET-AF [Rivaroxaban—Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism (target INR 2.0–3.0) for prevention of stroke and Embolism Trial in Atrial Fibrillation].5 Patients with very recent TIA within 3 days, acute stroke within 14 days, or severe disabling stroke within 3 months of randomization, were excluded.9 The study population in ROCKET-AF was at high risk of stroke; 55% of patients had a previous ischaemic stroke or TIA, and 90% had either a previous stroke or TIA, or three or more risk factors for stroke (Table 1). Patients were randomly assigned to receive fixed-dose rivaroxaban (20 mg daily, or 15 mg daily in patients with a creatinine clearance of 30–49 mL per minute) or adjusted-dose warfarin (target INR 2.0–3.0). The relative effects of rivaroxaban vs. warfarin in the 7468 patients with previous stroke or TIA were consistent with the effects of rivaroxaban vs. warfarin in the 6796 patients without previous stroke or TIA for major outcomes.9 Among the 7468 patients with prior stroke or TIA, the rate of stroke or SE was 2.79%/year with rivaroxaban vs. 2.96%/year warfarin (HR 0.94, 0.77–1.16).9 Among patients with prior stroke or TIA, the rate of major bleeding was 3.13%/year with rivaroxaban vs. 3.22%/year warfarin (HR 0.97, 0.79–1.19).9
Edoxaban vs. warfarin
The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial was a three-group, randomized, double-blind, double-dummy trial comparing two once-daily regimens of edoxaban [higher-dose edoxaban (60 mg once daily), or lower-dose edoxaban (30 mg once daily)] with adjusted-dose warfarin (INR 2.0–3.0) in 21 105 patients with moderate-to-high-risk AF (median follow-up, 2.8 years).6 Patients were excluded if they had a stroke within 30 days before randomization. The 60 mg dose of edoxaban was halved if any of the following characteristics were present at the time of randomization or during the study: estimated creatinine clearance of 30–50 mL per minute, a bodyweight of 60 kg or less, or the concomitant use of verapamil or quinidine (potent P-glycoprotein inhibitors). A total of 5973 (28.3%) patients had a previous ischaemic stroke or TIA, of whom 1976 were allocated higher dose edoxaban, and 1991 warfarin.10 As only the higher dose edoxaban is approved for use by regulatory authorities worldwide, the results below reflect the comparison of high dose edoxaban 60 mg once daily vs. warfarin. Among the 3967 patients with prior stroke or TIA, the rate of stroke or SE was 2.44%/year with higher-dose edoxaban vs. 2.85%/year warfarin (HR 0.86, 0.67–1.09).10 Among the 3967 patients with prior stroke or TIA, the rate of major bleeding was 3.25%/year with higher-dose edoxaban vs. 3.86%/year warfarin (HR 0.84, 0.67–1.06).10
Meta-analyses of major trials of non-vitamin K oral anticoagulants vs. warfarin in patients with prior stroke or transient ischaemic attack
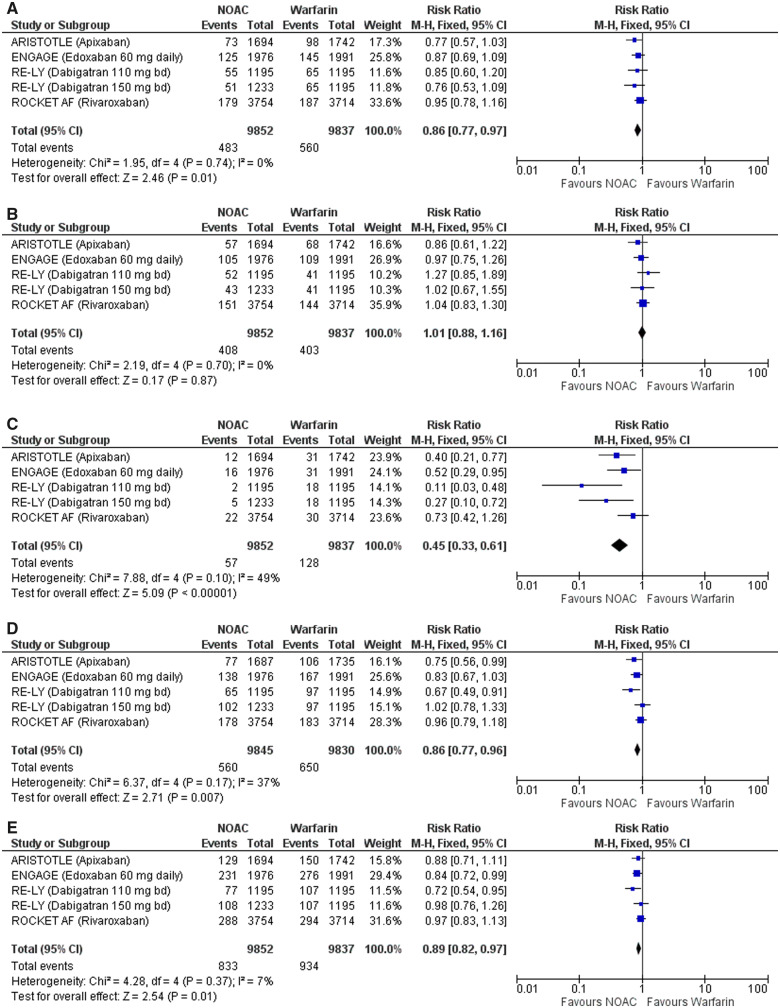
Meta-analyses of all 71 683 participants enrolled in the RE-LY, ROCKET AF, ARISTOTLE, and ENGAGE AF-TIMI 48 trials, included 17 299 (24%) patients with AF and a history of prior stroke or TIA.12,13 Adding the group of patients treated with a low dose of dabigatran resulted in 19 689 patients with AF and previous TIA or ischaemic stroke. The rate of stroke or SE, over a median follow-up ranging from 1.8 to 2.8 years, was 4.94% with a NOAC vs. 5.73% with warfarin (RR 0.86, 0.77–0.97). Figure 1A shows a forest plot of the four major clinical trials of the effect of NOACs vs. warfarin on stroke or SE. There was no significant difference in the relative effects of NOACs vs. warfarin on ischaemic stroke (RR 0.98, 0.85–1.13) (Figure 1B). The rate of haemorrhagic stroke was halved with a NOAC vs. warfarin (RR 0.45, 0.33–0.61) (Figure 1C). Among patients with AF and previous TIA or ischaemic stroke, the rate of major bleeding was 5.71% with a NOAC vs. 6.43% warfarin (RR 0.86, 0.77–0.96) (Figure 1D). Among patients with previous TIA or ischaemic stroke, there was a non-significant trend to a lower rate of death from any cause with a NOAC vs. warfarin (RR 0.89, 0.82–0.97) (Figure 1E).
Figure 1.
(A) Forest plot of the effect of non-vitamin K oral anticoagulants vs. warfarin on stroke or systemic embolism. (B) Forest plot of the effect of non-vitamin K oral anticoagulants vs. warfarin on ischaemic stroke. (C) Forest plot of the effect of non-vitamin K oral anticoagulants vs. warfarin on haemorrhagic stroke. (D) Forest plot of the effect of non-vitamin K oral anticoagulants vs. warfarin on major bleeding. (E) Forest plot of the effect of non-vitamin K oral anticoagulants vs. warfarin on death from any cause. Initiation or resumption of antithrombotic therapy after transient ischaemic attack or ischaemic stroke. AF, atrial fibrillation; NOAC, non-vitmain-K oral anticoagulant; VKA, vitamin-K antagonist.
Apixaban vs. aspirin
The AVERROES (Apixaban vs. Aspirin to Reduce the Risk of Stroke) study compared apixaban (5 mg twice daily) vs. aspirin (81–324 mg per day) in patients with AF who were thought to be unsuitable or unwilling to receive a vitamin K antagonist.14 The reasons for unsuitability for a vitamin K antagonist varied, but most (>70%) were related to issues with INR monitoring, INR instability, and patient refusal to take vitamin K antagonists. Stroke within the previous 10 days was an exclusion criteria.
The AVERROES trial showed overall that in patients with AF who were unable or unwilling to take a vitamin K antagonist, apixaban was more effective than aspirin for the prevention of stroke and systemic embolic events (1.6% per year apixaban vs. 3.7% per year aspirin HR 0.45, 0.32–0.62; P < 0.001), but it was also as safe as aspirin for major bleeding (1.4% per year apixaban vs. 1.2% aspirin; HR 1.13, 0.74–1.75; P = 0.57) and ICH (0.4% per year apixaban vs. 0.4% per year aspirin; HR 0.85, 0.38–1.90; P = 0.69).
The relative effects of apixaban vs. aspirin in the 764 patients with previous stroke or TIA were consistent with the effects of apixaban vs. aspirin in the 4832 patients without previous stroke or TIA.15 Among 764 patients with previous stroke or TIA, the rate of stroke or SE was 2.39% at 1 year with apixaban vs. 9.16% at 1-year aspirin (HR 0.29, 0.15–0.60).15 Among 764 patients with previous stroke or TIA, the rates of major bleeding were: 4.1% at 1-year apixaban vs. 2.89% at 1-year aspirin (HR 1.28, 0.58–2.82).15
Indirect comparison analysis
The efficacy and safety of one NOAC against another can only be definitively answered by a head-to-head randomized clinical trial (RCT). As no such trials have been performed, a number of indirect comparison analyses have been performed. For example, in secondary prevention, the comparison of apixaban with dabigatran (110 mg and 150 mg twice daily) showed a lower risk of myocardial infarction (HR 0.39, 95% CI 0.16–0.95) for apixaban compared to dabigatran 150 mg twice daily.16 No significant differences in efficacy and most safety endpoints were found between apixaban and dabigatran 150 mg compared to rivaroxaban. When comparing 110 mg b.i.d. dabigatran and rivaroxaban, there was less haemorrhagic stroke (HR 0.15, 0.03–0.66), vascular death (0.64, 0.42–0.99), severe bleeding (0.68, 0.47–0.99), and intracranial bleeding (0.27, 0.10–0.73) with dabigatran. No differences were found for the other efficacy endpoints and the risk of bleeding.
In summary, apixaban, rivaroxaban, and dabigatran appear to have broadly similar efficacy for the major endpoints in secondary prevention, although the endpoints of haemorrhagic stroke, vascular death, major bleeding, and ICH were less frequent with dabigatran 110 mg twice daily than with rivaroxaban.
Data from registries
Data from RCTs are the best evidence when comparing the efficacy and safety of an intervention. Following the RCT, the NOACs are licensed and used in everyday clinical practice, so-called ‘real world’ registries, which have provided much data comparing NOACs to warfarin, and to each other. Some have been based on claims datasets, while large prospective registries have also been published.
The largest of the retrospective claims datasets published was the ARISTOPHANES study (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients) which used multiple data sources to compare stroke/SE and major bleeding (MB) among a large number of non-valvular AF patients on NOACs or warfarin.17 The authors found that after propensity score matching, the NOACs had lower rates of stroke/SE and variable comparative rates of MB vs. warfarin, and subgroup analyses on patients with prior stroke/SE were generally consistent with the main results.
The study by Coleman et al.18 used MarketScan claims in the USA from January 2012 to June 2015 to analyse adults with newly initiated on oral anticoagulation and non-valvular AF and a history of previous ischaemic stroke/TIA. Data were analysed after 1:1 propensity score matching for apixaban vs. warfarin (n = 2514), dabigatran vs. warfarin (n = 1962), and rivaroxaban vs. warfarin (n = 5208). After a short mean observation time of 0.5 years, neither apixaban nor dabigatran reduced the combined primary endpoint of ischaemic stroke or ICH (HR 0.70, 95% CI 0.33–1.48 and HR 0.53, 95% CI 0.26–1.07). Rivaroxaban reduced the combined endpoint of ischaemic stroke or ICH (HR 0.45, 95% CI 0.29–0.72) without an effect on major bleeding (HR 1.07, 95% CI 0.71–1.61).
Using the Korean National Health Insurance Service claims database. Park et al.19 investigated the effectiveness and safety of NOACs for secondary prevention in 61 568 patients with AF. Compared with warfarin, NOACs were associated with lower risks of recurrent stroke (HR 0.67, 95% CI 0.62–0.72), major bleeding (HR 0.73, 95% CI 0.66–0.80), composite outcome (HR 0.69, 95% CI 0.65–0.73), and mortality. There was a consistent trend of improved outcomes in the subgroups of patients with severe, disabling, and recent stroke. Similar data were published from Taiwan’s National Health Insurance Research Database, where the reduced risks of thromboembolism and major bleeding for the four direct oral anticoagulants over warfarin persisted in AF patients with either primary or secondary stroke prevention.20
The PROSPER registry collected a cohort of Medicare patients with AF in the USA who had a history of ischaemic stroke21: 11 662 survivors of acute ischaemic stroke with a median age of 80 years were identified, of which 4041 (34.7%) patients were discharged with a NOAC and 7621 with warfarin. Patients on NOACs were less likely to experience major adverse cardiovascular events [adjusted HR (aHR) 0.89, 99% CI 0.83–0.96], and mortality was also reduced (aHR 0.88, 95% CI 0.82–0.95; P < 0.001). Concerning bleeding complications, haemorrhagic strokes (aHR 0.69, 95% CI 0.50–0.95; P = 0.02), and hospitalizations due to bleeding (aHR 0.89, 95% CI 0.81–0.97; P = 0.009) were reduced amongst those taking NOACs. The risk of gastrointestinal bleeding, however was increased (aHR 1.14, 95% CI 1.01–1.30; P = 0.03). The discrepancy between the two registry data could be due to the differences in mean age, which was 73 years in the Coleman et al. study and 80 years in PROSPER, as well as the short follow-up in the former study.
Recommendations from guidelines
The guidelines of the European Society of Cardiology from 2016 recommend NOACs in preference to vitamin-K antagonists (VKAs) or aspirin in patients with AF and a previous stroke (Class I, level B).22 The guidelines of the European Stroke Organization recommend in patients with non-valvular AF and previous ischaemic stroke or TIA, non-vitamin K antagonist oral anticoagulants over VKAs for secondary prevention of all events (quality of evidence: high, strength of recommendation: strong).23 The 2017 consensus of the Asia Pacific Heart Rhythm society states, that NOACs are preferred over VKA in Asian patients with a history of ischaemic stroke or TIA.24
Initiation or resumption of anticoagulation with non-vitamin K oral anticoagulants after ischaemic stroke or intracranial bleeding
Despite available guideline recommendations, the optimal time for administering anticoagulation therapy in acute cardioembolic stroke remains unclear. Guidelines from the European Society of Cardiology (ESC) for the management of AF recommend that in patients who suffer a moderate-to-severe ischaemic stroke while on anticoagulation, this treatment should be interrupted for 3–12 days to allow a multidisciplinary assessment of acute stroke and bleeding risk.25 The use of NOACs following a cardioembolic stroke has been assessed in several observational studies. The SAMURAI-NVAF study demonstrated that following NOAC initiation, within a median of 4 days post-stroke, no ICH was observed.26 Furthermore, in another observational study, no significant difference in recurrent ischaemic events was observed with post-stroke NOAC initiation, either less than or equal to 7 days or greater than 7 days following the initial event (P = 0.53).27 Early recurrences and major bleeding events (within 90 days) and the timing of these events in patients with an acute ischaemic stroke and AF who received a NOAC following the initial event were assessed in the prospective observational multicentre RAF-DOAC study. An early recurrent event occurred in 32 patients (2.8%) and major bleeding in 27 patients (2.4%). The composite rate of recurrence and major bleeding was 12.4% for patients initiating NOACs less than or equal to 2 days after the acute stroke, 2.1% for those initiating between days 3 and 14, and 9.1% for those initiating NOACs greater than 14 days after the initial stroke. The combined rate of recurrent and major bleeding was 5% in patients treated with NOACs following an acute stroke.28 An individual patient data analysis including 4912 patients treated with oral anticoagulation after recent cerebral ischaemia related to AF revealed the following major findings: First, treatment with NOACs commenced a median of 5 days after the index event has a lower risk of adverse outcomes compared to treatment with VKA; second, this benefit is mainly attributed to lower risks of ICH; and, third, the benefit is consistent across subgroups.29
Currently, several RCTs are investigating the risks and benefits of the early start of DOAC (Switzerland: ELAN ClinicalTrials.gov Identifier: NCT03148457; Sweden: TIMING ClinicalTrials.gov Identifier: NCT02961348; UK: OPTIMAS: EudraCT, 2018‐003859‐38 and USA: START ClinicalTrials.gov Identifier: NCT03021928).
Reinitiating oral anticoagulant after intracranial haemorrhage
Patients with AF who survive an ICH are at increased risk of subsequent ischaemic stroke.25 However, determining the best approach for reducing further risk of stroke in patients with AF and previous ICH, or other clinically relevant bleeds, requires careful consideration of the associated risks.30 All studies until now were done with VKAs. Currently, several RCTs are investigating the risks and benefits of start of DOAC after ICH: ENRICH-AF (ClinicalTrials.gov Identifier: NCT03950076), SoSTART2 (ClinicalTrials.gov Identifier: NCT03153150), NASPAF-ICH (ClinicalTrials.gov Identifier: NCT02998905), and Prestige AF (https://clinicaltrials.gov/ct2/show/NCT03996772).
A cohort study using national registry data suggested that oral anticoagulation was associated with a significant reduction in ischaemic stroke/all-cause mortality; therein supporting the reintroduction of OACs after resolution of ICH.30 One-year follow-up data (N = 1752) showed that the rate of ischaemic stroke/SE and all-cause mortality (per 100 person years) was 13.6 with oral anticoagulants (65% VKAs, 2% NOACs), 27.3 with no treatment, and 25.7 with antiplatelet therapy. The aHR of ischaemic stroke/SE and all-cause mortality was 0.55 (95% CI 0.39–0.78) in patients on oral anticoagulant treatment, compared with those on no treatment.
The question of starting or reinitiating oral anticoagulant (OAC) treatment in patients with AF after spontaneous ICH also depends on the underlying aetiology. The most frequent risk factor is arterial hypertension, and if adequate blood pressure control can be achieved, the benefit of OAC treatment in high-risk patients is likely to surpass the risk of further ICH- which is estimated at about 2% per year.31 In contrast, in patients with lobar ICH due to suspected cerebral amyloid angiopathy, the estimated risk of ICH recurrence is about 5–15% per year, making these patients ineligible for OAC treatment.
More recently, the results from an observational study have suggested that anticoagulant treatment can be initiated 7–8 weeks after ICH, in patients with AF to optimize the benefit from treatment and minimize stroke risk.32 Of 2619 ICH survivors with AF, anticoagulant treatment was associated with a reduced risk of vascular death and non-fatal stroke in patients deemed to be high-risk yet without any significantly increased risk of severe haemorrhage. Noteworthy, the ESC guidelines recommend that after ICH, oral anticoagulation in AF patients may be reinitiated after between 4–8 weeks provided the cause of bleeding or the relevant risk factor has been treated or controlled.22
Non-vitamin K oral anticoagulants in embolic stroke of undetermined aetiology (ESUS)
Cryptogenic ischaemic stroke describes the substantial fraction of ischaemic strokes that are of unclear cause. ESUS has been proposed for non-lacunar cryptogenic stroke with a presumed embolic mechanism.33 Unrecognized paroxysmal AF can be identified using cardiac rhythm monitoring during follow-up of patients with cryptogenic ischaemic stroke34,35 and suspected to have a role in recurrent stroke in ESUS patients.33
Two large, international randomized studies tested the hypothesis that NOACs are more effective than 100 mg aspirin in patients with ESUS: RE-SPECT ESUS (Randomized, Double-Blind, Evaluation in Secondary Stroke Prevention Comparing the Efficacy and Safety of the Oral Thrombin Inhibitor Dabigatran Etexilate vs. Acetylsalicylic Acid in Patients with Embolic Stroke of Undetermined Source)36 and NAVIGATE ESUS (New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial vs. ASA to Prevent Embolism in Embolic Stroke of Undetermined Source).37 For both trials, patients with AF or a history of AF were excluded, and participation required at least 20 h of cardiac rhythm monitoring prior to randomization to exclude AF (much longer in many participants).36,37 Both trials were neutral for their primary outcome of all recurrent strokes, although RE-SPECT ESUS reported an intriguing divergence of the Kaplan–Meier curves beginning after 1 year of follow-up that favoured dabigatran over aspirin.36
During the mean 11-month follow-up of 7213 participants in NAVIGATE ESUS, AF was reported in 3% of patients.37 Since participants were not systematically screened, this is almost certainly a substantial underestimate. At the time of recurrent ischaemic stroke, AF was identified in 12% (19/153) of those assigned to aspirin and 5% (8/156) of those assigned to rivaroxaban 15 mg daily, supporting a substantial efficacy of rivaroxaban for preventing recurrent stroke related to AF that was not apparent at the time of index ESUS.38 Nevertheless, the investigators concluded that occult AF was not a major cause of recurrent ischaemic stroke in NAVIGATE ESUS participants who had been screened to exclude AF as part of eligibility assessment.
The RE-SPECT ESUS trial involved 5390 who were randomized to either 150 mg dabigatran twice daily or, if age ≥75 years or estimated creatine clearance ≤50 mL/min, 110 mg dabigatran twice daily.36 During the mean follow-up of 19 months, 7.5% patients were reported to develop AF.39 At the time of recurrent ischaemic stroke, AF was identified in 15.4% (30/195) of those assigned to aspirin and 9.6% (20/208) of those assigned to dabigatran.39
While these two trials did not demonstrate a benefit of NOACs over aspirin for reducing recurrent stroke in unselected ESUS patients, they provide information about bleeding associated with NOACs vs. aspirin from randomized comparisons in large numbers of patients with recent ischaemic stroke. Severe bleeding complications [defined as International Society on Thrombosis and Haemostasis (ISTH) major bleeding] in the RE-SPECT ESUS study occurred at a rate of 1.7%/year in the dabigatran group and of 1.4%/year in those assigned aspirin 100 mg daily (HR 1.19, 95% CI 0.85–1.66; P = 0.30). In the NAVIGATE ESUS study, the rates of ISTH major bleeding was1.8%/year among those assigned rivaroxaban 15 mg daily and 0.7%/year among those assigned aspirin 100 mg daily (HR 2.72, 95% CI 1.68–4.39; P < 0.001).4,5
In conclusion, the two trials did not show a significant benefit of a NOAC over aspirin in ESUS patients. We hypothesize that the follow-up time was too short to allow sufficient numbers of participants to develop AF to demonstrate NOAC efficacy, with early recurrent strokes dominated by atheroembolic mechanisms.
Practical aspects
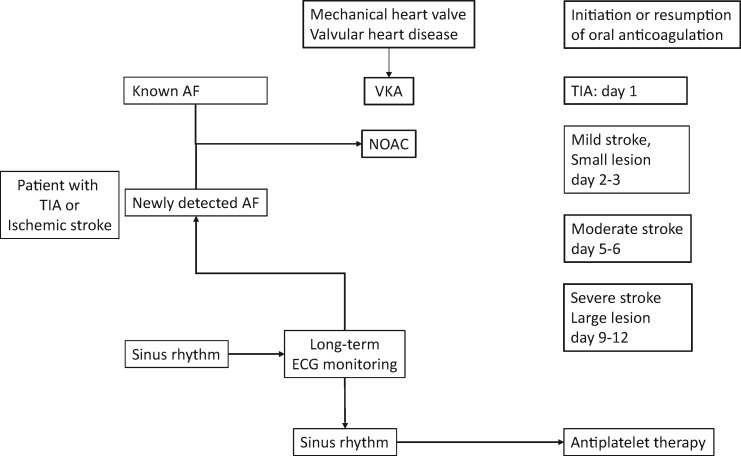
If a patient with known AF suffers a TIA or ischaemic stroke compliance with oral anticoagulation should be evaluated. In the majority of these patients, coagulation parameters are normal indicating non-compliance. Another subgroup of patients have been treated with too low a dose of a NOAC.40,41 Due to fear of bleeding these patients are treated with a reduced dose of a NOAC despite not fulfilling the criteria for dose reduction. Time of resumption of anticoagulation depends on the severity of stroke and the size of the ischaemic defect in brain imaging (see central figure). The highest risk of a recurrent stroke is in the first 10 days after the initial event. Therefore, early initiation or resumption of anticoagulation is warranted. In patients with a large area of the ischaemic stroke, early anticoagulation can lead to haemorrhagic transformation.42 Non-vitamin K oral anticoagulants are favoured over VKAs due to their improved efficacy and better safety, in particular concerning intracranial bleeds. Patients with TIA or ischaemic stroke in sinus rhythm should receive cardiac rhythm monitoring for at least 72 h to detect unrecognized paroxysmal AF. In elderly patients with cryptogenic stroke or ESUS long-term electrocardiogram monitoring is recommended.
Central figure.
Initiation or resumption of antithrombotic therapy after TIA or ischemic stroke. AF = atrial fibrillation, VKA = Vitamin-K antagonist; NOAC = non-vitmain-K oral anticoagulant.
Conflict of interest: H.-C.D. reports personal fees from Bayer, grants and personal fees from Boehringer Ingelheim, personal fees from Daiichi-Sankyo, personal fees from Pfizer, during the conduct of the study; personal fees from Abbott, personal fees from BMS, personal fees from Medtronic, grants and personal fees from Portola, personal fees from Sanofi-Aventis, personal fees from WebMD, outside the submitted work; and H.-C.D. received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. H.-C.D. served as editor of Neurologie up2date, Info Neurologie & Psychiatrie, Arzneimitteltherapie, as co-editor of Cephalalgia and on the editorial board of Lancet Neurology. H.-C.D. chairs the Treatment Guidelines Committee of the German Society of Neurology and contributed to the EHRA and ESC guidelines for the treatment of AF. G.J.H. reports personal fees from Bayer and from Johnson & Johnson during the conduct of the study; personal fees from American Heart Association outside the submitted work; and was a member of the executive committees of the ROCKET-AF trial, AMADEUS trial, and BOREALIS trial; and the adjudication committee for stroke outcome events of the RE-LY and AVERROES trials. J.D.E. reports personal fees from Bristol-Myers Squibb and Pfizer as a member of the executive committees of the ARISTOTLE trial. G.Y.H.L. reports consultancy and speaker fees from Bayer, Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi-Sankyo outside the submitted work. No fees received personally. R.G.H. reports other from Bayer AG, outside the submitted work; and Lead investigator in the NAVIGATE ESUS trial that was funded by Bayer AG testing rivaroxaban. V.C. reports grants from Boehringer Ingelheim, grants from Bristol-Meyer-Squibb, grants from Daiichi-Sankyo, from Bayer during the conduct of the study. This paper was published as part of a supplement financially supported by an unrestricted educational grant from Daiichi Sankyo Europe GmbH.
References
- 1.Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–1457. [PubMed] [Google Scholar]
- 2.European Atrial Fibrillation Trial (EAFT) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342:1255–1262. [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L.. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L.. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 7. Diener HC, Connolly SJ, Ezekowitz MD, Wallentin L, Reilly PA, Yang S, Xavier D, Di Pasquale G, Yusuf S.. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010;9:1157–1163. [DOI] [PubMed] [Google Scholar]
- 8. Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, Alings M, Goto S, Lewis BS, Rosenqvist M, Hanna M, Mohan P, Alexander JH, Diener HC; ARISTOTLE Committees and Investigators. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012;11:503–511. [DOI] [PubMed] [Google Scholar]
- 9. Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, Diener HC, Donnan GA, Halperin JL, Mahaffey KW, Mas JL, Massaro A, Norrving B, Nessel CC, Paolini JF, Roine RO, Singer DE, Wong L, Califf RM, Fox KA, Hacke W; ROCKET AF Steering Committee Investigators. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 2012;11:315–322. [DOI] [PubMed] [Google Scholar]
- 10. Rost NS, Giugliano RP, Ruff CT, Murphy SA, Crompton AE, Norden AD, Silverman S, Singhal AB, Nicolau JC, SomaRaju B, Mercuri MF, Antman EM, Braunwald E; ENGAGE AF-TIMI 48 Investigators. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: findings from ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Stroke 2016;47:2075–2082. [DOI] [PubMed] [Google Scholar]
- 11. Diener HC, Ntaios G, O’Donnell M, Easton JD.. Non-vitamin-K oral anticoagulants (NOACs) for the prevention of secondary stroke. Expert Opin Pharmacother 2018;19:1597–1602. [DOI] [PubMed] [Google Scholar]
- 12. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM.. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 13. Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P.. Nonvitamin-K-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized controlled trials. Int J Stroke 2017;12:589–596. [DOI] [PubMed] [Google Scholar]
- 14. Connolly SJ, Eikelboom J, Joyner C, Diener H-C, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim K-H, Lewis BS, Van Mieghem W, Lip GYH, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O'Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S.. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 15. Diener H-C, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, O'Donnell M, Hohnloser SH, Hankey GJ, Shestakovska O, Yusuf S.. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol 2012;11:225–231. [DOI] [PubMed] [Google Scholar]
- 16. Rasmussen LH, Larsen TB, Graungaard T, Skjoth F, Lip GY.. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ 2012;345:e7097–e7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K, Luo X, Mardekian J, Friend K, Nadkarni A, Pan X, Baser O, Deitelzweig S.. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke 2018;49:2933–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coleman CI, Peacock WF, Bunz TJ, Alberts MJ.. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke 2017;48:2142–2149. [DOI] [PubMed] [Google Scholar]
- 19. Park J, Lee SR, Choi EK, Kwon S, Jung JH, Han KD, Cha MJ, Ko SB, Oh S, Lip G.. Effectiveness and safety of direct oral anticoagulant for secondary prevention in asians with atrial fibrillation. J Clin Med 2019;8:2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan YH, Lee HF, See LC, Tu HT, Chao TF, Yeh YH, Wu LS, Kuo CT, Chang SH, Lip G.. Effectiveness and safety of four direct oral anticoagulants in asian patients with nonvalvular atrial fibrillation. Chest 2019;156:529–543. [DOI] [PubMed] [Google Scholar]
- 21. Xian Y, Xu H, O’Brien EC, Shah S, Thomas L, Pencina MJ, Fonarow GC, Olson DM, Schwamm LH, Bhatt DL, Smith EE, Hannah D, Maisch L, Lytle BL, Peterson ED, Hernandez AF.. Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the patient-centered research into outcomes stroke Patients Prefer and Effectiveness Research (PROSPER) study. JAMA Neurol 2019;76:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 23. Klijn CJ, Paciaroni M, Berge E, Korompoki E, Kõrv J, Lal A, Putaala J, Werring DJ.. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. Eur Stroke J 2019;4:198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiang CE, Okumura K, Zhang S, Chao TF, Siu CW, Wei Lim T, Saxena A, Takahashi Y, Siong Teo W.. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm 2017;33:345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caso V, Masuhr F.. A narrative review of nonvitamin k antagonist oral anticoagulant use in secondary stroke prevention. J Stroke Cerebrovasc Dis 2019;28:2363–2375. [DOI] [PubMed] [Google Scholar]
- 26. Toyoda K, Arihiro S, Todo K, Yamagami H, Kimura K, Furui E, Terasaki T, Shiokawa Y, Kamiyama K, Takizawa S, Okuda S, Okada Y, Kameda T, Nagakane Y, Hasegawa Y, Mochizuki H, Ito Y, Nakashima T, Takamatsu K, Nishiyama K, Kario K, Sato S, Koga M; SAMURAI Study Investigators. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: the SAMURAI-NVAF study. Int J Stroke 2015;10:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seiffge DJ, Traenka C, Polymeris A, Hert L, Peters N, Lyrer P, Engelter ST, Bonati LH, De Marchis GM.. Early start of DOAC after ischemic stroke: risk of intracranial hemorrhage and recurrent events. Neurology 2016;87:1856–1862. [DOI] [PubMed] [Google Scholar]
- 28. Paciaroni M, Agnelli G, Caso V, Tsivgoulis G, Furie KL, Tadi P, Becattini C, Falocci N, Zedde M, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D’Amore C, Mosconi MG, Cimini LA, Procopio A, Bovi P, Carletti M, Rigatelli A, Cappellari M, Putaala J, Tomppo L, Tatlisumak T, Bandini F, Marcheselli S, Pezzini A, Poli L, Padovani A, Masotti L, Vannucchi V, Sohn SI, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Ntaios G, Karagkiozi E, Athanasakis G, Makaritsis K, Vadikolias K, Liantinioti C, Chondrogianni M, Mumoli N, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Corea F, Ageno W, Bellesini M, Colombo G, Silvestrelli G, Ciccone A, Scoditti U, Denti L, Mancuso M, Maccarrone M, Orlandi G, Giannini N, Gialdini G, Tassinari T, De Lodovici ML, Bono G, Rueckert C, Baldi A, D’Anna S, Toni D, Letteri F, Giuntini M, Lotti EM, Flomin Y, Pieroni A, Kargiotis O, Karapanayiotides T, Monaco S, Baronello MM, Csiba L, Szabo L, Chiti A, Giorli E, Del Sette M, Imberti D, Zabzuni D, Doronin B, Volodina V, Michel P, Vanacker P, Barlinn K, Pallesen LP, Kepplinger J, Bodechtel U, Gerber J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Gourbali V, Yaghi S.. Prediction of early recurrent thromboembolic event and major bleeding in patients with acute stroke and atrial fibrillation by a risk stratification schema: the ALESSA score study. Stroke 2017;48:726–732. [DOI] [PubMed] [Google Scholar]
- 29. Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, Macha Md K, Tsivgoulis G, Ambler G, Arihiro S, Bonati LH, Bonetti B, Kallmunzer B, Muir KW, Bovi P, Gensicke H, Inoue M, Schwab S, Yaghi S, Brown MM, Lyrer P, Takagi M, Acciarrese M, Jager HR, Polymeris AA, Toyoda K, Venti M, Traenka C, Yamagami H, Alberti A, Yoshimura S, Caso V, Engelter ST, Werring DJ; RAF, RAF-DOAC, CROMIS-2, SAMURAI, NOACISP, Erlangen, and Verona registry collaborators. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol 2020; doi: 10.1002/ana.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY.. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation 2015;132:517–525. [DOI] [PubMed] [Google Scholar]
- 31. Mayer SA, Rincon F.. Treatment of intracerebral haemorrhage. Lancet Neurol 2005;4:662–672. [DOI] [PubMed] [Google Scholar]
- 32. Pennlert J, Overholser R, Asplund K, Carlberg B, Van Rompaye B, Wiklund PG, Eriksson M.. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke 2017;48:314–320. [DOI] [PubMed] [Google Scholar]
- 33. Hart RG, Diener H-C, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke EIWG. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–438. [DOI] [PubMed] [Google Scholar]
- 34. Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J; CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 35. Wachter R, Gröschel K, Gelbrich G, Hamann GF, Kermer P, Liman J, Seegers J, Wasser K, Schulte A, Jürries F, Messerschmid A, Behnke N, Gröschel S, Uphaus T, Grings A, Ibis T, Klimpe S, Wagner-Heck M, Arnold M, Protsenko E, Heuschmann PU, Conen D, Weber-Krüger M; Find-AF(randomised) Investigators and Coordinators. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol 2017;16:282–290. [DOI] [PubMed] [Google Scholar]
- 36. Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, Kreuzer J, Cronin L, Cotton D, Grauer C, Brueckmann M, Chernyatina M, Donnan G, Ferro JM, Grond M, Kallmunzer B, Krupinski J, Lee BC, Lemmens R, Masjuan J, Odinak M, Saver JL, Schellinger PD, Toni D, Toyoda K; RE-SPECT ESUS Steering Committee and Investigators. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med 2019;380:1906–1917. [DOI] [PubMed] [Google Scholar]
- 37. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD, Swaminathan B, Lavados P, Wang Y, Wang Y, Davalos A, Shamalov N, Mikulik R, Cunha L, Lindgren A, Arauz A, Lang W, Czlonkowska A, Eckstein J, Gagliardi RJ, Amarenco P, Ameriso SF, Tatlisumak T, Veltkamp R, Hankey GJ, Toni D, Bereczki D, Uchiyama S, Ntaios G, Yoon BW, Brouns R, Endres M, Muir KW, Bornstein N, Ozturk S, O’Donnell MJ, De Vries Basson MM, Pare G, Pater C, Kirsch B, Sheridan P, Peters G, Weitz JI, Peacock WF, Shoamanesh A, Benavente OR, Joyner C, Themeles E, Connolly SJ; NAVIGATE ESUS Investigators. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378:2191–2201. [DOI] [PubMed] [Google Scholar]
- 38. Ntaios G, Pearce LA, Veltkamp R, Sharma M, Kasner SE, Korompoki E, Milionis H, Mundl H, Berkowitz SD, Connolly SJ, Hart RG; on behalf of the NAVIGATE ESUS Investigators. Recurrent ischemic stroke after embolic stroke of undetermined source (ESUS) often remains ESUS and in same territory: insights from the NAVIGATE-ESUS trial. JAMA Neurol 2020;51:1797–1804. [DOI] [PubMed] [Google Scholar]
- 39. Granger C, Sacco R, Easton J, Meyerhoff J, Cronin L, Kleine E. Predictors of atrial fibrillation in patients with embolic stroke of undetermined source: an analysis of the RE-SPECT ESUS trial. In: ESC/WCC Congress 2019, 31 August–4 September, Paris (abstract);2019.
- 40. Cho MS, Yun JE, Park JJ, Kim YJ, Lee J, Kim H, Park DW, Nam GB.. Pattern and impact of off-label underdosing of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation who are indicated for standard dosing. Am J Cardiol 2020;125:1332–1338. [DOI] [PubMed] [Google Scholar]
- 41. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Kowey PR, Mahaffey KW, Naccarelli G, Reiffel J, Singer DE, Peterson ED, Piccini JP.. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol 2016;68:2597–2604. [DOI] [PubMed] [Google Scholar]
- 42. Lee JH, Park KY, Shin JH, Cha JK, Kim HY, Kwon JH, Oh HG, Lee KB, Kim DE, Ha SW, Cho KH, Sohn SI, Oh MS, Yu KH, Lee BC, Kwon SU.. Symptomatic hemorrhagic transformation and its predictors in acute ischemic stroke with atrial fibrillation. Eur Neurol 2010;64:193–200. [DOI] [PubMed] [Google Scholar]