Highlights
-
•
The serum IL-6 level is the best marker for the severity of COVID-19 of those tested.
-
•
IL-6 values greater than 35 pg/mL can differentiate between mild and severe COVID-19.
-
•
High serum IL-6 values are associated with increased risk of mortality and ICU admission.
Keywords: COVID-19 pneumonia, CURB-65 score, ICU admission, IL-6 serum levels, Mortality, Prognostic biomarkers
Abstract
The clinical presentation of COVID-19 is very heterogeneous, ranging from asymptomatic to severe, which could lead to the need for mechanical ventilation or even death.We analyzed the serum levels of IL-6 in patients with COVID-19 diagnosis and its relationship with the severity of the disease, the need for mechanical ventilation and with patient mortality. We assessed IL-6 in a cohort of 50 patients diagnosed with COVID-19 pneumonia with different degrees of disease severity, and compared it with clinical and laboratory findings.
We found higher levels of IL-6 in patients with more severe pneumonia according to CURB-65 scale (p = 0.001), with ICU mechanical ventilation requirements (p = 0.02), and who subsequently died (p = 0.003). Of the clinical and analytical parameters analyzed in the current study, the serum levels of IL-6 was the most effective predictor of disease severity. From the data obtained in ROC curve analysis, we defined a cut-off point for serum IL-6 levels of 35 pg/mL above which both the risk of mortality (OR = 20.00, 95 % CI 4.214-94-912, p = 0.0001) and ICU admission (OR = 12.750, 95 % CI 2,159-75,3,3, p = 0.005) were increased. Starting from blood IL-6 levels 27 out of 50 patients, with high levels and more severe symptoms, were treated with the IL-6 receptor antagonist Tocilizumab.
IL-6 serum levels appear to be a useful prognostic biomarker in patients with a diagnosis of COVID-19 pneumonia. A cut-off point of 35 pg/mL could clearly differentiate patients a with more severe disease.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection might cause immune-mediated inflammation that is associated with acute respiratory distress syndrome. The clinical presentation of COVID-19 is highly heterogeneous, ranging from asymptomatic to severe pneumonia with respiratory failure that could lead to invasive mechanical ventilation or death. The disease is characterised by an initial phase of viral replication that can be followed by a second phase driven by the host inflammatory response. The most critical patients can develop the so-called cytokine storm, characterised by an increased production of many cytokines that produce long-term damage and lung tissue fibrosis (George et al., 2020). Evidence shows that pro-inflammatory cytokines play a pivotal role in the pathophysiology in patients affected by COVID-19 (Ye et al., 2020). Patients affected by COVID-19 can develop a fulminant and damaging immune reaction sustained by cytokines leading to alveolar infiltration by macrophages and monocytes. Interleukin-6 (IL-6) is one of the main mediators of inflammation in immune response initiated by infection. Increased levels of IL-6 are found in many patients affected by COVID-19 (Herold et al., 2020; Zhang et al., 2020). A recent meta-analysis concluded that there is an evident association between high levels of serum IL-6 and a poor prognosis in the disease (Ulhaq and Soraya, 2020). However, until now it is not established whether IL-6 levels could be a better predictor than clinical and classical analytical data about the evolution of COVID-19. In the present study, we assessed IL-6 levels in conjunction with other analytical and clinical parameters to evaluate them according to the disease severity, the evolution to acute respiratory distress requiring mechanical ventilation, and the mortality of patients with COVID-19.
2. Material and methods
2.1. Study design and patients
A cohort of fifty patients (n = 50) diagnosed with pneumonia by SARS-Cov-2 infection upon admission in the Hospital General Universitario de Ciudad Real between April 1, 2020 and April 30, 2020 was investigated retrospectively from medical records. All patients were positively confirmed by SARS-Cov-2 by RT-PCR using samples from upper respiratory tract after admission. All the patients who were requested for the quantification of IL-6 in serum, during the period in which the study was carried out, were introduced in the study. All analytical determinations were performed simultaneously on the same blood sample. The samples were obtained during the course of the disease, when there was availability for the quantification of IL-6. The pneumonia severity of patients was categorized using the CURB-65 score (Lim et al., 2003) in the following risk groups: a) low risk or mild (score 1) (n = 10), b) moderate risk (score 2) (n = 34), and c) severe risk (score 3 or more) (n = 6). Patients with acute respiratory failure were admitted and intubated in the intensive care unit (ICU-patients, n = 8), whereas patients who did not require intubation were treated on the hospital with oxygen therapy (Non-ICU-patients, n = 42). Of the patients enrolled in the study, 14 deceased while 36 were discharged from hospital. Clinical features (demographic and comorbidities) as well as laboratory determinations were studied among the paired groups of patients to achieved prevalence and associations (Tables 1,2,3 ). IL-6 levels were measured by electrochemiluminescence immunoassay (ECLIA) using the Elecsys kit in a cobas e601 analyzer (Roche Diagnostics, Basel, Switzerland) (IL-6 normal limit in healthy individuals < 7 ng/mL). The absolute number and percentage of lymphocytes and neutrophils were measured in an automated analyzer of blood cell count (UniCel DxH800 Beckman Coulter Miami FL) from EDTA-anticoagulated samples. All patients included in the study had a standard treatment consisting of an antiviral and dexamethasone and/or hydroxychloroquine. Twenty-seven out of the 50 patients were subsequently treated with a IL-6 receptor antagonist treatment (Tocilizumab). All blood samples were obtained prior to initiation of Tocilizumab.
Table 1.
Clinical and laboratory findings of 50 patients diagnosed with COVID-19 disease according to pneumonia severity classified by the CURB-65 score as described in material and methods.The p values are obtained from the Chi-square test in qualitative variables and the Kruskal-Wallis for quantitative variables.
| Clinical and laboratory findings | Severe (n = 6) | Moderate (n = 34) | Mild (n = 10) | p |
|---|---|---|---|---|
| Age (years) | 64.50 ± 2.26 | 65.47 ± 2.05 | 56.20 ± 2.85 | ns |
| Gender | 7 M / 1 F | 28 M / 6 F | 8 M / 2 F | ns |
| Hypertension | 2 (33.33 %) | 19 (55.88 %) | 2 (20 %) | ns |
| Type 2Diabetes | 1 (16.67 %) | 7 (20.58 %) | 0 | ns |
| Cardiovascular disease | 2 (33.33 %) | 8 (23.53 %) | 1 (10 %) | ns |
| COPD | 0 | 7 (20.58 %) | 0 | ns |
| PMN (%) | 87.6 ± 1.9 | 77.4 ± 3.6 | 75.6 ± 3.2 | 0.04 |
| PMN x 103/μL | 8.8 ± 1.0 | 7.5 ± 0.6 | 6.1 ± 1.3 | ns |
| Lymphocytes (%) | 7.9 ± 1.1 | 10.8 ± 1.4 | 17.2 ± 2.4 | 0.02 |
| Lymphocytes x 103/μl | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.2 | ns |
| CRP (mg/dL) | 13.77 ± 5.15 | 7.00 ± 1.19 | 3.61 ± 1.36 | ns |
| D-Dimer (ng/mL) | 3267 ± 908 | 1293 ± 202 | 832 ± 241 | ns |
| Ferritine (ng/mL) | 1560.1 ± 591.6 | 1178.2 ± 180.2 | 910.2 ± 172.7 | ns |
| IL-6 (pg/mL) | 320.45 ± 220.96 | 35.48 ± 10.84 | 7.66 ± 4.52 | 0.001 |
Abbreviations; COPD Chronic obstructive pulmonary disease, PMN polymorphonuclear leukocytes.
2.2. Statistical analysis
The prevalence of categorical variables (gender and comorbidities) was calculated by Chi square with the Fisher’s exact test, with 2 × 2 contingency tables. For the continuous variables (age at admission, CRP, d-Dimer, Ferritine, and white blood cell count) the non-parametric Mann-Whitney U test was used, and when more than two categories were analyzed, the Kruskal-Wallis test was used Post-hoc pairwise comparisons were adjusted for multiple test by the Dunn-Bonferroni correction. The optimal cut-off point for the IL-6 levels to indicate greater severity in patients was obtained from the ROC curves for mortality and ICU admission. The likelihood of more severe disease in patients with elevated IL-6 were determined in logistic regression analysis by the odds ratios (ORs) with the confidence intervals (CIs) at 95 % All the statistical determinations were analyzed using SPSS version 22.0 (SPSS Inc., Chicago, Ill., USA). The differences were considered statistically significant at p-values of less than 0.05.
3. Results
3.1. Clinical characteristics and laboratory parameters among groups of patients
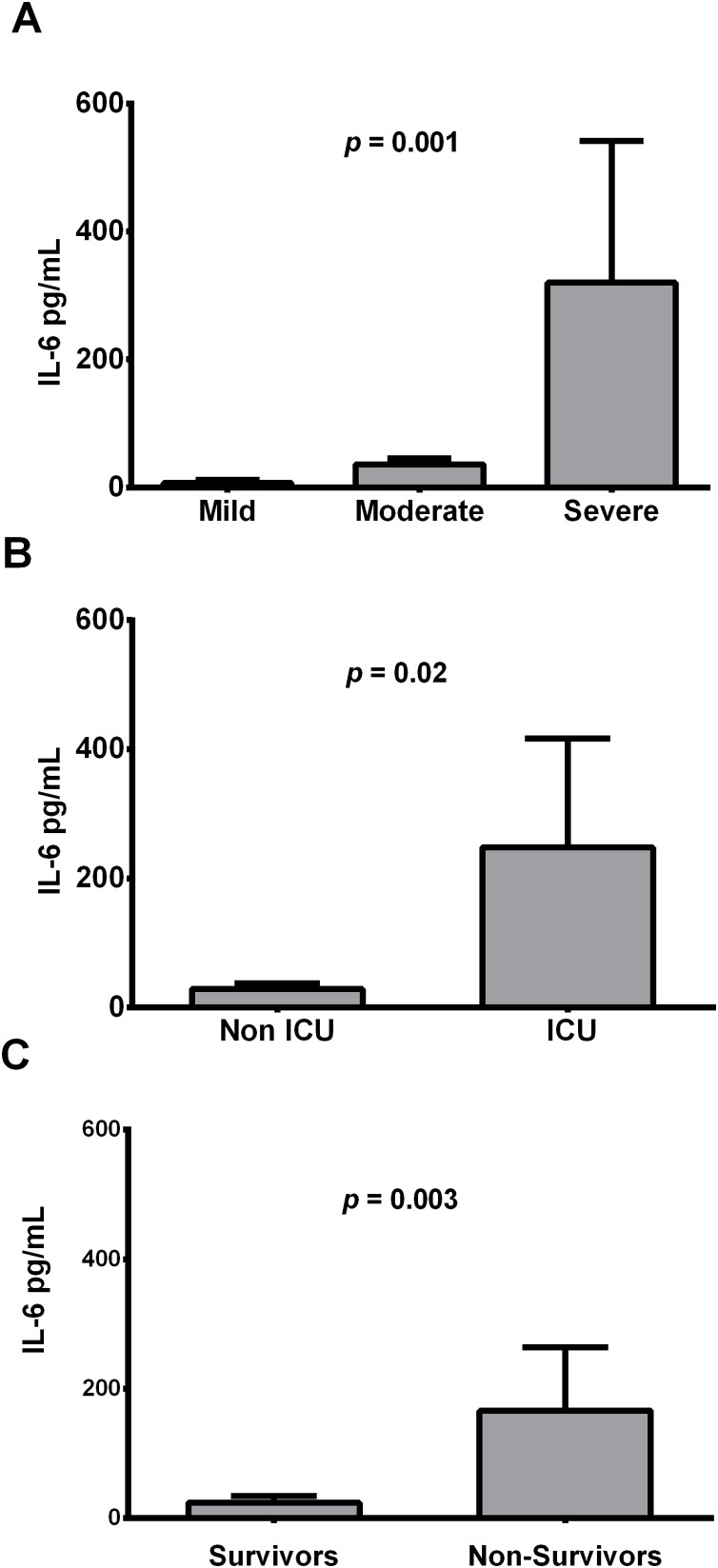
According to severity of COVID-19 pneumonia, patients were grouped as mild (n = 10), moderate (n = 34) and severe cases (n = 6) based on the CURB-65 score (Lim et al., 2003). Clinical and demographic features did not show differences in prevalence among the 3 groups of patients. Conversely, laboratory tests had significant differences both in the percentage of neutrophils (p = 0.04) and lymphocytes (p = 0.02) as well as in the plasmatic level of IL-6 cytokine (p = 0.001) (Table 1; Fig. 1 A). In the pairwise comparisons, both the percentage of polymorphonuclear (PMN) leukocytes and lymphocytes presented differences between groups: mild-severe for PMN (p = 0.04) and mild-moderate for lymphocytes (p = 0.04). Likewise, the IL-6 determination was a discriminator test between mild and moderate cases (p = 0.03) as well as between mild and severe cases (p = 0.001).
Fig. 1.
Mean in pg/mL of IL-6 serum levels according to disease severity (A), ICU admission (B), and mortality (C).
In addition, patients were grouped based on ICU admission and mortality. Patients needed of mechanical ventilation only presented differences in the IL-6 levels, with higher values than patients in hospitalization plant (p = 0.02) (Table 2; Fig. 1B). The group of non-survivors were older respect to discharged patients (69 years vs. 61 years; p = 0.03), presented a higher prevalence of type 2 diabetes (35.7 % vs. 8.33 %; p = 0.02), and also had higher percentage of neutrophils (85 % vs. 75.56 %; p = 0.03) respect to the survivors (Table 3). IL-6 levels presented the highest differences in the group of non-survivors regarding survivors (p = 0.003) (Table 3; Fig. 1C).
Table 2.
Parameters analyzed in the cohort of 50 patients diagnosed with COVID-19 disease based on mechanical ventilation requirements in ICU. The p values are obtained from the Chi-square test in qualitative variables and the U Mann-Wyitney for quantitative variables.
| Clinical and laboratory findings | ICU (n = 8) | Non-ICU (n = 42) | p |
|---|---|---|---|
| Age (years) | 62.13 ± 2.81 | 63.76 ± 1.80 | ns |
| Gender | 7 M / 1 F | 34 M / 8 F | ns |
| Hypertension | 3 (37.5 %) | 20 (47.61 %) | ns |
| Type 2 Diabetes | 2 (25 %) | 6 (14.28 %) | ns |
| Cardiovascular disease | 3 (37.5 %) | 8 (19.05 %) | ns |
| COPD | 0 | 7 (16.67 %) | ns |
| PMN (%) | 82.1 ± 3.0 | 77.5 ± 3.0 | ns |
| PMN x 103/μL | 7.6 ± 1.0 | 7.3 ± 0.6 | ns |
| Lymphocytes (%) | 12.1 ± 2.5 | 11.7 ± 1.3 | ns |
| Lymphocytes x 103/μL | 1.0 ± 0.2 | 0.9 ± 0.1 | ns |
| CRP (mg/dL) | 8.95 ± 4.24 | 6.79 ± 1.05 | ns |
| D-Dimer (ng/mL) | 1792 ± 314 | 1165 ± 419 | ns |
| Ferritine (ng/mL) | 1350.3 ± 550.2 | 1135.2 ± 139.2 | ns |
| IL-6 (pg/mL) | 248.36 ± 168.52 | 29.02 ± 8.97 | 0.02 |
Abbreviations: COPD Chronic obstructive pulmonary disease, PMN polymorphonuclear leukocytes.
Table 3.
Clinical and laboratory data in deceased and discharged patients with COVID-19 disease.The p values are obtained from the Chi-square test in qualitative variables and the U Mann-Wyitney for quantitative variables.
| Clinical and laboratory findings | Non-survivors (n = 14) | Survivors (n = 36) | p |
|---|---|---|---|
| Age (years) | 69.00 ± 3.09 | 61.36 ± 1.72 | 0.03 |
| Gender | 11 M / 3 F | 30 M / 6 F | ns |
| Hypertension | 8 (57.14 %) | 15 (41.67 %) | ns |
| Type 2 Diabetes | 5 (35.71 %) | 3 (8.33 %) | 0.02 |
| Cardiovascular disease | 4 (28.57 %) | 7 (19.44 %) | ns |
| COPD | 1 (7.14 %) | 6 (16.67 %) | ns |
| PMN (%) | 85.14 ± 2.10 | 75.56 ± 3.39 | 0.03 |
| PMN x 103/μL | 8.26 ± 0.70 | 7.02 ± 0.65 | ns |
| Lymphocytes (%) | 9.06 ± 1.67 | 12.89 ± 1.38 | ns |
| Lymphocytes x 103/μL | 0.79 ± 0.11 | 0.98 ± 0.11 | ns |
| CRP (mg/dL) | 9.57 ± 2.62 | 6.19 ± 1.12 | ns |
| D-Dimer (ng/mL) | 1863.38 ± 354.54 | 1230.46 ± 316.79 | ns |
| Ferritine (ng/mL) | 1362.21 ± 299.27 | 1093.50 ± 164.07 | ns |
| IL-6 (pg/mL) | 166.46 ± 97.36 | 24.31 ± 9.90 | 0.003 |
Abbreviations: COPD Chronic obstructive pulmonary disease, PMN polymorphonuclear leukocytes.
3.2. IL-6 cut-off of 35 pg/mL as prognostic biomarker
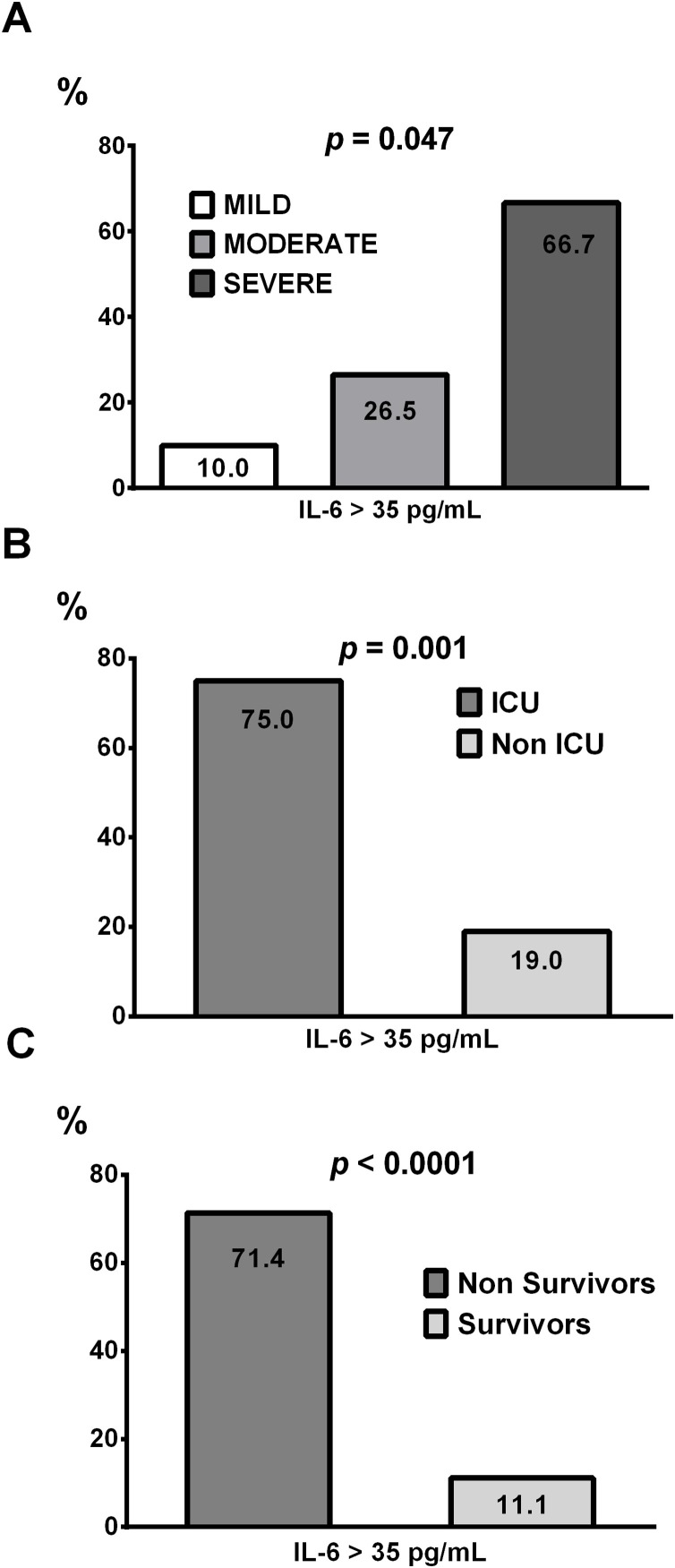
Analysis of ROC curves obtained for IL-6 according to patient mortality or ICU admission, yielded area under the (AUC) curve of 0.754 ;P = 0.024, and 0.760 ; P = 0.03 respectively. For both ROC curves a similar optimal cut-off point of 34.94 pg/mL was obtained. With this cut-off point according to ROC curves, high IL-6 levels provide a sensitivity of 0.71 and a specificity of 0.89 for mortality and 0.75 of sensitivity and 0.81 of specificity for ICU admission. We establish a cut-off of 35 pg/mL to discriminate between mild and severe cases of COVID-19 pneumonia. The cut-off point described in this study is completely consistent with the previously described (Herold et al., 2020) which discriminated against patients with a need for ICU admission. As shown in Fig. 2 A, using a cut-off for IL-6 (> 35 pg/mL) as discriminator biomarker of pneumonia COVID-19 severity, the percentage of patients with high IL-6 levels is increased with the severity of the disease according to the scale CURB-65. In addition, patients who required ICU and/or did not survive to the disease, in a high number had IL-6 values above the defined cut-off point as shown in Fig. 2 B and C respectively. In patients with COVID-19 pneumonia and IL-6 values higher than 35 pg/mL we found an increased risk of mortality (OR = 20.00, 95 % CI 4.214-94-912, p = 0.0001), and in the same way an increased risk to admission in ICU was observed (OR = 12.750, 95 % CI 2,159-75,3,3, p = 0.005). In the association analysis of elevated IL-6 and the pneumonia severity, equally we detected a raised risk (OR = 4.472, 95 % CI 1.146–17.446, p = 0.031). Therefore, IL-6 serum levels greater than 35 pg/mL of IL-6 are associated with increased risk of mortality, mechanical ventilation requirements, and increased severity of SARS-CoV-2 induced pneumonia.
Fig. 2.
Percentage of patients with IL-6 serum values above 35 pg/mL depending on the pneumonia severity CURB-65 score (A), ICU admission (B) and mortality (C).
3.3. Anti IL-6 treatment
Twenty-seven out of the 50 patients with COVID-19 diagnosis (54 %) were treated with the antagonist of IL-6 receptor Trocilizumab in addition to the standard treatments. As expected, patients with higher IL-6 serum values were subsequently treated (108.8 ± 52.4 vs 11.6 ± 7.3 pg/mL; p < 0.0001). Only one out of 10 mild patients was treated with Tocilizumab (10 %), while 26 out of 40 patients classified as moderate or severe (65 %) were treated (p = 0.005). Seven out of eight patients requiring ICU care were given Tocilizumab (87 %), while only 20 out of the remaining 42 (47 %) were treated (p = 0.038). Due to the clinical characteristics of the patients, many of them with severe pneumonia, there was a high mortality in the study group. Eight out 27 treated died (28 %) while six of the 23 untreated (26 %) died.
4. Discussion
IL-6 is a multifunctional cytokine that transmits cell signaling and regulates immune cells. This factor has a strong proinflammatory effect with multiple biological functions and plays an important role in inflammation, tumor, and hematological diseases (Tanaka et al., 2016). The inflammatory response plays a critical role in COVID-19, and inflammatory cytokine storm increases the severity of COVID-19. It has been described that cytokine storm is crucial to the progression of COVID-19 and can lead to severe complications and death (Soy et al., 2020). IL-6 along with other cytokines such as IL-2, TNFα and IL-10 are produced in significant quantities during inflammatory episodes, including SARS-CoV-2 infection (Chen et al., 2020). An increase in IL-6 levels has previously been observed in patients with respiratory dysfunction, implying a possible mechanism of cytokine-mediated lung damage caused by COVID-9 infection (McGonagle et al., 2020). A multicenter retrospective study of 150 confirmed COVID-19 cases confirmed that IL-6 is significantly increased in non-survivors (Ruan et al., 2020). A recent study in NY describes increases in IL-6 levels during the first week of hospital admission in patients who did not survive COVID-19 (Mikami et al., 2020).Together, these data demonstrate the important role that IL-6 plays in the pathophysiology of SARS-CoV-2 coronavirus infection. The present work evaluates the role that the quantification of IL-6 in serum plays in the clinical progress of patients diagnosed with COVID-19.
In this report, we provided evidence that IL-6 levels are closely linked to the severity of COVID-19 infection. The severity of the disease was assessed according to mortality, the need for mechanical ventilation, and by evaluating the severity of the pneumonia produced by the virus according to the CURB-65 score. In all three situations, IL-6 levels were associated with the worst clinical prognostics, highlighting the progression to death, where elevated IL-6 levels increase the risk twenty-fold. According to the CURB-65 scale the pneumonia exibited in patients with COVID-19 was classified as mild-moderate-severe. Patients classified as moderate had mean IL-6 value around 35 pg/mL which just coincides with the value previously described by Herold et al. as a predictor in COVID-19 patients of the need for mechanical ventilation (Herold et al., 2020).This value could be a valid predictor of worse prognosis in the disease, which would alert to the use of specific treatments. In patients treated with IL-6 receptor antagonist (Tocilizumab) it has been shown both a reduction in the risk of mortality and the need for mechanical ventilation (Guaraldi et al., 2020). However, other works have not been able to prove the beneficial effects of Tocilizumab (Campochiaro et al., 2020). In our work, patients with greater clinical severity were treated with Tocilizumab, an IL-6 receptor antagonist Although the mortality among treated and untreated patients was similar, it must be emphasized that only the most severe patients were treated with Tocilizumab and we do not have a untrated control group in the same clinical conditions, which does not allow us to conclude the effect of IL-6 antagonist tratment. The high mortality rate observed could be explained by the characteristics of patients with a high rate of severe pneumonia.
In our study the serum levels of IL-6 behave as a more appropriate biomarker of disease severity than others previously described. Leukocytosis and lymphopenia are also associated with the severity of COVID-19 disease as previously described (Yang et al., 2020) (Urra et al., 2020), but in our work the association observed for IL-6 is more evident. In addition to IL-6 levels, mortality is also influenced by age and the presence of type 2 diabetes in patients with COVID-19 disease. Although type 2 diabetes is not associated with increased SARS-CoV-2 infection, it is associated with a worse progression of COVID-19 (Fadini et al., 2020; Docherty et al., 2020).Type 2 diabetes is widely regarded as a chronic low-grade inflammatory disease, a metabolic syndrome with an excess of nutrients associated with obesity. Obesity-associated inflammation is characterized by an increased abundance and activation of immune cells in adipose tissue, along with a greater release of inflammatory factors such as cytokines at the local and systemic levels (Guo et al., 2020).
In conclusion, IL-6 serum levels appear to be a useful prognostic biomarker in patients with a diagnosis of COVID-19 disease. A cut-off point of 35 pg/mL could clearly differentiate patients with more severe disease.
Ethical approval
Ethical approval for this study was obtained from Ethical committee of Hospital General Universitario de Ciudad Real (ID C-374).
Declaration of Competing Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgements
This work was supported by Instituto de Salud Carlos III (ISCIII) co-founded by Fondo Europeo de Desarrollo Regional – FEDER for the Thematic Networks and Co-operative Research Centres: ARADyAL (RD 16/0006/0028).
References
- Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N., Tomelleri A., Baldissera E., Rovere-Querini P., Ruggeri A., Monti G., De Cobelli F., Zangrillo A., Tresoldi M., Castagna A., Dagna L. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang Xiaoyun, Chen H., Yu H., Zhang Xiaoping, Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. https://doi.org/10.1172%2FJCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Invest. 2020:1–3. doi: 10.1007/s40618-020-01236-2. https://doi.org/10.1007%2Fs40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., Santoro A., Gaetano M.D., Puzzolante C., Carli F., Bedini A., Corradi L., Fantini R., Castaniere I., Tabbì L., Girardis M., Tedeschi S., Giannella M., Bartoletti M., Pascale R., Dolci G., Brugioni L., Pietrangelo A., Cossarizza A., Pea F., Clini E., Salvarani C., Massari M., Viale P.L., Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020:0. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., Qin R., Wang H., Shen Y., Du K., Zhao L., Fan H., Luo S., Hu D. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., Bergwelt-Baildon Mvon, Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.S., van der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I., Lewis S.A., Macfarlane J.T. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T., Miyashita H., Yamada T., Harrington M., Steinberg D., Dunn A., Siau E. Risk factors for mortality in patients with COVID-19 in New York City. J. Gen. Intern. Med. 2020:1–10. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020:1–10. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Masuda K., Kishimoto T. Regulation of Cytokine Gene Expression in Immunity and Diseases. Springer; Dordrecht: 2016. Regulation of IL-6 in immunity and diseases; pp. 79–88. [DOI] [PubMed] [Google Scholar]
- Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra J.M., Cabrera C.M., Porras L., Ródenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin. Immunol. Orlando Fla. 2020;217 doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J.’an, Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-L., Hou Y.-L., Li D.-T., Li F.-Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand. J. Clin. Lab. Invest. 2020:1–7. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]