Editor—Finnerty and Buggy1 propose a role for lidocaine in coronavirus disease 2019 (COVID-19) patients involving neutrophil extracellular trap (NET)osis inhibition as a mechanism. We hypothesise that, given the anti-inflammatory effects of dexmedetomidine, it too may inhibit NETosis and so be beneficial in COVID-19 patients.
Dexmedetomidine, a selective α2-adrenergic receptor agonist, has been studied extensively for long-term ICU use.2, 3, 4, 5, 6 Based on studies investigating its effects in reducing sepsis-related lung injury and ischaemia–reperfusion injury of heart, kidney, brain, and intestine (organs commonly affected in COVID-19),7 and based on the mechanistic models of COVID-19 pathogenesis,8, 9, 10, 11 we suggest that dexmedetomidine may have therapeutic potential in COVID-19. Our hypothesis is supported by a case report of improved oxygenation with dexmedetomidine in a COVID-19 patient12 and by another encouraging report.13
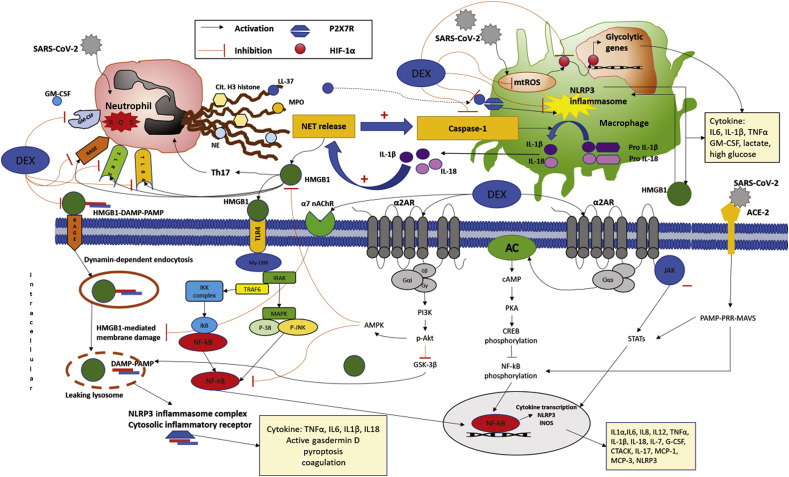
Although dexmedetomidine-mediated improvements in hypoxic pulmonary vasoconstriction and ventilation–perfusion ratios were proposed explanations for improved oxygenation after dexmedetomidine administration in COVID-19 patients,12 the anti-inflammatory properties of dexmedetomidine may also be instrumental in reducing disease severity. Such properties include favourable alterations of inflammation and immune function either directly via cell surface receptors or indirectly by altering sympathetic/parasympathetic imbalance.7 There are several putative mechanisms by which dexmedetomidine might be advantageous in COVID-19 patients (Fig. 1 ). These involve inhibition of Toll-like receptor, high-mobility group box 1, nod-like receptor family pyrin domain-containing protein 3 (NLPRP3) inflammasome, nuclear factor kappa light chain enhancer of activated B cells (NFκB), and Janus kinase/signal transducers and activators of transcription (JAK/STAT) signalling pathways,14, 15, 16, 17, 18 which may be responsible for feedforward interactions between neutrophils and macrophages stimulating NETosis and aggravating organ damage during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.8, 9, 10 Dexmedetomidine-mediated activation of cholinergic pathways and decreased sympathetic tone confer additional cytoprotective and anti-inflammatory benefits.13
Fig. 1.
Putative mechanisms of feedforward interactions between neutrophils, macrophages, and organ-specific cells potentiating NETosis during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and molecular mechanisms of organ protective and anti-inflammatory effects of dexmedetomidine. SARS-CoV-2 uses ACE-2 as its cell entry receptor and activates MAVS that stimulate viral-infected cells to secrete cytokines by activating NF-κB/STAT signalling pathways. SARS-CoV-2 infection results in NLRP3 inflammasome activation, and aggravates pyroptosis and production of DAMPs, such as HMGB1, that play potential key roles in establishing feedforward interactions between neutrophils, macrophages, and organ cells potentiating NETosis and pyroptosis during SARS-CoV-2 infection. Dexmedetomidine inhibits HMGB1/TLR and HMGB1/RAGE-mediated NETosis. Dexmedetomidine-mediated inhibition of NLPRP3 inflammasome, NF-κB, and JAK/STAT signalling pathways, and activation of cholinergic pathways confers anti-inflammatory and organ-protective effects and may reduce oxidative-stress-mediated pyroptosis and thrombotic complications of COVID-19 disease. Dexmedetomidine inhibits mt-ROS and may thereby prevent SARS-CoV-2-triggered mt-ROS production and stabilisation of HIF-1α and consequent sustained aerobic glycolysis mediating cytokine storm and inflammation. AC, adenylyl cyclase; ACE-2, angiotensin-converting enzyme 2; AMPK, adenosine monophosphate-activated protein kinase; cAMP, cyclic adenosine monophosphate; Cit. H3 histone, citrullinated H3 histone; COVID-19, coronavirus disease 2019; CREB, cyclic adenosine monophosphate response element-binding protein; CTACK, cutaneous T cell-attracting chemokine; DAMP, damage-associated molecular pattern; DEX, dexmedetomidine; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–monocyte colony-stimulating factor; GSK-3β, glycogen synthase kinase 3 beta; HIF-1α, hypoxia-inducible factor-1α; HMGB1, high-mobility group box 1; IκB, inhibitor of nuclear factor-κB; IKK complex, inhibitor of nuclear factor-κB kinase; IL1α, interleukin 1α; IL-1β, interleukin 1β; IL6, interleukin 6; IL-7, interleukin 7; IL8, interleukin 8; IL12, interleukin 12; IL-17, interleukin 17; IL-18, interleukin 18; iNOS, inducible nitric oxide synthase; JAK/STAT, Janus kinase/signal transducers and activators of transcription; LL-37, cathelicidin antimicrobial peptide; MAPK, mitogen-activated protein kinase; MAVS, mitochondrial antiviral-signalling protein; MCP-1, monocyte chemoattractant protein-1; MCP-3, monocyte chemoattractant protein-3; MPO, myeloperoxidase; My-D88, myeloid differentiation primary response 88; NE, neutrophil elastase; NET, neutrophil extracellular t rap; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; NLRP3, nod-like receptor family pyrin domain-containing protein 3; PAMP, pathogen-associated molecular pattern; p-Akt, phosphorylated protein kinase B; PI3K, phosphoinositide 3-kinases; P-JNK, phophorylated c-Jun N-terminal kinases; PKA, cyclic adenosine monophosphate-dependent protein kinase; PRR, pattern recognition receptor; P2X7R, P2X purinoceptor 7; RAGE, receptors for advanced glycation end-products; mt-ROS, mitochondrial reactive oxygen species; Th17, T-helper (Th) cell 17; TLR, toll-like receptor; TRAF6, tumor necrosis factor receptor associated factor 6; TNFα, tumor necrosis factor-alpha; α2AR, α2-adrenenergic receptor; α7 nAChR, α7-nicotinic acetylcholine receptor.
Use of dexmedetomidine for sedation in COVID-19 may have a theoretical advantage of skewing the immune response away from T-helper (Th) cell 17 (Th17),19 which has been linked with immunopathogenesis of severe COVID-19 pneumonia.20 Additionally, dexmedetomidine increases expression of natural killer cells, B-cells, CD4+ T cells, and the ratios of CD4+:CD8+ and Th1:Th2 cells, while decreasing CD8+ T cells.21 By increasing interferon gamma:interleukin 4 (INFγ:IL4) ratio,7 dexmedetomidine may possibly improve Th1 immune response against viral infections.7 , 21 Hypoxia-inducible factor-1α (HIF-1α)-induced changes in monocyte metabolism by SARS-CoV-2 infection have been identified to inhibit T-cell response directly and reduce epithelial cell survival.22 As dexmedetomidine can suppress mitochondrial reactive oxygen species formation and inhibit HIF-1α-dependent glycolysis in preclinical settings,23 we believe that early use of dexmedetomidine for sedation in COVID-19 patients admitted to ICU may have therapeutic value as well.
Finally, by upregulating silent information regulator/Forkhead box transcription factor family O 3a (SIRT/FOXO3a) signalling24 and by inhibiting stathmin-1/Bcl-2/caspase-9/caspase-3-mediated lidocaine-induced neurotoxicity,25 dexmedetomidine used in conjunction with lidocaine may provide additional anti-inflammatory benefits, while improving the safety profile of lidocaine.
Although the wealth of scientific evidence from preclinical and clinical studies supports the organoprotective and anti-inflammatory effects of dexmedetomidine,6 , 7 the role of dexmedetomidine as a novel therapeutic strategy to attenuate multi-organ dysfunction in COVID-19 remains hypothetical and awaits the outcome of clinical trials. Two such trials are ongoing and will assess whether or not dexmedetomidine has therapeutic benefits for patients with COVID-19. One of these trials (Immunomodulatory Profile of Dexmedetomidine Sedation in Patients Recovering After ARDS Covid-19; NCT04413864) seeks to study how dexmedetomidine changes the clinical status and immunomodulatory profile of intubated/ventilated patients with COVID-19 in ICU by determining changes in the interrelationship between cytokine levels and ICU delirium. The second study (Use of Dexmedetomidine in Light to Moderate Sedation in the Patient in the Palliative Situation of a SARS-CoV-2/COVID-19 Infection; NCT04350086) will be an investigation on patients with COVID-19 and respiratory failure, who are undergoing palliative care. Pending the results of these and other clinical trials investigating the immunomodulatory and therapeutic benefits of dexmedetomidine in patients with COVID-19, we advocate dexmedetomidine for sedation in patients with COVID-19 in ICU.
Declarations of interest
The authors declare that they have no conflicts of interest.
References
- 1.Finnerty D.T., Buggy D.J. A novel role for lidocaine in COVID-19 patients? Br J Anaesth. 2020;125 doi: 10.1016/j.bja.2020.07.015. e391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandharipande P.P., Pun B.T., Herr D.L., et al. Effect of sedation with dexmedetomidine versus lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z., Chen K., Ni H., Zhang X., Fan H. Sedation of mechanically ventilated adults in intensive care unit: a network meta-analysis. Sci Rep. 2017;7:44979. doi: 10.1038/srep44979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandharipande P.P., Sanders R.D., Girard T.D., et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawazoe Y., Miyamoto K., Morimoto T., et al. Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical trial. JAMA. 2017;317:1321–1328. doi: 10.1001/jama.2017.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashima T., Miyamoto K., Shima N., et al. Dexmedetomidine improved renal function in patients with severe sepsis: an exploratory analysis of a randomized controlled trial. J Intensive Care. 2020;8:1. doi: 10.1186/s40560-019-0415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanders C.A., Rocke A.S., Edwardson S.A., et al. The effect of dexmedetomidine and clonidine on the inflammatory response in critical illness: a systematic review of animal and human studies. Crit Care. 2019;23:402. doi: 10.1186/s13054-019-2690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Long X., Xu Q., et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. 2020;17:992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Berg D.F., Te Velde A.A. Severe COVID-19: NLRP3 inflammasome dysregulated. Front Immunol. 2020;11:1580. doi: 10.3389/fimmu.2020.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farsalinos K., Angelopoulou A., Alexandris N., Poulas K. COVID-19 and the nicotinic cholinergic system. Eur Respir J. 2020;56:2001589. doi: 10.1183/13993003.01589-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stockton J., Kyle-Sidell C. Dexmedetomidine and worsening hypoxemia in the setting of COVID-19: a case report. Am J Emerg Med Adv. 2020 doi: 10.1016/j.ajem.2020.05.066. https://doi:10.1016/j.ajem.2020.05.066 Access published on May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H., Davies R., Ma D. Potential therapeutic value of dexmedetomidine in COVID-19 patients admitted to ICU. Br J Anaesth Adv. 2020 doi: 10.1016/j.bja.2020.09.031. Access published on October 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji X., Guo Y., Zhou G., et al. Dexmedetomidine protects against high mobility group box 1-induced cellular injury by inhibiting pyroptosis. Cell Biol Int. 2019;43:651–657. doi: 10.1002/cbin.11140. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Jiang Z., Zhou X., et al. Dexmedetomidine preconditioning protects cardiomyocytes against hypoxia/reoxygenation-induced necroptosis by inhibiting HMGB1-mediated inflammation. Cardiovasc Drugs Ther. 2019;33:45–54. doi: 10.1007/s10557-019-06857-1. [DOI] [PubMed] [Google Scholar]
- 16.Wu C.Y., Lu Y.F., Wang M.L., et al. Effects of dexmedetomidine infusion on inflammatory responses and injury of lung tidal volume changes during one-lung ventilation in thoracoscopic surgery: a randomized controlled trial. Mediators Inflamm. 2018;2018:2575910. doi: 10.1155/2018/2575910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C., Zhang Z., Chen K., Zhang F., Peng M., Wang Y. Dexmedetomidine regulates inflammatory molecules contributing to ventilator-induced lung injury in dogs. J Surg Res. 2014;187:211–218. doi: 10.1016/j.jss.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Balkanay O.O., Goksedef D., Omeroglu S.N., Ipek G. The dose-related effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg. 2015;20:209–214. doi: 10.1093/icvts/ivu367. [DOI] [PubMed] [Google Scholar]
- 19.Kallioinen M., Scheinin A., Maksimow M., et al. The influence of dexmedetomidine and propofol on circulating cytokine levels in healthy subjects. BMC Anesthesiol. 2019;19:222. doi: 10.1186/s12871-019-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K., Wu M., Xu J., et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. 2019;123:777–794. doi: 10.1016/j.bja.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Codo AC, Davanzo GG, Monteiro LB, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32:437–446. doi: 10.1016/j.cmet.2020.07.007. published correction appears in Cell Metab 2020; 32: 498–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Q., Guo P., Jiang Z., Bo L., Bian J. Dexmedetomidine inhibits LPS-induced proinflammatory responses via suppressing HIF1α-dependent glycolysis in macrophages. Aging (Albany NY) 2020;12:9534–9548. doi: 10.18632/aging.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng L.-N., Guo F.-Q., Li Z.-S., et al. Dexmedetomidine protects against lidocaine-induced neurotoxicity through SIRT1 downregulation-mediated activation of FOXO3a. Hum Exp Toxicol. 2020;39:1213–1223. doi: 10.1177/0960327120914971. [DOI] [PubMed] [Google Scholar]
- 25.Tan Y., Bi X., Wang Q., et al. Dexmedetomidine protects PC12 cells from lidocaine-induced cytotoxicity via downregulation of Stathmin 1. Drug Des Devel Ther. 2019;13:2067–2079. doi: 10.2147/DDDT.S199572. [DOI] [PMC free article] [PubMed] [Google Scholar]