Abstract
Objective
Clinical implications of asymptomatic cases of the novel coronavirus disease 2019 (COVID-19) in nursing homes remain poorly understood. We assessed the association of symptom status and medical comorbidities on mortality and hospitalization risk associated with COVID-19 in residents across 15 nursing homes in Maryland.
Design
Retrospective cohort study.
Setting and Participants
1970 residents from 15 nursing home facilities with universal COVID-19 testing in Maryland.
Methods
We used descriptive statistics to compare baseline characteristics, logistic regression to assess the association of comorbidities with COVID-19, and Cox regression to assess the association of asymptomatic and symptomatic COVID-19 with mortality and hospitalization. We assessed the association of comorbidities with mortality and hospitalization risk. Symptom status was assessed at the time of the first test. Maximum follow-up was 94 days.
Results
Among the 1970 residents (mean age 73.8, 57% female, 68% black), 752 (38.2%) were positive on their first test. Residents who were positive for COVID-19 and had multiple symptoms at the time of testing had the highest risk of mortality [hazard ratio (HR) 4.44, 95% confidence interval (CI) 2.97, 6.65) and hospitalization (subhazard ratio 2.38, 95% CI 1.70, 3.33), even after accounting for comorbidity burden. Cases who were asymptomatic at testing had a higher risk of mortality (HR 2.92, 95% CI 1.95, 4.35) but not hospitalization (HR 1.06, 95% CI 0.82, 1.38) compared with those who were negative for COVID-19. Of 52 SARS-CoV-2–positive residents who were asymptomatic at the time of testing and were closely monitored for 14 days at one facility, only 6 (11.6%) developed symptoms.
Conclusions and Implications
Asymptomatic infection with SARS-CoV-2 in the nursing home setting was associated with increased risk of death, suggesting a need for closer monitoring of these residents, particularly those with underlying cardiovascular and respiratory comorbidities.
Keywords: COVID-19, SARS-CoV-2, long-term care facilities, epidemiology
Since the first outbreak of coronavirus disease 2019 (COVID-19) in a skilled nursing facility (SNF) in King County, Washington, in February 2020, these facilities have become epicenters for infection and transmission of the disease.1 Residents of these facilities are typically older adults and others with significant baseline comorbidities, and have experienced excess mortality associated with COVID-19.2, 3, 4, 5 By June 15, 2020, more than 50,000 residents living in SNFs had been reported dead from the 41 states reporting these data, accounting for 45% of the total reported COVID-19 deaths statewide.6
High mortality from COVID-19 infection in SNF residents and reports of rapid transmission of SARS-CoV-2 in these locations has led to guidance from the Centers for Disease Control and Prevention (CDC) and the Centers for Medicare & Medicaid Services recommending universal testing of residents and staff in facilities where 1 case has been identified.7 , 8 Although older adults may be particularly vulnerable to poor outcomes from this infection, early reports of point prevalence surveys in long-term care facilities show that a significant proportion of cases identified with universal testing have no documented symptoms at the time of testing.9 , 10
Implications and clinical relevance of asymptomatic COVID-19 is unclear, and controversy remains regarding the need for treatment or increased monitoring of these cases.11 There have been an increasing number of reports of asymptomatic nursing home residents testing positive during point prevalence testing. However, there is no current guidance on how to clinically manage these residents, and we still do not understand why some residents who may be otherwise deemed high risk do not develop any signs or symptoms or have milder cases of disease.
Based on case reports of COVID-19 submitted to the CDC surveillance network, adults with comorbidities such as diabetes, lung disease, or heart disease had a higher prevalence of COVID-19 and may develop more severe illness.9, 10, 11, 12 However, comorbidity data was missing for more than half of the reported cases. Another study looking at hospitalized patients with COVID-19 showed a relationship between increasing age and number of comorbidities with in-hospital mortality.13 In particular, a history of chronic kidney disease, lung disease, or cardiovascular disease was associated with higher mortality.13 Literature examining the effect of comorbidities on outcomes has predominantly focused on hospitalized cohorts, or those in middle age without testing of all individuals regardless of symptoms.5 , 14 , 15
On April 29, 2020, the Maryland Governor mandated that all residents of nursing homes in the state of Maryland must undergo testing for SARS-CoV-2. We assessed outcomes associated with SARS-CoV-2 infection among residents who were tested for SARS-CoV-2 RNA across one nursing home system with both long-term and post-acute rehabilitation services. Signs and symptoms of illness were obtained at the time of testing, and risk of infection, hospitalization, and death was analyzed based on symptoms and underlying comorbidities.
Methods
Study Population
All residents (N = 1970) from 15 SNFs who were universally tested for SARS-CoV-2 and had recorded test results between March 1, 2020, and June 12, 2020, were included in our study. Data were obtained from respiratory surveillance line list and manual chart review of the SNF electronic health record. A cohort of all residents at one facility who were asymptomatic at the time of testing were closely monitored by nursing home staff for development of symptoms over a 14-day period; this was documented in a dedicated line list and included as a subanalysis. This study protocol was approved by the institutional review board with a waiver of written consent.
Exposure
Nasopharyngeal samples were collected at the nursing homes for reverse transcription–polymerase chain reaction testing for SARS-CoV-2 RNA. When nasopharyngeal swabs were not available or if the resident would only consent to oropharyngeal swabs, an oropharyngeal sample was collected instead. All residents who consented to testing were tested. Residents who did not consent to testing were considered positive and isolated accordingly. Symptom status at the time of testing was determined based on review of respiratory surveillance line list documentation maintained by the nursing facility in the health system. The respiratory surveillance line list is used to monitor staff and resident symptoms during a respiratory disease outbreak or cluster. This monitoring tool represents each resident and staff member on a different row and tracks initial symptoms if present, date of confirmatory test, and other significant events, that is, hospitalization and death. Residents were added to respiratory surveillance line lists either for onset of signs or symptoms or at the time of testing for SARS-CoV-2 RNA.
Covariate Definitions
Age was defined as age on the date of testing. Sex, race, and smoking status were self-reported as recorded in the medical record. Comorbidities were abstracted based on ICD-10 codes in the medical record (Supplementary Table 1). Resuscitation preference was defined based on preferences expressed on medical orders for life-sustaining treatment forms. Symptomatic individuals were defined as having at least one of the following signs or symptoms: fever (temperature ≥100.4 °F), cough, sore throat, shortness of breath, headache, muscle aches, runny nose, nasal congestion, chest congestion, vomiting, diarrhea, chills, shaking, or loss of taste or smell. Asymptomatic individuals were those who did not have a documented sign or symptom on or prior to their date of testing.
Outcomes
The primary outcome was death in the full cohort. Some residents did not consent for hospital transfers from the nursing home. Therefore, time to first hospitalization was only assessed among those who consented to hospital transfer (n = 1845). Date of death or first hospitalization was abstracted from respiratory surveillance line list and confirmed with manual chart review. Follow-up began at the time of the resident's first SARS-CoV-2 test. The last date of follow-up was June 16, 2020. Residents were censored if they were discharged home or transferred to another system.
Statistical Analyses
We used 2-sample t tests to compare continuous variables and χ2 tests for categorical variables. Kaplan-Meier curves were used to compare hospitalization and mortality risk. We used Cox regression to model time to death and Fine and Gray to model time to hospitalization with death as a competing outcome. COVID-19 status was defined based on the results of the first test, and symptom status was defined based on recorded symptoms at the time of the first test. Residents who tested negative on first test remained in the negative cohort even if they subsequently tested positive. Model 1 included age, sex, race, and facility. Model 2 included covariates in model 1 and medical comorbidities [asthma, chronic obstructive pulmonary disease or emphysema, cancer, hypertension, atrial fibrillation, coronary artery disease, heart failure, peripheral vascular disease, history of deep vein thrombosis or pulmonary embolus, anemia, diabetes, hypothyroidism, human immunodeficiency virus (HIV), chronic hepatitis C, cerebrovascular disease, dementia, stage 1-5 chronic kidney disease, end-stage kidney disease, depression], or ventilator use, tracheostomy, and code status.
Results
There were 1970 residents in 15 facilities [median (interquartile range) age 73.9 (21.9, 105.4); 57% female; 68% black] who were tested at least once for SARS-CoV-2 RNA; of these, 752 (38.2%) initially tested positive between March 1, 2020, and June 12, 2020. Of the 1218 residents who initially tested negative, 558 (45.8%) had at least 1 additional test, and 169 (13.9%) eventually tested positive at some point before the end of follow-up. Residents who were positive for SARS-CoV-2 on their first test were significantly more likely to be hospitalized and die during follow-up than residents who tested negative (Table 1 ; P < .001).
Table 1.
Baseline Characteristics of Residents by COVID-19 and Symptom Status at First Test (N = 1970)
| SARS-CoV-2 RNA Negative (n = 1218) | Asymptomatic SARS-CoV-2 RNA Positive (n = 424) | Symptomatic SARS-CoV-2 RNA Positive (n = 328) | P Value∗ | P Value† | |
|---|---|---|---|---|---|
| Age, mean (SD) | 72.9 (14.2) | 74.7 (31.0, 104.6) | 76.5 (38.7, 102.4) | <.001 | <.001 |
| Male | 545 (44.7) | 170 (40.1) | 130 (39.6) | .035 | .11 |
| Race | .006 | <.001 | |||
| White | 377 (31.0) | 96 (22.6) | 87 (26.5) | ||
| Black | 799 (65.6) | 320 (75.5) | 224 (68.3) | ||
| Other or unknown | 42 (3.4) | 8 (1.9) | 17 (5.2) | ||
| Current smoker | 31 (2.5) | 22 (5.2) | 14 (4.3) | .008 | .022 |
| COVID-19 test location | .16 | .008 | |||
| Facility | 1098 (90.1) | 400 (94.3) | 292 (89.0) | ||
| Hospital | 120 (9.9) | 24 (5.7) | 36 (11.0) | ||
| Symptoms | |||||
| Fever | 113 (9.3) | – | 161 (49.1) | <.001 | |
| Cough | 152 (12.5) | – | 195 (59.5) | <.001 | |
| Sore throat | 10 (0.8) | – | 7 (2.1) | .007 | |
| Shortness of breath | 79 (6.5) | – | 69 (21.0) | <.001 | |
| Headache | 1 (0.1) | – | 0 (0.0) | .73 | |
| Muscle aches | 16 (1.3) | – | 28 (8.5) | <.001 | |
| Runny nose | 9 (0.7) | – | 8 (2.4) | .001 | |
| Nasal congestion | 9 (0.7) | – | 12 (3.7) | <.001 | |
| Chest congestion | 33 (2.7) | – | 24 (7.3) | <.001 | |
| Vomiting | 21 (1.7) | – | 14 (4.3) | <.001 | |
| Diarrhea | 41 (3.4) | – | 23 (7.0) | <.001 | |
| Chills | 1 (0.1) | – | 2 (0.6) | .062 | |
| Shaking | 0 (0.0) | – | 1 (0.3) | .082 | |
| Loss of taste and smell | 1 (0.1) | – | 0 (0.0) | .73 | |
| Number of symptoms | <.001 | <.001 | |||
| 0 | 905 (74.3) | 424 (100.0) | 0 (0.0) | ||
| 1 | 202 (16.6) | 0 (0.0) | 185 (56.4) | ||
| ≥2 | 110 (9.0) | 0 (0.0) | 143 (43.6) | ||
| Comorbidities | |||||
| Respiratory | |||||
| Asthma | 56 (4.6) | 26 (6.1) | 28 (8.5) | .015 | .019 |
| COPD or emphysema | 246 (20.2) | 74 (17.5) | 72 (22.0) | .67 | .28 |
| Cancer | |||||
| Cancer | 80 (6.6) | 25 (5.9) | 36 (11.0) | .20 | .012 |
| Cardiovascular | |||||
| Hypertension | 1027 (84.3) | 371 (87.5) | 293 (89.3) | .014 | .038 |
| Atrial fibrillation | 272 (22.3) | 82 (19.3) | 64 (19.5) | .12 | .31 |
| Coronary heart disease | 331 (27.2) | 132 (31.1) | 108 (32.9) | .024 | .068 |
| Heart failure | 324 (26.6) | 107 (25.2) | 91 (27.7) | .89 | .74 |
| Peripheral vascular disease | 206 (16.9) | 103 (24.3) | 65 (19.8) | .003 | .003 |
| DVT or PE | 143 (11.7) | 60 (14.2) | 47 (14.3) | .11 | .27 |
| Anemia | 547 (44.9) | 202 (47.6) | 187 (57.0) | .003 | <.001 |
| Endocrine | |||||
| Diabetes | 550 (45.2) | 209 (49.3) | 171 (52.1) | .020 | .050 |
| Hypothyroidism | 189 (15.5) | 64 (15.1) | 59 (18.0) | .62 | .49 |
| Infectious disease | |||||
| HIV | 28 (2.3) | 7 (1.7) | 3 (0.9) | .13 | .24 |
| Chronic hepatitis C | 32 (2.6) | 13 (3.1) | 8 (2.4) | .83 | .85 |
| Neurologic | |||||
| Cerebrovascular disease | 460 (37.8) | 162 (38.2) | 115 (35.1) | .68 | .62 |
| Dementia | 474 (38.9) | 218 (51.4) | 160 (48.8) | <.001 | <.001 |
| Kidney disease | |||||
| Chronic kidney disease, stage 1-5 | 261 (21.4) | 110 (25.9) | 90 (27.4) | .008 | .028 |
| End-stage kidney disease | 160 (13.1) | 38 (9.0) | 52 (15.9) | .45 | .014 |
| Psychiatric | |||||
| Depression | 598 (49.1) | 228 (53.8) | 193 (58.8) | .003 | .005 |
| Code status | |||||
| Full code | 725 (59.5) | 265 (62.5) | 181 (55.2) | .001 | .001 |
| DNR | 44 (3.6) | 3 (0.7) | 5 (1.5) | ||
| DNI | 302 (24.8) | 90 (21.2) | 92 (28.0) | ||
| Palliative | 147 (12.1) | 66 (15.6) | 50 (15.2) | ||
| Consent for hospital transfer | 1153 (94.7) | 399 (94.1) | 293 (89.3) | .019 | .002 |
| Any hospitalization | 260 (21.3) | 100 (23.6) | 125 (38.1) | <.001 | <.001 |
| Death | 87 (7.1) | 57 (13.4) | 98 (29.9) | <.001 | <.001 |
| Subsequent positive test | 169 (13.9) | – | – | – | – |
COPD, chronic obstructive pulmonary disease; DNI, do not intubate; DNR, do not resuscitate; DVT or PE, deep vein thrombosis or pulmonary embolus; HIV, human immunodeficiency virus; SD, standard deviation.
Symptoms were assessed at the time of testing. All n (%) unless otherwise noted.
P value comparing those who were SARS-CoV-2 RNA negative to those who were SARS-CoV-2 RNA positive, regardless of symptom status.
P value comparing those who were asymptomatic SARS-CoV-2 RNA positive to those who were symptomatic SARS-CoV-2 RNA positive.
Demographics and Comorbidities
Residents were more likely to test positive for COVID-19 if they were older, self-identified as black, were current smokers, or had certain comorbidities (asthma, cancer, hypertension, peripheral vascular disease, anemia, diabetes, dementia, renal disease, and depression) (Table 1). After accounting for age, sex, and facility (model 1), black residents were not at a higher risk of testing positive for SARS-CoV-2 [OR 1.07, 95% confidence interval (CI) 0.80, 1.42]. Peripheral vascular disease, diabetes, chronic kidney disease, and depression remained significantly associated with increased risk for infection after accounting for age, sex, and facility (Table 2 ).
Table 2.
Relative Risk (95% CI) of the Association of Comorbidities With COVID-19 and Outcomes, Adjusted for Age, Sex, Race, and Facility
| Comorbidity |
SARS-CoV-2 Infection (N = 1970) |
Hospitalization Among COVID-19 Cases (n = 692) |
Mortality Among COVID-19 Cases (n = 752) |
|---|---|---|---|
| Measure of Association | OR (95% CI) | SHR (95% CI) | HR (95% CI) |
| Respiratory | |||
| Asthma | 1.28 (0.81, 2.02) | 1.27 (0.74, 2.18) | 0.64 (0.30, 1.40) |
| COPD or emphysema | 0.82 (0.63, 1.07) | 1.31 (0.96, 1.80) | 1.55 (1.08, 2.24)∗ |
| Cancer | 0.80 (0.53, 1.20) | 1.22 (0.75, 1.99) | 1.22 (0.74, 2.01) |
| Cardiovascular | |||
| Hypertension | 1.33 (0.97, 1.83) | 1.04 (0.68, 1.59) | 0.78 (0.47, 1.27) |
| Atrial fibrillation | 0.92 (0.71, 1.20) | 1.16 (0.83, 1.62) | 0.86 (0.57, 1.30) |
| Coronary heart disease | 1.18 (0.94, 1.49) | 1.47 (1.10, 1.95)∗ | 0.83 (0.58, 1.19) |
| Heart failure | 1.12 (0.88, 1.42) | 1.39 (1.03, 1.87)∗ | 1.13 (0.79, 1.62) |
| Peripheral vascular disease | 1.30 (1.00, 1.70)∗ | 1.53 (1.12, 2.08)∗ | 1.15 (0.78, 1.69) |
| DVT or PE | 1.09 (0.81, 1.49) | 1.39 (0.99, 1.94) | 0.70 (0.42, 1.17) |
| Anemia | 1.11 (0.90, 1.37) | 1.43 (1.08, 1.90)∗ | 1.12 (0.80, 1.57) |
| Endocrine | |||
| Diabetes | 1.29 (1.04, 1.59)∗ | 1.40 (1.06, 1.85))∗ | 0.87 (0.63, 1.21) |
| Hypothyroidism | 1.06 (0.80, 1.42) | 0.84 (0.56, 1.27) | 1.06 (0.70, 1.61) |
| Infectious disease | |||
| HIV | 0.62 (0.29, 1.37) | 0.23 (0.03, 1.78) | 0.59 (0.08, 4.36) |
| Chronic hepatitis C | 0.84 (0.44, 1.62) | 1.75 (0.95, 3.22) | 0.94 (0.29, 3.02) |
| Neurologic | |||
| Cerebrovascular disease | 1.01 (0.81, 1.26) | 0.96 (0.72, 1.28) | 0.73 (0.51, 1.05) |
| Dementia | 1.23 (0.98, 1.54) | 0.82 (0.60, 1.12) | 0.99 (0.69, 1.42) |
| Kidney disease | |||
| Chronic kidney disease, stage 1-5 | 1.33 (1.03, 1.70)∗ | 1.26 (0.92, 1.73) | 1.02 (0.71, 1.46) |
| End-stage kidney disease | 1.32 (0.96, 1.81) | 1.57 (1.06, 2.32)∗ | 1.56 (0.95, 2.55) |
| Psychiatric | |||
| Depression | 1.33 (1.07, 1.65)∗ | 1.33 (1.01, 1.75)∗ | 1.08 (0.77, 1.50) |
COPD, chronic obstructive pulmonary; DVT or PE, disease deep vein thrombosis or pulmonary embolus; SHR, subhazard ratio.
P < .05.
After accounting for age, sex, race, and facility (model 1) among those who tested positive for SARS-CoV-2, coronary artery disease, heart failure, peripheral vascular disease, anemia, diabetes, end-stage kidney disease, and depression were at increased risk of hospitalization (Table 2). Only a history of chronic obstructive pulmonary disease or emphysema was significantly associated with higher mortality from COVID-19 after accounting for age, sex, and facility (Table 2).
Signs and Symptoms at Time of Testing
Of the 752 residents who tested positive, 56.4% (n = 424) had no documented signs or symptoms at the time of testing. Of the cases with documented signs or symptoms (n = 328), the most common were fever 49.1% (n = 161) and cough 59.5% (n = 195, Table 1), and 56.4% of residents (n = 185) had only 1 documented sign or symptom. Among residents with COVID-19, those with anemia, cancer, or end-stage renal disease were more likely to have signs and symptoms of illness at the time of testing and those with dementia and peripheral vascular disease were more likely to be asymptomatic (Table 1).
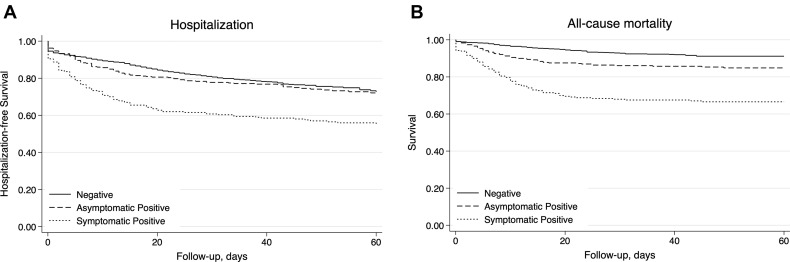
Over a maximum of 94 days of follow-up, there were 475 incident hospitalizations observed among the 1845 residents who consented to hospital transfer from the 15 facilities. The 30-day cumulative hospitalization rate was 45% among cases with multiple symptoms at testing, 35% among cases with 1 symptom at testing, 22% among cases asymptomatic at testing, and 21% among those who were negative. After accounting for all confounders, cases who were symptomatic at testing remained at significantly higher risk of hospitalization than those who were asymptomatic or negative ( Figure 1 A).
Fig. 1.
Kaplan-Meier Plot by COVID-19 test result and symptom status at testing of for (A) 60-day probability of staying out of the hospital among those who consented to for hospital transfer (n = 1845) and (B) survival (N = 1970).
There were 242 total deaths among the 1970 residents over the 94-day follow-up period; 155 (64%) were in those who tested positive for SARS-CoV-2. Mortality rates were highest among residents who tested positive for SARS-CoV-2 and had COVID-19 signs or symptoms (Figure 1B). The 30-day cumulative mortality was 39% among cases with 2 or more signs or symptoms at testing, 27% among cases with 1 sign or symptom at testing, 14% among cases who were asymptomatic at testing, and 7% among those who were negative for SARS-CoV-2. After accounting for demographics, comorbidities, and resuscitation preference (model 2), cases who were symptomatic at testing remained at highest risk of mortality, and cases asymptomatic at testing were at intermediate risk [hazard ratio (HR) 2.92, 95% CI 1.95, 4.35] compared to those who were negative (Table 3 ). Those with multiple signs or symptoms also had a higher risk of mortality compared to those with a single sign or symptom (model 1 HR 1.52, 95% CI 1.01, 2.99; model 2 HR 1.52, 95% CI 0.99, 2.35).
Table 3.
Adjusted HRs (95% CIs) for Mortality and SHRs (95% CIs) for Incident Hospitalization
| Mortality (N = 1970) |
Hospitalization (n = 1845) |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| SARS-CoV-2 negative | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| SARS-CoV-2 positive: 0 symptoms | 1.48 (1.03, 2.14) | 1.59 (1.10, 2.31) | 1.02 (0.79, 1.32) | 1.06 (0.82, 1.38) |
| SARS-CoV-2 positive: 1 symptom | 2.86 (1.93, 4.25) | 2.92 (1.95, 4.35) | 1.75 (1.27, 2.42) | 1.69 (1.21, 2.35) |
| SARS-CoV-2 positive: 2 symptoms | 4.35 (2.95, 6.42) | 4.44 (2.97, 6.65) | 2.39 (1.73, 3.31) | 2.38 (1.70, 3.33) |
SHR, subhazard ratio.
Death was considered a competing risk for hospitalization models.
Model 1: age, sex, race, facility; model 2: model 1 + asthma, chronic obstructive pulmonary disease or emphysema, cancer, hypertension, atrial fibrillation, coronary artery disease, heart failure, peripheral vascular disease, history of deep vein thrombosis or pulmonary embolus, anemia, diabetes, hypothyroidism, HIV, chronic hepatitis C, cerebrovascular disease, dementia, stage 1-5 chronic kidney disease, end-stage kidney disease, depression, ventilator use, tracheostomy, code status.
One facility had 52 cases who were asymptomatic at the time of testing and were closely monitored for 14 days for development of signs or symptoms. Of these, only 6 (11.5%) developed any documented symptoms over the 14-day follow-up from point prevalence testing. Of the 6 residents that became symptomatic, one developed a nonproductive cough at day 9 post diagnosis and remained stable in the facility. Three residents were hospitalized: 1 developed malaise and shortness of breath at day 12 and was transferred to the hospital, then returned 5 days later; 1 developed an elevated temperature (99 °F) on day 10—he was transferred to the hospital on day 12 when his oxygen saturation reached 91% and expired the next day. One resident developed chills, shortness of breath, and diminished lung sounds on day 12 and expired during transfer to the hospital. The remaining 2 residents passed away abruptly in the facility—both were noted to rapidly develop restlessness and shortness of breath and expired shortly thereafter (1 on day 6 post diagnosis and 1 on day 11).
Discussion
Our findings highlight that all nursing home residents infected with SARS-CoV-2 are at a significantly higher risk of both hospitalization and death; even if asymptomatic. Asymptomatic infection has profound implications in this population as there was a higher risk of death, but not hospitalization, among cases who were asymptomatic at the time of point prevalence testing compared to those who tested negative for COVID-19. Comorbidities associated with increased risk of infection after accounting for demographic factors included peripheral vascular disease, diabetes, chronic kidney disease, and depression; renal disease, diabetes, and depression were also associated with increased risk for hospitalization. The only comorbidity significantly associated with increased risk of death after adjusting for confounders in our cohort was underlying lung disease. After adjusting for facility, we did not observe differences in infection rates or outcomes by race, which is consistent with observations from a hospitalized cohort in Louisiana.16 These findings have major policy implications. Multiple studies, and national and state reporting data have all demonstrated that mortality from COVID-19 is higher in under-represented minority groups. Our findings suggest that this mortality differential among blacks is predominantly due to increased prevalence, and possibly severity of underlying diseases, rather than a COVID-19–specific cause.
The clinical implications of COVID-19 detection among asymptomatic people remains poorly understood.17 The published prevalence of asymptomatic cases varies greatly from population to population, ranging from 1.6% in China18 to 88% in a Boston homeless shelter.19 In our study population of residents of long-term care facilities undergoing point prevalence testing, more than half of the cases detected were asymptomatic at testing, which is consistent with other early reports in this setting.9 , 10 , 20
Despite a lack of documented symptoms at the time of testing, our data show that residents who are asymptomatic at testing have up to 2 times the mortality risk of residents who test negative for SARS-CoV-2. However, there was no difference in risk of hospitalization between residents who tested negative and asymptomatic residents who tested positive. This may suggest that staff are unable to accurately elicit symptoms, or that infected individuals are decompensating so rapidly that nursing home staff are not able to identify a clinical decline and transfer them to a higher level of care prior to death. Indeed, residents with dementia or cerebrovascular disease or history of stroke were more likely to be deemed asymptomatic than others, suggesting that assessing symptom status in this population is particularly challenging and may require unique attention. Nevertheless, because asymptomatic infections have important clinical ramifications, including death, the objective of testing expands beyond simple case finding and needs to occur frequently.
Paradoxically, residents with kidney disease and diabetes were more likely to be symptomatic. These individuals may have more opportunities for close monitoring and screening, such as prior to undergoing hemodialysis or receiving insulin or glucose checks, which increase the likelihood of observing or reporting symptoms. Observations from different cohorts have reported differential distributions of symptoms among older adults with COVID-1912 , 15 including self-reported symptoms such as falls, mental status changes, and loss of appetite, leading to recommendations from the American Medical Director's Association for alternative screening processes for COVID-19 in this population.21
A consistent anecdotal finding in some reports investigating the pathogenesis of COVID-19 is the development of hypoxia without dyspnea, or “silent hypoxia.”22 , 23 Several reports discuss a mismatch between the appearance of the patient and what is seen on the monitor, such as a hospitalized patient chatting comfortably on his phone with an oxygen saturation that is considered “incompatible with life.”24 Although the underlying pathogenesis remains poorly understood, this phenomenon could explain why patients in the nursing home may appear comfortable without evidence of dyspnea early in the disease and decompensate rapidly when oxygen saturation reaches critical levels. During this investigation, the approach to management of patients with COVID-19 was still evolving. Some facilities had the capability of monitoring pulse oximetry on a regular basis; however, this practice was not formerly protocolized across facilities and depended on the timing of the outbreak in respect to our understanding of the disease. These data suggest that consistent monitoring of pulse oximetry in nursing home residents who test positive for SARS-COV-2 may be an important protocol to implement, regardless of symptom status.
This study is subject to limitations. Comprehensive day-to-day symptom tracking was not documented for most residents in the system and thus some of those who were defined as asymptomatic may have been presymptomatic. This could result in a lead-time bias whereby asymptomatic testing detected cases earlier in their disease trajectory and thus was associated with improved clinical outcomes. Estimates based on close tracking at a single facility suggests that the conversion rate from asymptomatic to symptomatic was relatively low during the brief follow-up. Another limitation is the known false negative rate associated with nasopharyngeal and oropharyngeal swab samples for reverse transcription–polymerase chain reaction testing.25 This, however, would lead to a conservative bias whereby the effects detected would be smaller in magnitude as some cases may be misclassified as being negative for COVID-19. Finally, SARS-CoV-2 infection status and COVID-19 symptom status were treated as time-fixed in our analyses, resulting in a conservative bias as those who became positive over follow-up remained in the negative group.
There are also several strengths of our study. Universal testing was completed at all facilities regardless of symptoms or clinical suspicion for COVID-19 mitigating concerns of selection bias in testing. Furthermore, there was comprehensive documentation of comorbidities for all residents. In contrast, national databases are often limited because of significant missing data on comorbidity status and differences in comorbidity assessment and documentation across data sources. To our knowledge, this is the largest cohort of nursing homes residents with individual-level data that have been reported during the COVID-19 pandemic. Moreover, there was a majority of black SNF residents, whereas most other long-term care cohorts have historically consisted of mostly white residents.
Conclusions and Implications
Infections with SARS-CoV-2 detected on asymptomatic screening in the nursing home setting are not benign, underscoring the importance of universal testing, especially in high-risk subgroups. Reliance on signs and symptoms for SARS-CoV-2 risk assessment alone may not be sufficient, as residents living with dementia may be at a higher risk of infection but less likely to report or exhibit signs and symptoms, and the natural history of this disease remains to be fully established, particularly in the setting of hypoxia without dyspnea. In addition to the obvious benefits of case identification to assist with infection control practices, our data suggest that asymptomatic residents are at higher risk of death than residents who tested negative and may benefit from close monitoring, such as regular pulse oximetry, as well as any future treatments.
Footnotes
O.T. and B.F.B. contributed equally to this work.
M.J.K. receives grant funding from the Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality. O.T. is supported by a grant from the National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases (5F30DK120160).
The authors declare no conflicts of interest.
Supplementary Data
Supplementary Table 1.
ICD-10 Codes for Identification of Comorbidities in Long-Term Care Facility Residents
| Medical Comorbidity | ICD-10 Codes |
|---|---|
| Asthma | J45 |
| COPD or emphysema | J43, J44 |
| Cancer | C00-C95 |
| Hypertension | I10-I13 |
| Atrial fibrillation | I48.0-I48.2, I48.91 |
| Coronary artery disease | I21, I22, I25, I24.8, I25.1, I25.2, I25.7, I25.8 |
| Heart failure | I50, I11.0 |
| Peripheral vascular disease | I73.9 |
| Deep vein thrombosis or pulmonary embolism | I26, I27.82, I82 |
| Anemia | D50, D51, D52, D53, D54, D55, D59, D60, D61, D62, D63, D64 |
| Hypothyroidism | E03 |
| Chronic kidney disease, stage 1-5 | D63.1, E10.22, E13.22, I22, I13, N18.1, N18.2, N18.3, N18.4, N18.5, N18.9, I12.9 |
| End-stage kidney disease | Z99.2, Z94.0, T86.12, N18.6, I12.0 |
| Cerebrovascular disease (not including dementia) | I63, I67, G46, I69.3, I69.8, I69.9 |
| Dementia | F01, F02, F03, G30, G31.0, G31.83 |
| Diabetes | E08, E10, E11, E13 |
| HIV | B20 |
| Chronic hepatitis C | B18.2 |
| Depression | F32, F33 |
COPD, chronic obstructive pulmonary disease.
References
- 1.McMichael T.M., Clark S., Pogosjans S. COVID-19 in a long-term care facility—King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:339–342. doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonanad C., García-Blas S., Tarazona-Santabalbina F. The effect of age on mortality in patients with COVID-19: A meta-analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21:915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez G.V., Biedron C., Fink L.R. Initial and repeated point prevalence surveys to inform SARS-CoV-2 infection prevention in 26 skilled nursing facilities—Detroit, Michigan, March–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:882–886. doi: 10.15585/mmwr.mm6927e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann-Podczaska A., Al-Saad S.R., Karbowski L.M. COVID 19—clinical picture in the elderly population: A qualitative systematic review. Aging Dis. 2020;11:988–1008. doi: 10.14336/AD.2020.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H., Ning R., Tao Y. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: A retrospective study. J Am Geriatr Soc. 2020;68:E19–E23. doi: 10.1111/jgs.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett M.L., Hu L., Martin T., Grabowski D.C. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324:2019–2021. doi: 10.1001/jama.2020.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Testing guidelines for nursing homes. https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-testing.html Available at: Published 2020.
- 8.Centers for Medicare & Medicaid Services Nursing home reopening recommendations frequently asked questions. www.coronavirus.gov Available at:
- 9.Bigelow B.F., Tang O., Barshick B. Outcomes of universal COVID-19 testing following detection of incident cases in 11 long-term care facilities. JAMA Intern Med. 2020 Jul 14 doi: 10.1001/jamainternmed.2020.3738. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh S., O’Laughlin K., Ehrlich H.Y. Point prevalence testing of residents for SARS-CoV-2 in a subset of Connecticut nursing homes. JAMA. 2020;324:1101–1103. doi: 10.1001/jama.2020.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Z., Xu Y., Sun C. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2020 May 15 doi: 10.1016/j.jmii.2020.05.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow N., Fleming-Dutra K., Gierke R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with Coronavirus Disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imam Z., Odish F., Gill I. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020;288:469–476. doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., He W., Yu X. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 19.Baggett T.P., Keyes H., Sporn N., Gaeta J.M. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323:2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel M.C., Chaisson L.H., Borgetti S. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis. 2020 Jun 16 doi: 10.1093/cid/ciaa763. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Society for Postacute and Long-term Care Medicine Common and less common signs and symptoms of COVID-19 in older adults. https://paltc.org/sites/default/files/Active Screening apr 28_revised.pdf Available at: Published 2020.
- 22.Couzin-Frankel J. The mystery of the pandemic’s “happy hypoxia”. Science. 2020;368:455–456. doi: 10.1126/science.368.6490.455. [DOI] [PubMed] [Google Scholar]
- 23.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitan R. Opinion: The infection that’s silently killing coronavirus patients. The New York Times. 2020 April 22 [Google Scholar]
- 25.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]