Abstract
Objectives
To investigate the genomic context of a novel resistance island (RI) in multiply antibiotic-resistant Acinetobacter baumannii clinical isolates and global isolates.
Methods
Using a combination of long and short reads generated from the Oxford Nanopore and Illumina platforms, contiguous chromosomes and plasmid sequences were determined. BLAST-based analysis was used to identify the RI insertion target.
Results
Genomes of four multiply antibiotic-resistant A. baumannii clinical strains, from a US hospital system, belonging to prevalent MLST ST2 (Pasteur scheme) and ST281 (Oxford scheme) clade F isolates were sequenced to completion. A class 1 integron carrying aadB (tobramycin resistance) and aadA2 (streptomycin/spectinomycin resistance) was identified. The class 1 integron was 6.8 kb, bounded by IS26 at both ends, and embedded in a new target location between an α/β-hydrolase and a reductase. Due to its novel insertion site and unique RI composition, we suggest naming this novel RI AbGRI4. Molecular analysis of global A. baumannii isolates identified multiple AbGRI4 RI variants in non-ST2 clonal lineages, including variations in the resistance gene cassettes, integron backbone and insertion breakpoints at the hydrolase gene.
Conclusions
A novel RI insertion target harbouring a class 1 integron was identified in a subgroup of ST2/ST281 clinical isolates. Variants of the RI suggested evolution and horizontal transfer of the RI across clonal lineages. Long- and short-read hybrid assembly technology completely resolved the genomic context of IS-bounded RIs, which was not possible using short reads alone.
Introduction
The success of Acinetobacter baumannii as a nosocomial pathogen has been attributed to its unusual environmental persistence, drug resistance, bacterial virulence factors and host immune evasion.1–3 A. baumannii has remarkable abilities to acquire drug resistance genes through multiple pathways including horizontal transfer by the acquisition of transposons, integrons or plasmids.4 Composite transposons and integrons comprising a variable assortment of resistance genes have been identified at multiple chromosomal locations.5 These genomic regions harbouring specific resistance genes and target genes are known as resistance islands (RIs).6 The majority of globally disseminated clones are represented by global clone 1 (GC1) and global clone 2 (GC2). In GC1 isolates, the AbaR0 and AbaR3 RIs have been reported to be progenitors of most RIs identified at the comM locus.5 Amongst GC2 isolates, three major RIs have been identified: AbGRI1,7 AbGRI28 and AbGRI3.9 The three AbGRIs are widely separated across different quadrants of the circular A. baumannii chromosome, either within or juxtaposed to comM, an amino acid permease gene and a putative GNAT family N-acetyltransferase gene. Also, MDR isolates may carry any or all of the AbGRIs.10–12
AbGRI1 has a complex origin with various combinations of transposon Tn6022 (orf4-sup-uspA-orf-tniE-tniD-tniB-tniA-tniC), a deletion derivative Tn6022Δ and the defective transposon Tn6172, strA-strB (streptomycin resistance), tetA(B) (tetracycline resistance) or blaOXA-23 (carbapenem resistance) from Tn2006.7,12,13 AbGRI1 is inserted into the comM locus, which encodes an ATPase.14 AbGRI2 consists of a class 1 integron backbone bounded by copies of IS26 (intl1-aacC1-orfP-orfP-orfQ-aadA1-qacEΔ1-sul1),8 where aacC1, aadA1 and sul1 confer resistance to gentamicin, streptomycin and sulphonamides, respectively. Additionally, variants of AbGRI2 may carry the blaTEM (ampicillin resistance) and aphA1b (kanamycin and neomycin resistance).15,16 The progenitor of AbGRI2 was identified in A320.15 The third type of RI, AbGRI3, carries an IS26-bounded transposon (Tn6180) harbouring a 16S rRNA methyltransferase (armA) and an integron.9 The integron carries int1Δ-aacA4-catB8-aadA1-sul1 with aacA4-catB8-aadA1 being the resistance gene cassettes, where aacA4 confers resistance to gentamicin/tobramycin and catB8 confers resistance to chloramphenicol. AbGRI3 inserts into a putative GNAT family N-acetyltransferase gene.
Molecular approaches, such as PCR and amplicon sequencing, are traditionally used to characterize unique target gene loci to systematically dissect the RI backbone of A. baumannii isolates. Recent advances of next-generation sequencing in the last decade have offered genome-level analysis capabilities; however, the draft genomes produced were often fragmented into several hundreds of contigs when using short-read platforms. Fragmentation of genomic contigs can be partly attributed to repetitive elements, such as the presence of IS elements and transposons (e.g. ∼1 kb), which cannot be resolved using short reads alone (i.e. 150–300 bp read length). RIs of A. baumannii are often bounded by IS elements, posing assembly challenges that commonly result as unanchored solo contigs in draft genome assemblies.10 The recent introduction of long-read sequencing platforms, such as those from Pacific Biosciences (PacBio) and Oxford Nanopore Technologies (ONT), have provided the ability to produce long sequencing reads (i.e. 10–100 kb or longer). The long sequence reads can dramatically improve the contiguity and completeness of chromosome and plasmid assemblies for the analyses of genotype and gene content, and also the structure, composition and genomic context of antimicrobial resistance (AMR) features associated with prophage regions, AMR genes and genomic islands.17–20
A class 1 integron-type RI (intl1-aadB-aadA2-qacEΔ1-sul1), located on unanchored genome contigs, was recently reported in multiply antibiotic-resistant isolates collected through a multi-year A. baumannii surveillance study at a US hospital system.21 In the current study, we generated complete and polished chromosomes of four selected clade F isolates belonging to MLST ST2 (Pasteur scheme) and ST281 (Oxford scheme) (referred to as ST2/ST281 hereafter), using a combination of long reads (ONT) and short reads (Illumina). Based on the complete genome sequences, we identified a novel target gene (an α/β-hydrolase gene) where the class 1 integron-type RI insertion was detected. We propose naming the RI insertion and its novel genomic context AbGRI4. Further examination of publicly available A. baumannii genomes revealed multiple variants of AbGRI4. This study provides new insights into the horizontal transmission and evolution of aminoglycoside resistance determinants in A. baumannii.
Materials and methods
Genome sequencing and assembly
High molecular weight genomic DNA was prepared from A. baumannii overnight cultures using the QIAGEN Gentra Puregene Yeast/Bact Kit. MinION sequencing libraries were prepared using the Rapid Barcoding kit (SQK-RBK004) and sequenced on a MinION R9.4 flow cell. Paired-end Illumina sequencing libraries had been previously generated21 using the NexteraXT library kit and Illumina reads had been generated on a NextSeq 500 (300 cycles, 2 × 150 bp). Hybrid MinION–Illumina de novo assembly was performed using the Unicycler pipeline (v0.4.7)22 under default settings. Pilon (v1.22)23 was used iteratively to polish the assemblies with Illumina reads until no additional nucleotide corrections could be made. The genomes were annotated by the NCBI Prokaryotic Genome Annotation Pipeline (PGAP).24,25 MLST typing of A. baumannii isolates was performed using LOCUST.26 Sequence reads for the four whole-genome sequenced isolates are available from the NCBI Sequence Read Archive (SRA) (Isolate/Illumina/ONT): ABUH763/SRR3666894/SRR10173076; ABUH773/SRR3666904/SRR10173072, SRR10173073, SRR10173075, SRR10173077; ABUH793/SRR3666207/SRR10173103; and ABUH796/SRR3666896/SRR10173078. Assembled chromosome and plasmid sequences are available under: ABUH763/CP035051–3, ABUH773/CP035049–50, ABUH793/CP035045–8 and ABUH796/CP035043–4.
BLAST-based RI analysis
To screen for RI insertions at target genes, BLAST-based analysis was used to detect inflated total gene lengths due to target site duplications, as previously described.9–11 This approach does not require complete genomes and works for draft genome assemblies, which represent the majority (96%) of over 6000 sequenced A. baumannii isolates collected at the Pathosystems Resource Integration Center (PATRIC) as of September 2019.27 Briefly, full-length intact versions of target genes, in which RIs are commonly inserted, were collected and used as reference sequences, including: comM (ACINNAV82_0188) for AbGRI1, an amino acid permease gene (AB57_1175) and an NAD(P)/FAD-dependent oxidoreductase gene (AB57_1209) for AbGRI2 and a GNAT family N-acetyltransferase gene (DMO12_07266) for AbGRI3. Each target gene reference was mapped against the A. baumannii genomic contigs using BLASTN28 and total query gene hit lengths were computed. The presence of an RI insertion in the query target gene would interrupt the gene sequence and produce two gene fragment hit lengths extended by a copy of the target site duplication. To determine the type of RI present among the four whole-genome sequenced A. baumannii clinical isolates, genomic contigs were mapped against previously characterized RIs including: AbGRI1 and AbGRI2,8 and AbGRI3.10,11 The representative GenBank accessions and nucleotide coordinates for AbGRI1 were A91: JN968483.3, 56871–66688; and MDR-TJ: CP003500, 671921–3694731. Representatives for AbGRI2 were WM99c: JX869489, 12072–17437; MDR-TJ: CP003500, 2664312–2669960; and MDR-ZJ06: CP001937, 1342193–1346858. Representatives for AbGRI3 were TYTH-1: CP003856, 2824315–2828926; and Tn1548-armA: EU014811, 1979–2752. The sequence of the inverted repeat near intI1 (IRi) used in searches was ‘TGTCGTTTTCAGAAGACGGCTGCAC’. The sequences of the inverted repeat right of IS1326 (IRR1326) and the inverted repeat left of Tn2 (IRL2) were obtained from ISFinder.29
To determine whether the new RI AbGRI4 was present among A. baumannii global isolates, over 3000 A. baumannii genome assemblies were downloaded from NCBI (September 2018) and searched against a full-length version of the newly identified α/β-hydrolase target gene (e.g. AB994_0727) and the AbGRI4 reference sequence from this study using BLASTN. The AbGRI4 reference sequence was extracted from ABUH796 (CP035043, 1515737–1524576).
Phylogenetic analysis
A whole-genome alignment was inferred from SNPs identified by the Northern Arizona SNP Pipeline (NASP) v1.0.230 and Gubbins v2.2.131 using A. baumannii A320 (CP032055) as the reference genome and A. baumannii AB0057 (CP001182) as the outgroup. A maximum-likelihood phylogenetic tree was generated using RAxML32 under the GTRCAT model with 100 bootstrap replicates. The resulting tree was rendered with metadata annotated using the Interactive Tree of Life (iTOL).33
Results
Study strains
A subset of A. baumannii isolates belonging to the ST2/ST281 clade F group21 showed two different RI configurations at the comM locus based on analysis of the Illumina short read-based genomic assemblies. In this study, four isolates were selected for WGS to completion using ONT long reads. The isolates ABUH763, ABUH793 and ABUH796 were collected from patients with pneumonia and ABUH773 from a patient with a urinary tract infection. The isolates were collected in 2015 within a US hospital system (Cleveland, OH, USA) and the antibiotic susceptibility profiles were previously reported.21
Identification of known RIs and elements
Complete chromosomes and plasmids were generated for the four isolates using a combination of long and short reads (Table S1, available as Supplementary data at JAC Online). Using a BLAST-based analysis workflow we have previously developed for large-scale RI typing of A. baumannii isolates,10 we performed analysis for AbGRI1, AbGRI2 and AbGRI3 on the four complete genomes. A. baumannii RIs were identified based on the detection of target gene fragments as a result of RI insertions and the detection of RI sequences.
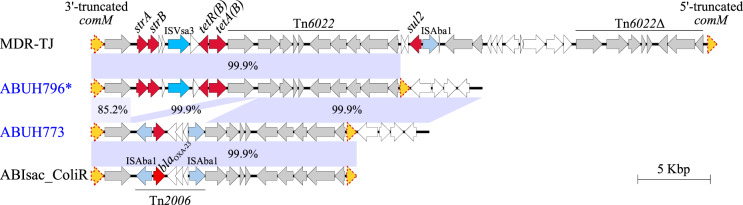
For three isolates (ABUH763, ABUH793 and ABUH796), RI analysis identified the insertion of strA-strB (streptomycin resistance) and tetR(B)-tetA(B) (tetracycline resistance) genes at the comM locus (Figure 1). A copy of the transposon Tn6022 was identified downstream of the resistance genes. The entire 22 kb RI region that was inserted into the ABUH796 comM locus shared 99.9% nucleotide sequence identity with the 5′ portion of the comM RI region in the MDR MDR-TJ isolate (Figure 1)13 and also with an AbGRI1 variant reported in the LT-V1 isolate.34 In ABUH773, an RI of size 18.3 kb identified as AbaR4 was detected at the comM locus, which was identical (99.9%) to the comM RI previously reported in the isolates ABIsac_ColiR35 (Figure 1) and D36.36 No RI insertions were identified for AbGRI2 or AbGRI3 across all four whole-genome sequenced ABUH isolates. At the genomic region corresponding to AbGRI2, a 22 kb deletion accompanied by a single IS26 element with respect to ACICU was detected in ABUH796 (Figure S1a). No extra sequences were detected at the corresponding region of AbGRI3 in ABUH796 (Figure S1b).
Figure 1.
RI configurations at the comM locus of ABUH796 and ABUH773. The reference isolates MDR-TJ and ABIsac_ColiR carried distinct AMR gene sequences at the comM locus. ABUH796 shared close-to-perfect nucleotide sequence identity with MDR-TJ, and ABUH773 with ABIsac_ColiR, respectively. The genomic regions shown were extracted from ABUH796: EP550_17795 to EP550_17895; ABUH773: EQH48_17425 to EQH48_17510; MDR-TJ: ABTJ_RS17575 to ABTJ_RS17785; and ABIsac_ColiR: BN50_RS01315 to BN50_RS01215 and displayed using Easyfig.52 The asterisk is representative of isolates ABUH763, ABUH793 and ABUH796. comM, orange-filled arrows with dotted line; AMR genes, red-filled arrows; IS elements, blue-filled arrows; Tn6022 and related ORFs, grey-filled arrows. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
For plasmids, both TnaphA6 (aphA6, conferring aminoglycoside resistance, flanked by two direct copies of ISAba125) and blaOXA-23 (carbapenemase resistance, flanked by two direct copies of ISAba1 in reverse orientation that were 15 kb apart) were identified in a 74 kb plasmid in two of the sequenced isolates (ABUH763 and ABUH793). The 74 kb plasmid showed nearly perfect identity to a previously reported plasmid group named pABUH1.11 Another plasmid (13 kb) carrying a copy of TnaphA6 was identified in ABUH796. The 13 kb plasmid was near-identical to plasmid pS21-1 (MG954376). A summary of the RIs and elements identified in these plasmids is shown in Table 1.
Table 1.
Summary of AMR elements of four whole-genome sequenced A. baumannii isolates
| A. baumannii isolate | MLST (Pasteur/ Oxford or Replicon) | Location of AMR elements (RI, Tn, IS) | Resistance genes |
|---|---|---|---|
| ABUH763 | |||
| chromosome (CP035051) | 2/281 | AbGRI1 (3668886–3690858) | strA-strB, tetR(B)-tetA(B), sul2 |
| AbGRI2 (not present) | none | ||
| AbGRI3 (not present) | none | ||
| AbGRI4 (1518797–1527636) | aadB, aadA2, sul1 | ||
| plasmid p74.1 Kbp (CP035052) | repAci6 | TnaphA6 (42861–45871) ISAba1-blaOXA-23 (7234–9244) | aphA6, blaOXA-23 |
| ABUH793 | |||
| chromosome (CP035045) | 2/281 | AbGRI1 (77749–55777) | strA-strB, tetR(B)-tetA(B), sul2 |
| AbGRI2 (not present) | none | ||
| AbGRI3 (not present) | none | ||
| AbGRI4 (2228102–2219263) | aadB, aadA2, sul1 | ||
| plasmid p74.1 Kbp (CP035047) | repAci6 | TnaphA6 (42861–45871) ISAba1-blaOXA-23 (7234–9244) | aphA6, blaOXA-23 |
| ABUH796 | |||
| chromosome (CP035043) | 2/281 | AbGRI1 (3669082–3691054) | strA-strB, tetR(B)-tetA(B), sul2 |
| AbGRI2 (not present) | none | ||
| AbGRI3 (not present) | none | ||
| AbGRI4 (1515737–1524576) | aadB, aadA2, sul1 | ||
| plasmid p13.0 Kbp (CP035044) | repAci2 | TnaphA6 (7091–10101) | aphA6 |
| ABUH773 | |||
| chromosome (CP035049) | 2/281 | AbGRI1 (3616915–3635219) | bla OXA-23 |
| AbGRI2 (not present) | none | ||
| AbGRI3 (not present) | none | ||
| AbGRI4 (not present) | none | ||
| plasmid p11.8 Kbp (CP035050) | repAci2 | TnaphA6 (not present) | none |
A novel class 1 integron RI, AbGRI4
Based on the complete whole-genome sequences, we determined the genomic context of a previously identified, but unanchored, class 1 integron RI detected in the isolates ABUH763, ABUH793 and ABUH796. As all three isolates carried the same identical class 1 integron RI, the discussion hereafter will focus on ABUH796.
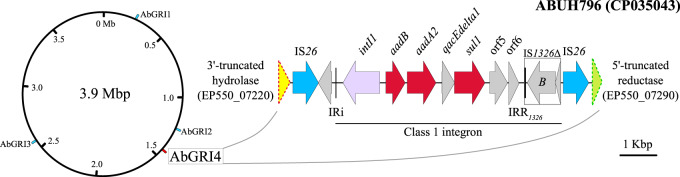
The 6.8 kb class 1 integron-type RI was bounded by IS26 and located between two neighbouring genes: an α/β-hydrolase gene fragment (EP550_07220) and an FMN-dependent NADH-azoreductase gene fragment (EP550_07290) in the ABUH796 isolate (CP035043). The genomic region was approximately 200 kb downstream from the genomic hotspot where AbGRI2 was previously reported (Figure 2). The new RI carried a class 1 integron backbone and aadB-aadA2 AMR gene cassettes and was flanked by two direct copies of IS26, a member of the IS6 family (Figure 2). IS26/IS6 family members are known to play key roles in the dissemination of antibiotic resistance genes in Gram-negative bacteria, as discussed in a recent review.37 Because of the unique combination of the class 1 integron backbone and the resistance gene cassettes, and a novel genomic location, we propose naming this new RI AbGRI4.
Figure 2.
AbGRI4 carries a class 1 integron harbouring the aadB-aadA2 resistance gene cassettes. The gene orders are: IS26-intl1-aadB-aadA2-qacEΔ1-sul1-orf5/6-istB-IS26. In AbGRI4-positive isolates, the AbGRI4 RI juxtaposed 3′-truncated hydrolase and 5′-truncated reductase gene fragments. The genomic location of AbGRI4 was projected onto a 3.9 Mbp circular genome backbone relative to the locations of previously reported RIs AbGRI1, AbGRI2 and AbGRI3. The AbGRI4 genomic region was displayed using Easyfig.52 Hydrolase gene, yellow-filled arrow with red dotted line (truncated); reductase gene, green-filled arrow with green dotted line (truncated); AMR genes, red-filled arrows; IS26 elements, blue-filled arrows.
In class 1 integrons, two conserved segments (CSs) of sequence flank the variable AMR cassette array, with the 5′-CS including intI1 and the 3′-CS corresponding to qacEΔ1, sul1, orf5 and orf6.38 The ORF qacEΔ1 encodes a quaternary ammonium compound efflux AMR transporter and sul1 a sulphonamide-resistant dihydropteroate synthase, which confer resistance to older generations of antibiotics. The AbGRI4 nucleotide sequence, when compared with the class 1/In0 subclass (U49101), consisted of intl1 and qacEΔ1-sul1-orf5/6-istB, albeit missing three ORFs downstream of istB (i.e. istA, tniBΔ1 and tniA). The intl1 and qacEΔ1-sul1-orf5/6-istB sequences of AbGRI4 were 98.4% and 100% identical to the class1/In0 subfamily, respectively.
The AMR gene cassettes of AbGRI4 consisted of aadB (kanamycin/gentamicin/tobramycin resistance) and aadA2 (streptomycin/spectinomycin resistance). In A. baumannii, aadB (aminoglycoside resistance) is not commonly detected and so far has only been reported in pRAY* plasmids,39 a genomic island in the D4 isolate40 and a class 1 integron in a few ST3/ST928 isolates.41 In contrast to AbGRI4, the aadB gene cassette in pRAY* is not presented within a class 1 integron, but instead is flanked by ORFs of unknown function and mobilization genes mobA and mobC. In the D4 isolate (ST25/ST364), a 30 kb genomic island AGI1 has been identified,40 but is distinct from AbGRI4. AGI1 carries multiple class 1 integrons, each individually harbouring aadB or aadA2, and the genomic island is inserted into the 3′ end of trmE. Finally, an 18 kb RI identified in ST3/ST928 isolates carries aadB in a class 1 integron backbone but at a different genomic location than AbGRI4.10,42
Large genomic islands similar to AGI1 have also been identified in the genomes of Salmonella enterica serovars and Proteus mirabilis at the trmE gene locus.43–45 It is interesting to note that the AbGRI4 class 1 integron (from IRi to 996 bases through IS1326) including the aadB-aadA2 cassettes aligned at near-perfect nucleotide identity (99.7%) to a subregion of the P. mirabilis genomic islands PGI1 (81.1 kb) and GIPmi1 (55.8 kb) (Figure S2). These genomic islands have been discovered in P. mirabilis clinical isolates PmCHA, PmCHE and PmPHI, collected in 2012 in France.46,47 Possible horizontal transfer of AMR between A. baumannii and P. mirabilis warrants further investigation.
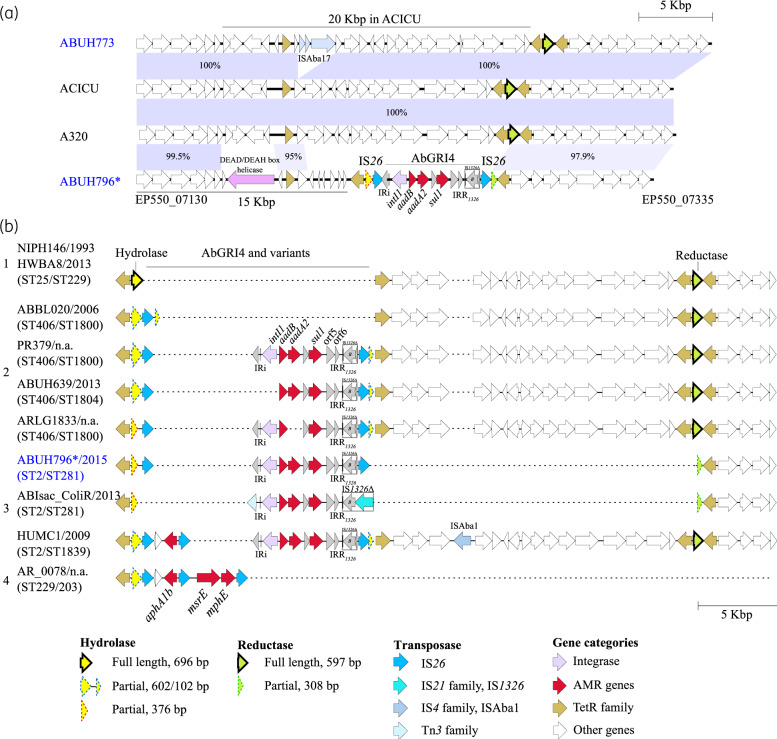
The fourth ST2/ST281 clade F isolate ABUH773 did not carry a class 1 integron in the syntenic region where AbGRI4 was detected. Instead, the alignment of ABUH773 against the ST2/ST437 ACICU reference genome revealed the insertion of an IS66 family ISAba17 (ORFs EQH48_07190, EQH48_07195 and EQH48_07200) in the genomic region of interest, which was otherwise nearly identical to ACICU (Figure 3a). Further, a comparison of the AbGRI4-positive isolate ABUH796 against ACICU and ABUH773 revealed the replacement of a 20 kb region in ACICU (DMO12_04170 to DMO12_04230) by a genomic fragment that carried genes for a helicase, multiple TetR family proteins and hypothetical proteins, followed by a 3′-truncated hydrolase gene, the AbGRI4 RI and a 5′-truncated reductase gene (Figure 3a). In all, syntenic analysis showed that the 20 kb region of interest was highly variable across the ST2 (Pasteur) isolates ABUH796, ABUH773 and ACICU and could be a chromosomal hotspot of IS insertions and recombinations. Finally, we noted that genes for TetR family proteins were found immediately flanking both of the RI target genes, for hydrolase and reductase (Figure 3a). The TetR family proteins, in addition to their wide association with antibiotic resistance, are known to be involved in one-component signal transduction and transcriptional regulation.48 Whether the TetR family proteins, with genes residing in close proximity to RIs, are functionally involved in IS insertion and recombination events requires future investigation.
Figure 3.
AbGRI4 configuration in ST2/ST281 and other clonal lineages. (a) Pairwise alignments of AbGRI4 and genomic regions amongst: ABUH796 (AbGRI4 positive), ABUH773 (AbGRI4 negative) and the reference genomes A320 and ACICU (both AbGRI4 negative). The genomic regions shown were extracted from ABUH796: EP550_07130 to EP550_07335; ABUH773: EQH48_07125 to EQH48_07330; ACICU: DMO12_04152 to DMO12_04257; and A320: A320_02326 to A320_02291 and displayed using Easyfig.52 (b) Variants of AbGRI4 identified in ST2/ST281, the ST406 group and other isolates. All isolates shared a conserved region (15 kb) [not shown in (b)] upstream of the hydrolase gene spanning a DEAD/DEAH box helicase gene up to EP550_07130 in ABUH796 as indicated in panel (a) (see Figure S3 for extended alignment of the region). In ST25 (Group 1), an intact hydrolase gene was detected. For ST406 (Group 2), putative precursor or intermediate forms of AbGRI4 were identified. The isolates also carried a 20 kb genomic region downstream of AbGRI4 including an intact reductase gene. ST2 isolates (Group 3), including ABUH796 together with ABUH763 and ABUH793, were whole-genome sequenced and finished in this study and carried exemplar copies of AbGRI4 flanked by IS26 elements. The AbGRI4 in ABIsac_ColiR was flanked by a recombinase gene and an IS1326 element instead of IS26. The AbGRI4 in HUMC1 carried an aphA1b resistance gene, upstream of the AbGRI4, and an ISAba1 insertion in the 20 kb genomic region. In AR_0078, a completely different set of resistance genes was identified including aphA1b, msrE and mphE. AR_0078 carried a hydrolase gene fragment (AM457_11345), but the reductase gene was not detected in the corresponding region. The genomic regions bounded by the hydrolase and reductase genes were as follows: NIPH146, F979_RS13625 to F979_RS13685; HWBA8, B7L46_16170 to B7L46_16265; ABBL20, APC64_12840 to APC64_12940; PR379, B9Y25_04090 to B9Y25_04245; ABUH639, BTH23_04090 to BTH23_04235; ARLG1833, CAS91_11670 to CAS91_11825; ABUH796, EP550_07220 to EP550_07290; ABIsac_ColiR, BN50_RS06860 to BN50_RS06920; and HUMC1, AWC45_RS08085 to AWC45_RS08260. Alignments of the genomic regions were performed using Easyfig.52 The asterisk is representative of isolates ABUH763, ABUH793 and ABUH796. Isolates shown in blue were whole-genome sequenced and finished in this study; n.a., not available. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
AbGRI4 in global A. baumannii isolates
To determine whether AbGRI4 RIs are present across global A. baumannii isolates, a total of 3113 publicly available genome assemblies were searched against a full-length hydrolase target gene and the AbGRI4 nucleotide sequence (Table S2). Multiple distinct variants of hydrolase gene fragments were detected. First, a group of 147 out of 312 ST2/ST281 isolates were identified as AbGRI4 positive, carrying 5′-hydrolase gene fragments of the exact length of 376 bp and AbGRI4 sequences, including three of the four isolates sequenced in this study (i.e. ABUH763, ABUH793 and ABUH796). Second, a new group of 22 out of 50 ST406/ST1800 and ST1088/ST1804 (collectively referred to as the ST406 group hereafter) were AbGRI4-positive isolates carrying 5′-hydrolase gene fragments, of various distinct fragment lengths, and AbGRI4 sequences. Third, intact hydrolase gene sequences were detected in a group of ST25 (Pasteur) isolates that included multiple different ST types under the Oxford scheme. A sequence alignment analysis of an extended 90 kb genomic region around AbGRI4 of ABUH796 confirmed a common recombination region across the global isolates described below (Figure S3).
To investigate clonal lineage differences of the AbGRI4-positive isolates, a phylogenetic tree was constructed from whole-genome alignment of SNPs with respect to the genome of reference isolate A320. The SNP tree showed that ST2/ST281 isolates were separated from the ST406 group, with each forming a distinct lineage (Figure S4). The ST2/ST281 isolates were mostly collected from US hospital systems between 2012 and 2016,21 while isolates from the ST406 group were collected from the USA during an earlier period between 2005 and 2013. Considering the distinct genome backbones of the ST2/ST281 and the ST406 group lineages, the different timing of collection and the varying lengths of the hydrolase gene fragments between the two lineages, all evidence taken together supports independent acquisition of the AbGRI4 RI in ST2/ST281 and the ST406 groups except for HUMC1, whose hydrolase gene shares the same insertion site as some ST406 strains, which suggests acquisition from a common source.
Investigations of AbGRI4 structural variation at the nucleotide sequence level were performed using a 36 kb AbGRI4-containing genomic region derived from the isolate ABUH796 (EP550_07130 to EP550_07335) (Figure 3a). Multiple variants of AbGRI4 were identified in global isolates including ABIsac_ColiR and ABIsac_ColiS, HUMC1 and AR_0078. The clone pair of ABIsac_ColiR and ABIsac_ColiS (both ST2/ST281) were collected in Marseille, France in 201335 (Figure 3b, Group 3). From the RI analysis, it was determined that the isolates carried a 376 bp hydrolase gene fragment and a copy of AbGRI4 at 99.4% nucleotide sequence identity to that of ABUH796. Surprisingly, the RI was partially flanked by a different type of IS element (i.e. IS21 family/IS1326), which was distinct from the IS26 found in AbGRI4. The IS21 family element does not share sequence similarity with IS26. The HUMC1 (ST2/ST1839) isolate was collected in 2009 and carried two hydrolase gene fragments, 602 bp and 102 bp, split by AbGRI4, and an additional upstream aphA1b resistance gene (Figure 3b, Group 3). Finally, in AR_0078 (ST229/203), AbGRI4 was not detected; however, a completely different group of AMR genes were identified downstream of the 602 bp hydrolase gene fragment (Figure 3b, Group 4). The isolate harboured multiple IS26 copies that flanked the AMR genes including aphA1b [APH(3′)-I family aminoglycoside O-phosphotransferase], msrE [ABC-F type ribosomal protection protein Msr(E)] and mphE (macrolide phosphotransferase). The year of collection for AR_0078 was not available.
Among the ST406 group of isolates, multiple AbGRI4 variants were identified when compared with the version found in ABUH796 (Figure 3b, Group 2). In the ABBL020 isolate (ST406/ST1800), the hydrolase gene was interrupted by a single copy of IS26 and split into two fragments of length 602 bp and 102 bp. A DR of 8 bp (5′-AAAAATGT-3′) was detected at either end, flanking the 821 bp single IS26 element. The DR appeared to result from a target site duplication event due to IS insertion. The DRs were also detected in other ST406 group isolates that carried both of the 602 bp and 102 bp hydrolase gene split fragments (PR379 and ABUH639) and also HUMC1, which belongs to ST2. The PR379 (ST406/ST1800) isolate carried a full-length AbGRI4, including a class 1 integron, AMR cassettes and IS elements, as an insertion that interrupted the hydrolase gene, which was again split into two fragments of length 602 bp and 102 bp. In PR379 and other isolates belonging to the ST406/ST1800 group (Figure 3b, Group 2), the reductase gene was intact, together with a 20 kb region downstream of the 102 bp hydrolase 3′ gene fragment. It is notable that the corresponding 20 kb genomic region was absent from most of the AbGRI4-positive ST2/ST281 isolates (e.g. ABUH796) with the exception of HUMC1 (Figure S3).
Other AbGRI4 variants included a missing integrase ORF in ABUH639 (ST406/ST1804) and the absence of aadA2 from the AMR gene cassette array in ARLG1833 (ST406/ST1800), which could be possible intermediates of AbGRI4 (Figure 3b, Group 2). Finally, ST25/ST229 isolates, collected two decades apart in 1993 (NIPH146) and 2013 (HWBA8), both carried a full-length intact copy of the hydrolase gene and no AbGRI4 (Figure 3b, Group 1). It would be interesting to monitor possible future RI insertions at the hydrolase gene hotspot in ST25/ST229 isolates.
Discussion
In this study, a new RI, AbGRI4, was identified in 47% of ST2/ST281 isolates (147 out of 312), including clinical isolates collected from US hospitals over multiple years of A. baumannii surveillance, and 44% of ST406/ST1800 and ST1088/ST1804 isolates (22 out of 50). The high prevalence of AbGRI4 in each clonal lineage may reflect the fitness advantage of AbGRI4 when under antibiotic pressure in a clinical setting.
A plausible evolutionary scenario of the AbGRI4 unit in the ST406 group was a single IS26 insertion into the hydrolase gene (e.g. similar to ABBL020), followed by class 1 integron insertion via the IS26 element (e.g. similar to PR379) and mobilization as a composite transposon. However, a single copy of IS26 is capable of mobilizing resistance genes via intermolecular replicative transposition. An existing copy of IS26 can also be targeted by intermolecular conservative transposition, resulting in the creation of an array of ‘translocatable units’ containing multiple copies of IS26, like that observed in HUMC1 and AR_0078 in Figure 3(b).38,49,50 In the ST2/ST281 lineage, acquiring the AbGRI4 unit by horizontal transfer from a yet-to-be-identified source and further rearrangement events including the deletion of a 20 kb genomic fragment downstream and chromosomal recombination would have given rise to additional truncated versions of both the hydrolase and reductase genes that differed from ST406. Another possibility for acquisition of AbGRI4 is by homologous recombination, as has been described for AbGRI3.51
Comparing the timing of occurrences of the ST406 group and ST2/ST281 revealed interesting observations. The ST406 group isolates were collected during a relatively early period between 2005 and 2013, whereas the ST2/ST281 isolates were collected between 2012 and 2016 and carried a version of AbGRI4 missing a 20 kb neighbouring genomic region. The ABIsac_ColiR and ABIsac_ColiS isolate pairs were collected in 2009 and the HUMC1 isolate in 2013, both belonging to or closely related to ST/ST281. It is tempting to propose a scenario where a progenitor version of AbGRI4 had been horizontally transmitted across the clonal lineages with insertion/deletion of RI components during IS-mediated recombination.
Conclusions
We have provided genomic evidence showing multiple occurrences and possible evolution paths of a novel A. baumannii genomic region that confers resistance to aminoglycosides via a class 1 integron. The complete genomes obtained via WGS and characterization of the AbGRI4 sequence and its chromosomal insertion target altogether provide new AMR landmarks that will be useful for performing geographical surveillance and epidemiological analysis and investigating AMR evolution mechanisms of emerging drug-resistant A. baumannii isolates across global clonal lineages.
Supplementary Material
Acknowledgements
We thank Roshan D’Souza for performing genomic DNA extraction for the bacterial isolates used in this study.
Funding
The research reported in this publication has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Department of Health and Human Services, under award number U19AI110819 to the J. Craig Venter Institute. This work was also supported by the NIH-NIAID to R.A.B. under Award Numbers R01AI100560, R01AI063517 and R01AI072219. In addition, this work was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, Award Number 1I01BX001974 to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development, and the Geriatric Research Education and Clinical Center VISN 10.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs.
Supplementary data
Tables S1 and S2 and Figures S1 to S4 are available as Supplementary data at JAC Online.
References
- 1. Wong D, Nielsen TB, Bonomo RA et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30: 409–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harding CM, Hennon SW, Feldman MF. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol 2018; 16: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris FC, Dexter C, Kostoulias X et al. The mechanisms of disease caused by Acinetobacter baumannii. Front Microbiol 2019; 10: 1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pagano M, Martins AF, Barth AL. Mobile genetic elements related to carbapenem resistance in Acinetobacter baumannii. Braz J Microbiol 2016; 47: 785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamidian M, Hall RM. The AbaR antibiotic resistance islands found in Acinetobacter baumannii global clone 1—structure, origin and evolution. Drug Resist Updat 2018; 41: 26–39. [DOI] [PubMed] [Google Scholar]
- 6. Fournier P-E, Vallenet D, Barbe V et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2006; 2: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nigro SJ, Hall RM. Antibiotic resistance islands in A320 (RUH134), the reference strain for Acinetobacter baumannii global clone 2. J Antimicrob Chemother 2012; 67: 335–8. [DOI] [PubMed] [Google Scholar]
- 8. Nigro SJ, Farrugia DN, Paulsen IT et al. A novel family of genomic resistance islands, AbGRI2, contributing to aminoglycoside resistance in Acinetobacter baumannii isolates belonging to global clone 2. J Antimicrob Chemother 2013; 68: 554–7. [DOI] [PubMed] [Google Scholar]
- 9. Blackwell GA, Holt KE, Bentley SD et al. Variants of AbGRI3 carrying the armA gene in extensively antibiotic-resistant Acinetobacter baumannii from Singapore. J Antimicrob Chemother 2017; 72: 1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan AP, Sutton G, DePew J et al. A novel method of consensus pan-chromosome assembly and large-scale comparative analysis reveal the highly flexible pan-genome of Acinetobacter baumannii. Genome Biol 2015; 16: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wright MS, Haft DH, Harkins DM et al. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. MBio 2014; 5: e00963–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamidian M, Hall RM. Origin of the AbGRI1 antibiotic resistance island found in the comM gene of Acinetobacter baumannii GC2 isolates. J Antimicrob Chemother 2017; 72: 2944–7. [DOI] [PubMed] [Google Scholar]
- 13. Huang H, Yang Z-L, Wu X-M et al. Complete genome sequence of Acinetobacter baumannii MDR-TJ and insights into its mechanism of antibiotic resistance. J Antimicrob Chemother 2012; 67: 2825–32. [DOI] [PubMed] [Google Scholar]
- 14. Post V, White PA, Hall RM. Evolution of AbaR-type genomic resistance islands in multiply antibiotic-resistant Acinetobacter baumannii. J Antimicrob Chemother 2010; 65: 1162–70. [DOI] [PubMed] [Google Scholar]
- 15. Blackwell GA, Nigro SJ, Hall RM. Evolution of AbGRI2-0, the progenitor of the AbGRI2 resistance island in global clone 2 of Acinetobacter baumannii. Antimicrob Agents Chemother 2016; 60: 1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nigro SJ, Hall RM. Loss and gain of aminoglycoside resistance in global clone 2 Acinetobacter baumannii in Australia via modification of genomic resistance islands and acquisition of plasmids. J Antimicrob Chemother 2016; 71: 2432–40. [DOI] [PubMed] [Google Scholar]
- 17. Hamidian M, Hall RM. Genetic structure of four plasmids found in Acinetobacter baumannii isolate D36 belonging to lineage 2 of global clone 1. PLoS One 2018; 13: e0204357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Judge K, Harris SR, Reuter S et al. Early insights into the potential of the Oxford Nanopore MinION for the detection of antimicrobial resistance genes. J Antimicrob Chemother 2015; 70: 2775–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bickhart DM, Watson M, Koren S et al. Assignment of virus and antimicrobial resistance genes to microbial hosts in a complex microbial community by combined long-read assembly and proximity ligation. Genome Biol 2019; 20: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chavda KD, Chen L, Fouts DE et al. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. MBio 2016; 7: e02093–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams MD, Wright MS, Karichu JK et al. Rapid replacement of Acinetobacter baumannii strains accompanied by changes in lipooligosaccharide loci and resistance gene repertoire. MBio 2019; 10: e00356–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wick RR, Judd LM, Gorrie CL et al. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom 2017; 3: e000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker BJ, Abeel T, Shea T et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 2014; 9: e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tatusova T, DiCuccio M, Badretdin A et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 2016; 44: 6614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haft DH, DiCuccio M, Badretdin A et al. RefSeq: an update on prokaryotic genome annotation and curation. Nucleic Acids Res 2018; 46: D851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brinkac LM, Beck E, Inman J et al. LOCUST: a custom sequence locus typer for classifying microbial isolates. Bioinformatics 2017; 33: 1725–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wattam AR, Abraham D, Dalay O et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 2014; 42: D581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camacho C, Coulouris G, Avagyan V et al. BLAST+: architecture and applications. BMC Bioinformatics 2009; 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siguier P, Perochon J, Lestrade L et al. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 2006; 34: D32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sahl JW, Lemmer D, Travis J et al. NASP: an accurate, rapid method for the identification of SNPs in WGS datasets that supports flexible input and output formats. Microb Genom 2016; 2: e000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Croucher NJ, Page AJ, Connor TR et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 2015; 43: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30: 1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Letunic I, Bork P. Interactive Tree of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 2019; 47: W256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seputiene V, Povilonis J, Suziedeliene E. Novel variants of AbaR resistance islands with a common backbone in Acinetobacter baumannii isolates of European clone II. Antimicrob Agents Chemother 2012; 56: 1969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rolain J-M, Diene SM, Kempf M et al. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob Agents Chemother 2013; 57: 592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamidian M, Hall RM. AbaR4 replaces AbaR3 in a carbapenem-resistant Acinetobacter baumannii isolate belonging to global clone 1 from an Australian hospital. J Antimicrob Chemother 2011; 66: 2484–91. [DOI] [PubMed] [Google Scholar]
- 37. Harmer CJ, Hall RM. An analysis of the IS6/IS26 family of insertion sequences: is it a single family? Microb Genom 2019; 5: e0.000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Partridge SR, Kwong SM, Firth N et al. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 2018; 31: e00088–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamidian M, Nigro SJ, Hall RM. Variants of the gentamicin and tobramycin resistance plasmid pRAY are widely distributed in Acinetobacter. J Antimicrob Chemother 2012; 67: 2833–6. [DOI] [PubMed] [Google Scholar]
- 40. Hamidian M, Holt KE, Hall RM. Genomic resistance island AGI1 carrying a complex class 1 integron in a multiply antibiotic-resistant ST25 Acinetobacter baumannii isolate. J Antimicrob Chemother 2015; 70: 2519–23. [DOI] [PubMed] [Google Scholar]
- 41. Domingues S, Harms K, Fricke WF et al. Natural transformation facilitates transfer of transposons, integrons and gene cassettes between bacterial species. PLoS Pathog 2012; 8: e1002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karah N, Dwibedi CK, Sjöström K et al. Novel aminoglycoside resistance transposons and transposon-derived circular forms detected in carbapenem-resistant Acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother 2016; 60: 1801–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmed AM, Hussein AIA, Shimamoto T. Proteus mirabilis clinical isolate harbouring a new variant of Salmonella genomic island 1 containing the multiple antibiotic resistance region. J Antimicrob Chemother 2007; 59: 184–90. [DOI] [PubMed] [Google Scholar]
- 44. Siebor E, Neuwirth C. Emergence of Salmonella genomic island 1 (SGI1) among Proteus mirabilis clinical isolates in Dijon, France. J Antimicrob Chemother 2013; 68: 1750–6. [DOI] [PubMed] [Google Scholar]
- 45. Doublet B, Poirel L, Praud K et al. European clinical isolate of Proteus mirabilis harbouring the Salmonella genomic island 1 variant SGI1-O. J Antimicrob Chemother 2010; 65: 2260–2. [DOI] [PubMed] [Google Scholar]
- 46. Siebor E, Neuwirth C. Proteus genomic island 1 (PGI1), a new resistance genomic island from two Proteus mirabilis French clinical isolates. J Antimicrob Chemother 2014; 69: 3216–20. [DOI] [PubMed] [Google Scholar]
- 47. Siebor E, de Curraize C, Neuwirth C. Genomic context of resistance genes within a French clinical MDR Proteus mirabilis: identification of the novel genomic resistance island GIPmi1. J Antimicrob Chemother 2018; 73: 1808–11. [DOI] [PubMed] [Google Scholar]
- 48. Cuthbertson L, Nodwell JR. The TetR family of regulators. Microbiol Mol Biol Rev 2013; 77: 440–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harmer CJ, Hall RM. IS26-mediated precise excision of the IS26-aphA1a translocatable unit. MBio 2015; 6: e01866–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harmer CJ, Moran RA, Hall RM. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. MBio 2014; 5: e01801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hamidian M, Hawkey J, Wick R et al. Evolution of a clade of Acinetobacter baumannii global clone 1, lineage 1 via acquisition of carbapenem- and aminoglycoside-resistance genes and dispersion of ISAba1. Microb Genom 2019; 5: e0.000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics 2011; 27: 1009–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.