Abstract
Over the last 10 years since the introduction of non-vitamin K antagonist oral anticoagulants (NOACs) into routine clinical practice our experience with these drugs has increased tremendously, also in the context of patients undergoing electrophysiology procedures. While some open questions remain, the available evidence indicates that for the majority of cases, these interventions can safely be performed on NOACs if study-based standard operating procedures are in place and followed. This review summarizes the most current trial evidence and guidelines on the use of NOACs for patients undergoing cardioversion, atrial fibrillation ablation, and device implantations, based on previous work of the author and others.
Keywords: NOAC, Atrial fibrillation, Cardioversion, Ablation Pacemaker, ICD
Introduction
Based on the outcomes of four landmark randomized clinical trials, non-vitamin K antagonist oral anticoagulants (NOACs) have become the standard of care for stroke prevention in atrial fibrillation (AF).1–6 As a result, many patients undergoing electrophysiological procedures, including cardioversion, ablation, and device implantations, are treated with NOACs. The management of these patients may differ from peri-interventional management around other procedures such as general or orthopaedic surgery in that they involve direct (ablation) or indirect (cardioversion, device implantation) manipulation of the heart and, importantly, the left atrium. As such, anticoagulation management requires an even more refined balance between stroke prevention and the risk of peri-interventional bleeding. Over the last years, experience on the efficacy and safety of NOACs in the context of patients undergoing electrophysiology (EP) procedures has increased tremendously. This review summarizes the most current trial evidence, guidelines, and open questions regarding the use of NOACs for patients undergoing cardioversion, AF ablation, and device implantations, based on previous work of the author and others.7
Cardioversion
Patients with AF of ≥48 h (or unknown) duration who are undergoing electrical or pharmacological cardioversion need to have effective oral anticoagulation established for at least 3 weeks prior to cardioversion based on current ESC guidelines.5 Alternatively, LA/LAA thrombus may be ruled out, e.g. by transoesophageal echocardiography (TOE). Oral anticoagulation has to be continued for at least another 4 weeks following cardioversion. These recommendations are independent of the mode of cardioversion (electrical vs. medical) as well as the CHA2DS2-VASc score and have hence to be followed also in patients with a score of 0 who otherwise do not qualify for long-term anticoagulation.5,6 The rationale behind this seeming inconsistency has traditionally been the belief that the risk of thromboembolism is increased after cardioversion due to dislodgement of a pre-existing thrombus or temporary reduction in atrial function resulting in new thrombus formation.8 Recent evidence has challenged the causal relationship of the increased thrombo-embolic risk around the time of cardioversion indicating that the latter may at least in part be due to overall clinical deterioration of these patients (resulting in the need for cardioversion).9 As such, the mere necessity for cardioversion may (also) be a marker of a larger overall disease burden rather of than (purely) a causal factor for ischaemic events.
In patients requiring cardioversion, two different scenarios have to be distinguished:
Cardioversion in patients newly diagnosed with AF and naïve to anticoagulation.
Cardioversion in patients on chronic treatment with a NOAC
Cardioversion in patients not on non-vitamin K antagonist oral anticoagulants therapy
Dedicated studies have been performed for the scenario of cardioversion in AF patients not on a NOAC. The EMANATE,10 ENSURE-AF,11 and X-VeRT12 trials with apixaban, edoxaban, and rivaroxaban, respectively, included 100%, 27%, and 57% of oral anticoagulation (OAC)-naïve patients, respectively.7 The majority of patients included in these trials had AF of ≥48 h (or unknown) duration. Two different strategies were investigated: Patients were either cardioverted early (after exclusion of LA/LAA thrombus via TOE) or delayed after 3–8 weeks of anticoagulation before cardioversion. Except for a lower rate of ischaemic events with apixaban in the EMANATE trial, there were no differences in ischaemic or bleeding events between the NOAC- and the group using VKA. Similarly, no difference between the early and delayed group was observed. Overall, event rates were (expectedly) low and as such, the trials were formally not powered for non-inferiority. In the EMANATE trial, an initial loading dose of 10 mg of apixaban (5 mg if does-adjustment criteria were met) was administered in 45% of patients.
Taken together, these data indicate that performing a cardioversion in a patient with AF of ≥48 h duration either after 3 weeks of NOAC therapy or using at least a single NOAC dose ≥4 h before cardioversion (≥2 h after apixaban loading dose) after exclusion of a LA/LAA thrombus appears safe and effective.7 Importantly, adherence needs to be assured in patients cardioverted after 3 weeks of NOAC therapy and, if in doubt, exclusion of LA/LAA thrombus is nonetheless recommended.
Observational studies have indicated a lower incidence of ischaemic events using anticoagulation even in patients with AF duration of <48 h, especially in individuals with an elevated CHA2DS2-VASc score ≥2 and AF duration ≥12 h.7,13,14 These patients were not included to a great enough extent in the above-mentioned pivotal cardioversion trials in order to make any firm recommendations. In the pre-NOAC era, they were usually cardioverted following administration of a single dose of LMWH, followed by VKA treatment for at least 4 weeks (if CHA2DS2-VASc score 0 in men or 1 in women) or life-long (in all other patients). In view of the similar pharmacokinetic and pharmacodynamic properties of NOACs vs. LMWH and the similar results in patients on NOACs vs. LMWH/VKA in patients cardioverted after ≥48 h duration AF, the use of NOACs appears justifiable also in these situations.7 Independent of the mode of therapy, LA/LAA thrombus should be ruled out or anticoagulation installed for ≥3 weeks prior to cardioversion in very high risk patients (i.e. CHA2DS2-VASc ≥ 4) and in those in whom there is any doubt about the onset of AF.7
Cardioversion in atrial fibrillation patients on non-vitamin K antagonist oral anticoagulant therapy for ≥3 weeks
Several thousand cardioversions were performed in the landmark phase III NOAC trials in patients on apixaban,3 dabigatran,1 edoxaban,15 or rivaroxaban.2 Overall, the risk of ischaemic as well as bleeding events following cardioversion were very low and similar compared to patients undergoing cardioversion under warfarin. These results were later confirmed in the above-discussed dedicated cardioversion trials as well as in various meta-analyses and ‘real world’ studies.16–18 Although none of these studies include sufficient patient numbers to formally demonstrate statistically non-inferiority, the available evidence strongly suggests that cardioversion under regular and continued NOAC intake is reasonably safe.5,7 One important prerequisite, however, is adequate adherence and correct (on-label) dosing of NOAC therapy prior to cardioversion. If this cannot be guaranteed LA/LAA thrombus should be ruled out with imaging prior to cardioversion. Finally, thrombi may also develop under adequate anticoagulation therapy,19–21 which is why one may opt to anyways perform imaging even in case of long-standing anticoagulation prior to cardioversion. Conversely, not all strokes in patients with AF may be due to thrombi in the LA/LAA; as such, any (rare) ischaemic event in the time period after cardioversion—even on adequate anticoagulation—may not be the result of an undetected LA/LAA thrombus.
Duration of anticoagulation post-cardioversion
Patients with a CHA2DS2-VASc score of ≥2 (women) or ≥1 (men) have a Class IIa indication for life-long anticoagulation independent of cardioversion, while a Class I indication is given in case of a score ≥3 (women) and ≥2 (men).5 However, in patients with a CHA2DS2-VASc score of 1 (women) or 0 (men), the situation is less clear. While current guidelines recommend 4 weeks of anticoagulation if AF duration was ≥48 h prior to cardioversion data are scarce for patients with shorter durations, especially <12 h.7 Importantly, these patients may in addition have self-limiting (i.e. ‘self-cardioverting’) bouts of AF of several hours’ duration (which may be asymptomatic) for which it is unclear whether OAC should be recommended. Given the overall very low risk of thromboembolism in these patients and the usually comparatively good general health, longer and particularly life-long anticoagulation does generally not seem to be mandated, but further studies are necessary.
Management of left atrial/left atrial appendage thrombus
In the absence of an emergency indication cardioversion should be deferred in patients in whom an LA/LAA thrombus is detected. There are no (and likely never will be any) adequately powered endpoint trials comparing a LMWH/VKA based regimen to NOACs for the treatment of LA/LAA thrombi. Increasing observational evidence is indicating a similar degree of thrombus resolution using a NOAC vs. a LMWH/VKA based regimen.22–24 In the prospective X-TRA study, thrombus resolution was observed in 22 out of 53 patients (41.5%) with standard dose rivaroxaban,25 which is comparable to the resolution rate under heparin/VKA in the retrospective CLOT-AF registry (60/96 patients, 62.5%).25 Similarly the rate of thrombus resolution was similar in patients treated with apixaban (52%, 12/23) as compared to LMWH/VKA (56%, 10/18) in the EMANATE trial.26 The prospective RE-LATED AF study with dabigatran has recently been terminated due to recruitment problems (NCT02256683, accessed 10 January 2020). Importantly, mal-adherence as well as off-label use of reduced NOAC dose were shown to be among the main predictors for the development of LA/LAA thrombus ‘under’ NOAC therapy.24 Conversely, increased dosage or changing the anticoagulant strategy appeared to be successful in thrombus resolution.24
Taken together, increasing evidence indicates that NOACs may be a valid option for LA/LAA thrombus resolution. This appears particularly appealing in patients in whom VKA are not well tolerated or in whom an adequate time in therapeutic range cannot be achieved.7
Atrial fibrillation ablation
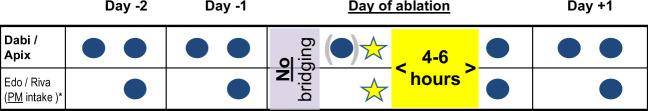
Although the absolute incidence in experienced hands is decreasing, left atrial catheter ablation still carries a risk of both severe ischaemic and bleeding complications, mainly related to transseptal puncture and ablation in the left atrium.27,28 In patients on VKA, ablation is recommended to be performed on uninterrupted therapy with a target international normalized ratio 2–2.5,5,27 as this has been shown to lead to less thromboembolic as well as bleeding complications compared to a LMWH/UFH heparin-based bridging strategy.29 Over the last years, dedicated randomized clinical trials for apixaban (AXAFA),30 dabigatran (RE-CIRCUIT),31 edoxaban (ELIMINATE),32 and rivaroxaban33 have been performed each comparing uninterrupted NOAC to uninterrupted VKA treatment around catheter ablation for AF. Importantly, bid-based NOACs (apixaban, dabigatran) were administered in the morning of the procedure, whereas the last dose of once-daily based NOACs were recommended (rivaroxaban) or mandated (edoxaban) to be administered in the evening before the procedure (Figure 1). Although the studies were underpowered to detect statistically significant differences in hard endpoints, the overall event rate in the NOAC arms of the trials were low, while large variations in the event rate in the VKA arm of the trials were observed. A lower rate of bleeding events was confirmed in a recent meta-analysis of 29 studies comprising over 12 000 patients.34 As such, it may be concluded that the use of uninterrupted NOAC therapy can be considered safe and effective and should likely be preferred to uninterrupted VKA treatment in patients undergoing AF ablation. Whether to give or withhold the morning dose before ablation may depend on various factors. In spite of the positive trial evidence operators may not be entirely comfortable performing groyne puncture, transseptal puncture and left-sided ablation at peak NOAC plasma levels. On the flipside, however, patients may be exposed to very low anticoagulant levels following the procedure if the morning dose is withheld, particularly if protamine is delivered prior to sheath removal. It may therefore be advisable to administer the morning dose prior to ablation if intraprocedural heparin administration is routinely reversed prior to sheath removal, or if the first dose of heparin is administered only after successful transseptal puncture. If, conversely, the peri-interventional protocol includes administration of a first bolus of unfractionated heparin just prior to the first transseptal puncture, closure of the groyne puncture (e.g. with a figure-of-eight stitch or a dedicated closure device) without administration of protamine, and resumption of full dose NOAC therapy 4–6 h post sheath removal, withholding the morning dose of the NOAC appears justifiable as the patient is unlikely to be exposed to low anticoagulant levels at any point in time. Randomized clinical trial evidence comparing these two approached, however, are not available. In once-daily based NOACs, switching NOAC intake to the evening well in advance (e.g. 1 week) of the intervention appears reasonable in order to follow a similar protocol as in the respective clinical trials.32,33
Figure 1:
Management of non-vitamin K antagonist oral anticoagulants around atrial fibrillation ablation (based on reference 7). See text for details. Consider administration of twice-daily dosed non-vitamin K antagonist oral anticoagulants in the morning of the procedure especially if no administration of heparin prior to transseptal puncture is administered and when periprocedural heparin anticoagulation is reversed prior to sheath removal. *Patients on once-daily dosed non-vitamin K antagonist oral anticoagulants (edoxaban, rivaroxaban) are recommended to be switched to evening intake well before the planned atrial fibrillation ablation.
Current expert consensus statements generally recommend to routinely consider exclusion of an LA/LAA thrombus prior to AF ablation.7,27 While TOE (or intracardiac imaging) is currently considered the standard of care to rule out LA/LAA thrombus, computed tomography (CT) may represent an attractive alternative35 especially since a large number of patients will anyways undergo CT imaging prior to ablation for LA anatomy assessment and 3D reconstruction. Performance of the CT shortly prior to the ablation (as for TOE), implementation of a dedicated CT scanning protocol, and extensive experience in the interpretation of CTs in this indication are crucial prerequisites. In case of doubts or inconclusive CT imaging results, performance of a TOE is mandatory.
During AF ablation, intravenous heparin with a target activated clotting time (ACT) of 300–350 s should be administered.7,27,36 The total dose of heparin and time to reach the target ACT is generally higher in patients undergoing AF ablation on NOACs vs. VKA,37–39 reflecting a difference in whole blood coagulability.
Device implantation procedures
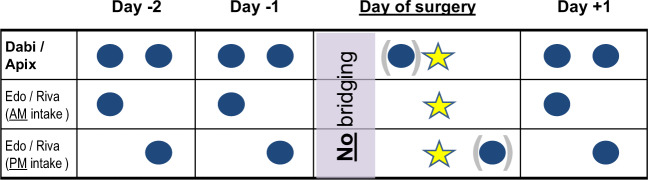
In experienced hands, implantation of cardiac device (i.e. pacemaker, implantable cardioverter defibrillator (ICD), and cardiac resynchronization therapy (CRT) devices) and generator exchanges are generally procedures with a low bleeding risk. Uninterrupted VKA treatment has been shown to lead to fewer bleeding events as compared to heparin-based bridging in patients undergoing device implantation on VKA in the prospective randomized BRUISE-CONTROL trial.40 The BRUISE-CONTROL 2 trial demonstrated that patients on uninterrupted NOAC therapy (including intake in the morning of the procedure) have a similarly low rate of relevant bleeding complications as those with a last intake 48 h before the procedure.41 Other complications were rare, including one stroke and one symptomatic pericardial effusion. Further evidence for a low overall complication rate, including both bleeding and ischaemic events, comes from several registries42 as well as from the PAUSE43 and the EMIT studies in which a various amount of patients undergoing cardiac device procedures on either continued or minimally uninterrupted NOAC therapy have been included, and in which low incidence of bleeding and ischaemic event rates were confirmed. As such, the standard strategy suggested for ‘low bleeding risk’ procedures in the current EHRA44 Practical Guide on the use of NOACs with intake of the last NOAC dose in the morning or evening of the day before the procedure seems reasonable in most cases, followed by restarting one day afterwards (adequate haemostasis provided, Figure 2).7
Figure 2:
Management of non-vitamin K antagonist oral anticoagulants around cardiac device implantation/generator exchange (based on reference 7). See text for details.
The role of NOAC plasma level measurements prior to device surgery (or, for that matter, any type of surgery) is currently unclear due to the lack of adequately powered prospective clinical trial data. Importantly, it is unclear whether the benefit of withholding NOACs for a longer time period based on an elevated NOAC plasma level outweighs the risk of prolonged interruption of anticoagulation.7 Based on the available evidence discussed above, routine NOAC plasma level assessments are not recommended. Whether such a strategy may be of use in very high risk situations such as severe renal insufficiency, multiple possible drug–drug interactions, very high risk interventions (including difficult lead extractions) etc. will require further studies.
Summary and conclusions
Over the last 10 years since the introduction of NOACs into routine clinical practice, our experience with these drugs has increased tremendously, also in the context of patients undergoing EP procedures. Some open questions remain: how long should low-risk patients (CHA2DS2-VASc 0 in men, 1 in women) be anticoagulated following cardioversion? Can NOACs safely and effectively be used to ‘dissolve’ a thrombus in the LA/LAA? Is ‘truly’ uninterrupted NOAC therapy safer and more effective as leaving out the morning dose of the NOAC before AF ablation? What is the optimal NOAC treatment strategy around higher risk cardiac device interventions such as transvenous lead extraction? These questions will require further data, ideally in the form of dedicated randomized trials to be solved; additionally, data from partly already ongoing large registries will be of value (with the main limitation of unmeasured confounding). This notwithstanding the available evidence today indicates that for the majority of cases, EP procedures like cardioversion, ablation, and device implantations can safely be performed on NOACs if study-based standard operating procedures are in place and followed.
Conflict of interest: Dr J.S. has received consultant and/or speaker fees from Abbott, Amgen, Astra-Zeneca, Bayer, Berlin-Chemie/Menarini, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Pfizer, Sanofi-Aventis, and WebMD. He reports ownership of CorXL. J.S. has received grant support through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic. This paper was published as part of a supplement financially supported by an unrestricted educational grant from Daiichi Sankyo Europe GmbH.
References
- 1. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L.. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L.. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM.. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369: 2093–2104. [DOI] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW.. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 7. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H, Lip GYH, Weitz J, Fauchier L, Lane D, Boriani G, Goette A, Keegan R, MacFadyen R, Chiang C-E, Joung B, Shimizu W; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 8. Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW, Davidoff R, Erbel R, Halperin JL, Orsinelli DA, Porter TR, Stoddard MF.. Assessment of cardioversion using transesophageal echocardiography I. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001;344:1411–1420. [DOI] [PubMed] [Google Scholar]
- 9. McIntyre WF, Connolly SJ, Wang J, Masiero S, Benz AP, Conen D, Wong JA, Beresh H, Healey JS.. Thromboembolic events around the time of cardioversion for atrial fibrillation in patients receiving antiplatelet treatment in the ACTIVE trials. Eur Heart J 2019;40:3026–3032. [DOI] [PubMed] [Google Scholar]
- 10. Ezekowitz MD, Pollack CV, Sanders P, Halperin JL, Spahr J, Cater N, Petkun W, Breazna A, Kirchhof P, Oldgren J.. Apixaban compared with parenteral heparin and/or vitamin K antagonist in patients with nonvalvular atrial fibrillation undergoing cardioversion: rationale and design of the EMANATE trial. Am Heart J 2016;179:59–68. [DOI] [PubMed] [Google Scholar]
- 11. Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J, Mercuri MF, Grosso MA, Fernandez V, Al-Saady N, Pelekh N, Merkely B, Zenin S, Kushnir M, Spinar J, Batushkin V, de Groot JR, Lip GY.. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet 2016;388:1995–2003. [DOI] [PubMed] [Google Scholar]
- 12. Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, Talajic M, Scanavacca M, Vardas PE, Kirchhof P, Hemmrich M, Lanius V, Meng IL, Wildgoose P, van Eickels M, Hohnloser SH; X-VeRT Investigators. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J 2014;5:3346–3355. [DOI] [PubMed] [Google Scholar]
- 13. Hansen ML, Jepsen RM, Olesen JB, Ruwald MH, Karasoy D, Gislason GH, Hansen J, Kober L, Husted S, Torp-Pedersen C.. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace 2015;17:18–23. [DOI] [PubMed] [Google Scholar]
- 14. Gronberg T, Hartikainen JE, Nuotio I, Biancari F, Ylitalo A, Airaksinen KE.. Anticoagulation, CHA2DS2VASc score, and thromboembolic risk of cardioversion of acute atrial fibrillation (from the FinCV Study). Am J Cardiol 2016;117:1294–1298. [DOI] [PubMed] [Google Scholar]
- 15. Plitt A, Ezekowitz MD, De Caterina R, Nordio F, Peterson N, Giugliano RP; ENGAGE AF-TIMI 48 Investigators. Cardioversion of atrial fibrillation in ENGAGE AF-TIMI 48. Clin Cardiol 2016;39:345–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Telles-Garcia N, Dahal K, Kocherla C, Lip GYH, Reddy P, Dominic P.. Non-vitamin K antagonists oral anticoagulants are as safe and effective as warfarin for cardioversion of atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol 2018;268:143–148. [DOI] [PubMed] [Google Scholar]
- 17. Mincu RI, Mahabadi AA, Totzeck M, Rassaf T.. Novel anticoagulants versus vitamin K antagonists for cardioversion of non- valvular atrial fibrillation - a meta-analysis of more than 17000 patients. Sci Rep 2019;9:3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frederiksen AS, Albertsen AE, Christesen AMS, Vinter N, Frost L, Moller DS.. Cardioversion of atrial fibrillation in a real-world setting: non-vitamin K antagonist oral anticoagulants ensure a fast and safe strategy compared to warfarin. Europace 2018;20:1078–1085. [DOI] [PubMed] [Google Scholar]
- 19. McCready JW, Nunn L, Lambiase PD, Ahsan SY, Segal OR, Rowland E, Lowe MD, Chow AW.. Incidence of left atrial thrombus prior to atrial fibrillation ablation: is pre-procedural transoesophageal echocardiography mandatory? Europace 2010;12:927–932. [DOI] [PubMed] [Google Scholar]
- 20. Puwanant S, Varr BC, Shrestha K, Hussain SK, Tang WH, Gabriel RS, Wazni OM, Bhargava M, Saliba WI, Thomas JD, Lindsay BD, Klein AL.. Role of the CHADS2 score in the evaluation of thromboembolic risk in patients with atrial fibrillation undergoing transesophageal echocardiography before pulmonary vein isolation. J Am Coll Cardiol 2009;54:2032–2039. [DOI] [PubMed] [Google Scholar]
- 21. Scherr D, Dalal D, Chilukuri K, Dong J, Spragg D, Henrikson CA, Nazarian S, Cheng A, Berger RD, Abraham TP, Calkins H, Marine JE.. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2009;20:379–384. [DOI] [PubMed] [Google Scholar]
- 22. Lee WC, Fang CY, Chen YL, Fang HY, Chen HC, Liu WH, Fu M, Chen MC.. Left atrial or left atrial appendage thrombus resolution after adjustment of oral anticoagulant treatment. J Stroke Cerebrovasc Dis 2019;28:90–96. [DOI] [PubMed] [Google Scholar]
- 23. Niku AD, Shiota T, Siegel RJ, Rader F.. Prevalence and resolution of left atrial thrombus in patients with nonvalvular atrial fibrillation and flutter with oral anticoagulation. Am J Cardiol 2019;123:63–68. [DOI] [PubMed] [Google Scholar]
- 24. Yang Y, Du X, Dong J, Ma C.. Outcome of anticoagulation therapy of left atrial thrombus or sludge in patients with nonvalvular atrial fibrillation or flutter. Am J Med Sci 2019;358:273–278. [DOI] [PubMed] [Google Scholar]
- 25. Lip GY, Hammerstingl C, Marin F, Cappato R, Meng IL, Kirsch B, van Eickels M, Cohen A, Study XT; X-TRA study and CLOT-AF registry investigators. Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J 2016;178:126–134. [DOI] [PubMed] [Google Scholar]
- 26. Ezekowitz MD, Pollack CV Jr, Halperin JL, England RD, VanPelt Nguyen S, Spahr J, Sudworth M, Cater NB, Breazna A, Oldgren J, Kirchhof P.. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J 2018;39:2959–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot N, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T.. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haeusler KG, Kirchhof P, Endres M.. Left atrial catheter ablation and ischemic stroke. Stroke 2012;43:265–270. [DOI] [PubMed] [Google Scholar]
- 29. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S, Rossillo A, Lakkireddy D, Reddy M, Hao S, Hongo R, Beheiry S, Zagrodzky J, Rong B, Mohanty S, Elayi CS, Forleo G, Pelargonio G, Narducci ML, Dello Russo A, Casella M, Fassini G, Tondo C, Schweikert RA, Natale A.. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of Coumadin in preventing thromboembolism in atrial fibrillation (Af) patients undergoing catheter ablation (COMPARE) randomized trial. Circulation 2014;129:2638–2644. [DOI] [PubMed] [Google Scholar]
- 30. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A, Fetsch T, Van Gelder IC, Gentlesk P, Grimaldi M, Hansen J, Hindricks G, Al-Khalidi HR, Massaro T, Mont L, Nielsen JC, Nolker G, Piccini JP, De Potter T, Scherr D, Schotten U, Themistoclakis S, Todd D, Vijgen J, Di Biase L.. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, Okumura K, Serota H, Nordaby M, Guiver K, Biss B, Brouwer MA, Grimaldi M.. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–1636. [DOI] [PubMed] [Google Scholar]
- 32. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Mont L, Morillo CA, Abozguia K, Grimaldi M, Rauer H, Reimitz PE, Smolnik R, Monninghoff C, Kautzner J.. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J 2019;40:3013–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ, Ma CS, Hess S, Wells DS, Juang G, Vijgen J, Hugl BJ, Balasubramaniam R, De Chillou C, Davies DW, Fields LE, Natale A; VENTURE-AF Investigators. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ge Z, Faggioni M, Baber U, Sartori S, Sorrentino S, Farhan S, Chandrasekhar J, Vogel B, Qadeer A, Halperin J, Reddy V, Dukkipati S, Dangas G, Mehran R.. Safety and efficacy of nonvitamin K antagonist oral anticoagulants during catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Cardiovasc Ther 2018;36:e12457. [DOI] [PubMed] [Google Scholar]
- 35. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ.. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 2013;6:185–194. [DOI] [PubMed] [Google Scholar]
- 36. Torn M, Rosendaal FR.. Oral anticoagulation in surgical procedures: risks and recommendations. Br J Haematol 2003;123:676–682. [DOI] [PubMed] [Google Scholar]
- 37. Bassiouny M, Saliba W, Rickard J, Shao M, Sey A, Diab M, Martin DO, Hussein A, Khoury M, Abi-Saleh B, Alam S, Sengupta J, Borek PP, Baranowski B, Niebauer M, Callahan T, Varma N, Chung M, Tchou PJ, Kanj M, Dresing T, Lindsay BD, Wazni O.. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Di Biase L, Lakkireddy D, Trivedi C, Deneke T, Martinek M, Mohanty S, Mohanty P, Prakash S, Bai R, Reddy M, Gianni C, Horton R, Bailey S, Sigmund E, Derndorfer M, Schade A, Mueller P, Szoelloes A, Sanchez J, Al-Ahmad A, Hranitzky P, Gallinghouse GJ, Hongo RH, Beheiry S, Pürerfellner H, Burkhardt JD, Natale A.. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm 2015;12:1162–1168. [DOI] [PubMed] [Google Scholar]
- 39. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E.. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–38. [DOI] [PubMed] [Google Scholar]
- 40. Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, Simpson CS, Ayala-Paredes F, Coutu B, Leiria TL, Essebag V; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013;368:2084–2093. [DOI] [PubMed] [Google Scholar]
- 41. Birnie DH, Healey JS, Wells GA, Ayala-Paredes F, Coutu B, Sumner GL, Becker G, Verma A, Philippon F, Kalfon E, Eikelboom J, Sandhu RK, Nery PB, Lellouche N, Connolly SJ, Sapp J, Essebag V.. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur Heart J 2018;39:3973–3979. [DOI] [PubMed] [Google Scholar]
- 42. Black-Maier E, Kim S, Steinberg BA, Fonarow GC, Freeman JV, Kowey PR, Ansell J, Gersh BJ, Mahaffey KW, Naccarelli G, Hylek EM, Go AS, Peterson ED, Piccini JP; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators. Oral anticoagulation management in patients with atrial fibrillation undergoing cardiac implantable electronic device implantation. Clin Cardiol 2017;40:746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ, Vanassche T, Verhamme P, Shivakumar S, Gross PL, Lee AYY, Yeo E, Solymoss S, Kassis J, Le Templier G, Kowalski S, Blostein M, Shah V, MacKay E, Wu C, Clark NP, Bates SM, Spencer FA, Arnaoutoglou E, Coppens M, Arnold DM, Caprini JA, Li N, Moffat KA, Syed S, Schulman S.. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med 2019;179:1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colonna P, von Heymann C, Santamaria A, Saxena M, Vanassche T, Wolpert D, Laeis P, Wilkins R, Chen C, Unverdorben M. Routine clinical practice in the periprocedural management of edoxaban therapy is associated with low risk of bleeding and thromboembolic complications: The prospective, observational, and multinational emit-af/vte study. Clin Cardiol 2020;doi:10.1002/clc.23379. [DOI] [PMC free article] [PubMed] [Google Scholar]