Highlights
-
•
Pneumothorax may be a frequent and fatal complication in critically ill patients with COVID-19.
-
•
Pneumothorax was likely to occur 2 weeks after the beginning of dyspnea in senile male patients.
-
•
Mechanical ventilation, recruitment maneuver, forced inhalation, severely coughing, and changes of lung structure and function, contribute to the occurrence of pneumothorax. Lung recruitment maneuver should be cautiously considered.
Keywords: COVID-19, Pneumothorax, Epidemiology, Mortality, Mechanism, Prevention, Coronavirus disease 2019(COVID-19)
Abstract
Background
The clinical characteristics of the patients with COVID-19 complicated by pneumothorax have not been clarified.
Objectives
To determine the epidemiology and risks of pneumothorax in the critically ill patients with COVID-19.
Methods
Retrospectively collecting and analysing medical records, laboratory findings, chest X-ray and CT images of 5 patients complicated by pneumothorax.
Results
The incidence of pneumothorax was 10% (5/49) in patients with ARDS, 24% (5/21) in patients receiving mechanical ventilation, and 56% (5/9) in patients requiring invasive mechanical ventilation, with 80% (4/5) patients died. All the 5 patients were male and aged ranging from 54 to 79 years old. Pneumothorax was most likely to occur 2 weeks after the beginning of dyspnea and associated with reduction of neuromuscular blockers, recruitment maneuver, severe cough, changes of lung structure and function.
Conclusions
Pneumothorax is a frequent and fatal complication of critically ill patients with COVID-19.
Introduction
From the outbreak of coronavirus disease 2019 (COVID-19) in December 2019 to Mar 27, 2020, and the number of confirmed patients and deaths outside of China is increasing rapidly all around the world.1 The autopsy of a patient who died from severe infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) showed evident desquamation of pneumocytes, hyaline membrane formation and pulmonary edema, suggesting acute respiratory distress syndrome (ARDS).2 Xun et al. reported that all of 25 patients with COVID-19 died of SARS-CoV-2 infection complicated by respiratory failure, suggesting that lung is the main target organ, followed by multiple organ dysfunction, including heart, kidney and liver.3 Therefore, refractory hypoxemia and ARDS are the leading causes of death for critically ill patients with COVID-19.
Pneumothorax is a common complication of ARDS. The incidence of pneumothorax varies from 1.7% to 77%, significantly increasing the mortality of patients.4, 5, 6, 7 However, there are no detailed reports on pneumothorax in critically ill patients with COVID-19, but a report by Yang et al. mentioned that 1 of 37 patients treated with mechanical ventilation developed pneumothorax, indicating a low incidence of pneumothorax in critically ill patients with COVID-19.8 Nonetheless, this is inconsistent with what we have found in clinical observations. Meanwhile, the clinical characteristics of the patients with COVID-19 complicated by pneumothorax have not been clarified. Therefore, we have summarized the clinical characteristics of 5 critically ill COVID-19 patients with pneumothorax in our center, which may shed light on early prevention and identification of pneumothorax in critically ill patients with COVID-19 and reduce the mortality.
Methods
Study design and patients
The diagnosis of COVID-19 was performed according to New Coronavirus Pneumonia Diagnosis and Treatment Program (Trial Fifth Edition). Two negative nucleic acid tests of respiratory tract specimens (with an interval of at least 1 day) were considered the virus was eliminated.9 Diagnosis of ARDS was based on Berlin standards .10 According to the mechanism of pneumothorax of patients with ARDS, the risks of pneumothorax are classified into four aspects, including increased alveolar pressure, increased negative pressure of the pleural cavity, shear stress, and changes of lung structure and function. Probability of the risk was defined as irrelevant, possible and probable (Table 1 ). This study was approved by the Medical Ethical Committee of the First Affiliated Hospital of Chongqing Medical University (approval number 20200601). Due to the special reasons of the epidemic, the patients’ informed consent was not obtained.
Table 1.
Evaluation of the risks related to pneumothorax.
| Irrelevant | Possible | Probable | |
|---|---|---|---|
| Increased alveolar pressure | Without mechanical ventilation | Protective mechanical ventilation | Recruitment maneuver or Pplat > 35 cmH2O |
| Increased negative pressure of the pleural cavity | RR < 20/min | 20/min < RR < 30/min | RR > 30 min, severe cough, fored inhaled or paradoxical thoracoabdominal motion |
| Alveolar shear stressa | Normal | Uniform exudation | Heterogenic distributionb |
| Changes of lung structure and functionb | Normal | Only ground-glass opacification | Consolidation, fibrosis, pulmonary cysts, or emphysema |
According to the chest CT images before development of pneumothorax
Patchy infiltrates interspersed with areas of normal-appearing lung, or distributed in gravity dependent lung regions
Data collection
From January 23, 2020 to March 15, 2020, we retrospectively screened 248 COVID-19 patients, and collected detailed medical records, laboratory findings, chest CT scan and X-ray images of critically ill COVID-19 patients complicated by pneumothorax at Chongqing Three Gorges Central Hospital.
Statistical analysis
Continuous variables were presented with range. Categorical variables were presented with percentage.
Results
From January 23, 2020 to March 15, 2020, a total of 248 patients with COVID-19 were admitted to the hospital, and 49 of them met the diagnostic criteria of ARDS. Twenty-one of these patients were treated with mechanical ventilation, and 9 of them received invasive mechanical ventilation (IMV), 5 of whom developed pneumothorax. The overall incidence of pneumothorax was 2.01% (5/248), 10% (5/49) in patients with ARDS, 24% (5/21) in patients receiving mechanical ventilation, and 56% (5/9) in patients requiring IMV. Four of the 5 patients died during hospitalization, with a mortality as high as 80% (4/5), and the remaining one was still in hospital with a prolonged length of stay of 39 days (supplmentary).
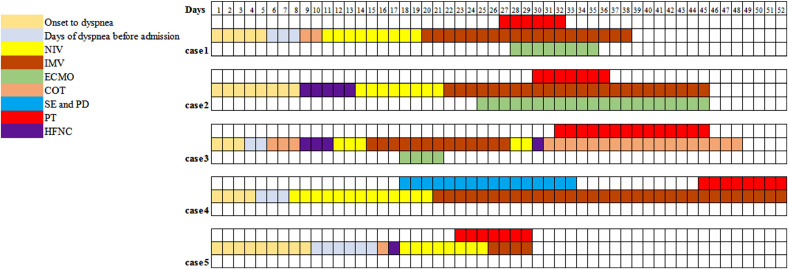
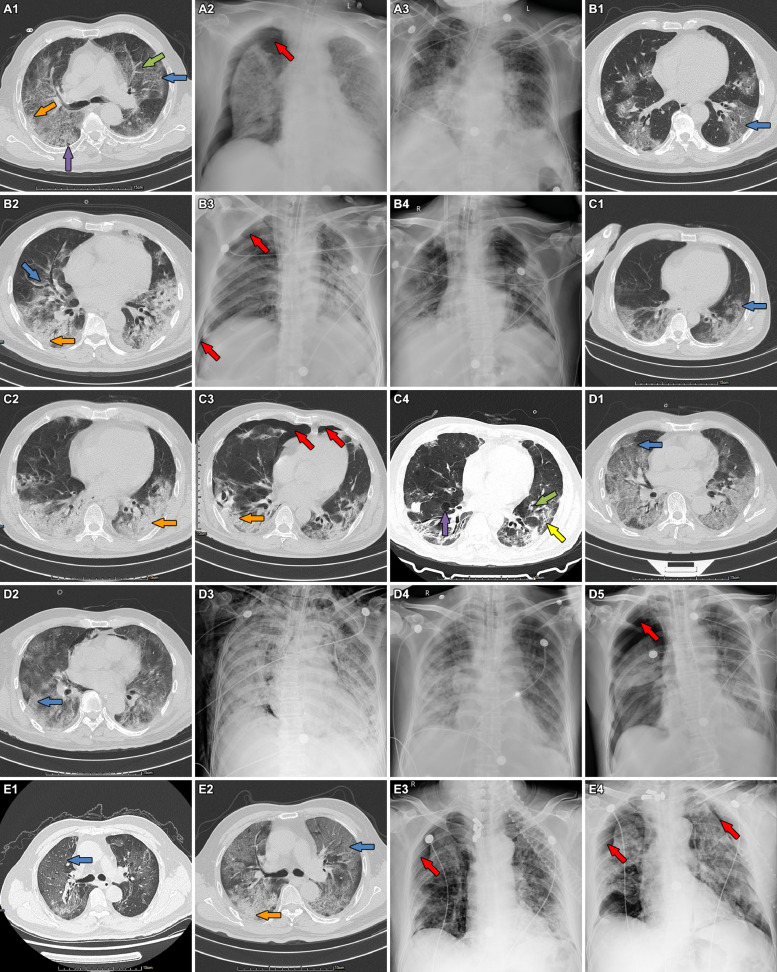
The 5 patients were all male, their age ranging from 54 to 79 years old. The time from onset to dyspnea ranged from 3 to 9 days, and the interval from dyspnea to the occurrence of pneumothorax varied from 15 to 40 days. PO2/FiO2 on admission varied between 127-200 mmHg according to arterial blood gas (ABG) tests, which, in combination with history and chest radiography, was consistent with the diagnostic criteria of ARDS. None of the 5 patients had chronic lung diseases or smoking history. The emphysema showed in the chest CT images at admission of case 1 was considered as senile emphysema, as the patient has no history of chronic coughing, sputum or dyspnea. They received treatment of mechanical ventilation with protective ventilation strategies. Chest CT scan and X-ray images of the 5 patients showed remarkably heterogenic distribution, with patchy glass-ground opacification, interspersed with normal-appearing areas or consolidation, especially distributing in gravity dependent lung regions. The fibrosis of cases 3 and 4, consolidation of case 2, 3 and 5, and pulmonary cysts and emphysema of case 3 were newly developed during the treatment. Case 1 and 4 developed right pneumothorax after reducing the usage of neuromuscular blocking agents, with increased respiratory rates (RR) and paradoxical thoracoabdominal motion. Right pneumothorax of case 2 occurred after an ineffective recruitment maneuver when he was treated by IMV combined with venous-venous extracorporeal membrane oxygenation (VV-ECMO). Pneumothorax of case 3 occurred after a consecutively and severely coughing, although he was already weaning from IMV combined with VV-ECMO. Case 5 developed bilateral pneumothorax when he was treated by noninvasive ventilation (NIV), with RR of 34 breaths/min along with paradoxical thoracoabdominal motion. Except case 5 were treated by NIV when pnemothorax occured, the other 4 patients treated by IMV were used neuromuscular blockers. Case 1 encountered refractory hypoxemia and acidosis after pneumothorax and was treated by VV-ECMO. Case 5 was switched NIV to IMV due to severe hypoxemia after the occurrence of pneumothorax. Observation was used in 2 patients with small-amount pneumothorax, and traditional tube thoracostomy was performed in the other 3 patients with large pneumothorax and severe dyspnea. Air leaks persisted in 2 of the 4 dead patients until their death (Figs. 1 and 2 , Table 2 ).
Fig. 1.
The clinical course and treatment of the 5 patients.
Fig. 2.
Chest CT scan and X-ray images of the 5 patients showed remarkably heterogenic distribution, with patchy glass-ground opacification, interspersed with normal-appearing areas or consolidation, especially distributing in gravity dependent lung regions. (A1-3) Emphysema (green arrow), pulmonary cysts (purple arrow), consolidation (orange arrow) and ground-glass opacification (blue arrow), pneumothorax (red arrow) and pneumothorax resolved in case 1. (B1-4) Newly developed consolidation (orange arrow), right pneumothorax (red arrow) and pneumothorax resolved in case 2. (C1-4) Newly developed fibrosis (yellow arrow), consolidation (orange arrow), pulmonary cysts (purple arrow) and emphysema (green arrow) and bilateral pneumothorax (red arrow), and pneumothorax resolved in case 3. (D1-5) Newly developed pneumomediastinum, subcutaneous emphysema, and right pneumothorax (red arrow) after pneumomediastinum and subcutaneous emphysema resolved in case 4. (E1-4) Newly developed consolidation (orange arrow), right pneumothorax (red arrow) and bilateral pneumothorax (red arrow) in case 5. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Clinical characteristics, treatments and outcomes pneumothorax.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Male |
| Age, years | 79 | 56 | 54 | 69 | 63 |
| Smoking history | No | No | No | No | No |
| Chronic lung diseases | No | No | No | No | No |
| Description of air leaks | |||||
| Side of PTa | Right | Right | Bilateral | Right | Bilateral |
| SEb | No | No | No | Yes | No |
| PDc | No | No | No | Yes | No |
| Smoking history | No | No | No | No | No |
| Chronic lung diseases | No | No | No | No | No |
| RR (breaths /min) | 24 | 10 | 32 | 35 | 34 |
| Arterial blood gas tests before air leaks | |||||
| pH | 7.32 | 7.36 | 7.44 | 7.43 | 7.46 |
| FiO2 | 90% | 50%+VV-ECMO | 37% | 70% | 55% |
| PO2/FiO2, mmHg | 129 | NA | 192 | 110 | 131 |
| PO2, mmHg | 116 | 72 | 71 | 77 | 72 |
| PCO2, mmHg | 76 | 40 | 38 | 72 | 42 |
| Severity of ARDS | moderate | severe | moderate | moderate | moderate |
| Paradoxical thoracoabdominal motion | Yes | No | No | Yes | Yes |
| Mechanical ventilation before air leaks | IMV | IMV+VV-ECMO | NIV+IMV (terminated) | IMV | NIV |
| Ventilation parameters | |||||
| VTd, ml/kg | 5.8 | 5 | NA | 5.5 | NA |
| PEEPe, cmH2O | 12 | 12 | NA | 8 | 5 |
| IPAPf, cmH2O | 24 | 25 | NA | 22 | 15 |
| Pplatg, cmH2O | <30 | <30 | NA | <30 | NA |
| Neuromuscular blockers | Yes (decreasing in dose) | Yes | No | Yes (decreasing in dose) | No |
| Recruitment maneuver | No | Yes | No | No | No |
| Lung-protective ventilation | Yes | Yes | No | Yes | Yes |
| ECMO | No | Yes | No | No | No |
| Days of CT before PT | 17 | 22/10 | 25/0 | 37 /27 | 11/6 |
| CT features | |||||
| Ground-glass opacity | Yes | Yes/ Yes | Yes /Yes | Yes /Yes | Yes /Yes |
| Consolidation | Yes | No/ Yes | No /Yes | No /No | No /Yes |
| Fibrosis | No | No /No | No /Yes | No /No | No/No |
| Pulmonary cysts | Yes | No /No | No/ Yes | No /No | No /No |
| Emphysema | Yes | No /No | No/ Yes | No /No | No /No |
| From dyspnea to occurence of air leaks, days | 21 | 21 | 28 | 40 | 15 |
| From mechanical ventilation to occurence of air leaks, days | 18 | 20 | 18 | 37 | 5 |
| Chest drainage | Yes | No | No | Yes | Yes (Bilateral) |
| ECMO | Yes | Yes | No | No | No |
| Pneumothorax resolved | Yes | Yes | Yes | No | No |
| Virus eliminated | Yes | Yes | Yes | Yes | No |
| Outcome | Died | Died | Alive | Died | Died |
| From occurrence of pneumothora to death, days | 11 | 15 | NA | 7 | 4 |
| Length of hospital stay, days | 29 | 38 | 42 | 44 | 13 |
PT= pneumothorax,
SE= subcutaneous emphysema,
PD= pneumomediastinum,
VT = tidal volume,
PEEP= positive end-expiratory pressure
IPAP= inspiratory positive airway pressure,
Pplat = end-inspiratory pressures.
Discussion
The incidence and poor prognosis of pneumothorax in ctitical patients with ARDS
Pneumothorax is a potentially fatal complication in patients with ARDS, especially in those who receivemechanism ventilation. However, the incidence of pneumothorax varies widely, from 1.7% to 10%,7 , 11 according to previous literature, with an astonishing 77% in an early report.6 In our study, the incidence of pneumothorax was 10% (5/49) in COVID-19 patients with ARDS, 24% (5/21) in patients receiving mechanical ventilation, 56% (5/9) in patients treated with IMV, significantly higher than 2.70% (1/37) as reported by Yang X et al. in COVID-19 patients with mechanical ventilation8 and 1.7% (6/356) as reported by Sihoe et al. in severe acute respiratory syndrome (SARS) patients with ARDS.7 Pneumothorax has been known to increase the mortality in patients with ARDS. In a study of 84 patients with severe ARDS by Gattinoni et al., the incidence of pneumothorax was 48.8% (40/84), and the mortality was higher in patients with pneumothorax than those without it (66% vs. 46%).5 On the basis of our data, pneumothorax is a frequent and fatal complication in critically ill COVID-19 patients with ARDS, resulting in a mortality rate as high as 80% (4/5) and prolonged length of hospital stay. Therefore, prevention, prompt recognition and treatment of pneumothorax are very important to minimize the mortality of critically ill COVID-19 patients with ARDS, especially those who require mechanical ventilation.
Mutiple risk factors and mechanism contributing to the occurrence of pneumothorax
The occurrence of pneumothorax in patients with ARDS is associated with multiple factors, including chronic pulmonary diseases, smoking, severity and duration of ARDS, mechanical ventilation settings, changes of lung structure and function during ARDS.12 While, none of the 5 patients in the current study had a history of smoking or chronic pulmonary diseases.
Several ventilation parameters are considered significant in the pathology of pneumothorax, including peak inspiratory pressure, PEEP, RR and VT. Lung-protective ventilation strategies were applied in all 5 patients while they were on mechanical ventilation, with VT set according to predicted body weight (<6 ml/kg) and static P plat less than 30 cm H2O.13 All patients except case 5 were treated with neuromuscular blockers to reduce oxygen consumption, RR, nagetive pressure in pleural cavity, transpulmonary pressure and human-machine confrontation during IMV.14 , 15 However, case 2 developed right pneumothorax after ineffective recruitment maneuver when he was on IMV combined with VV-ECMO treatment. Recruitment maneuver may increase alveolar pressure and lead to pneumothorax, in line with previous reports.16 Case 1 and 4 developed pneumothorax in the process of reducing neuromuscular blocker dose. Moreover, paradoxical thoracoabdominal motion and increased RR appeared in case 1, 4 and 5, suggesting forced inhalation while they suffered from severe ARDS. Pneumothorax occurred in case 3 due to a severely coughing when he was treated by COT after extubated. Severe cough and forced inhalation significantly increase the negative pressure in the pleural cavity17 and transpulmonary pressure, thereby leading to alveolar overdistension18 and causing pneumothorax.
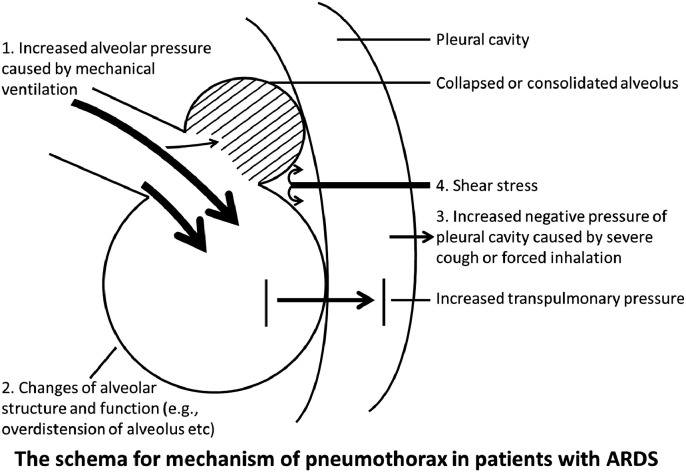
However, pneumothorax is more common in intubated patients with ARDS than in those without ARDS.19 Therefore, in addition to mechanical ventilation parameters, there are many other factors promoting pneumothorax, such as formation of pulmonary cysts and emphysema-like changes.20 , 21 The autopsy of COVID-19 patients showed pulmonary consolidation, fibrosis and lung lobe dilation.22 These signs were also partially shown in the CT images of the 5 patients, similar to SARS-related ARDS.23 Fibrosis was seen in 3 of the 5 patients, which seems more common compared to patients with SARS (3/8).23 Moreover, the fibrosis of cases 3 and 4, consolidation of case 2, 3 and 5 and pulmonary cysts and emphysema of case 3 were newly developed during the treatment. Chest CT images of the 5 patients showed heterogenic distribution, with patchy glass-ground opacification interspersed with areas of normal-appearing or consolidation, especially distributing in gravity dependent lung regions. The shear stress at the junction between aerated and collapsed lung aggravates the structural damages during the recruitment and de-recruitment of lung units.18 Hence, the abnormal alveolar pressure and negative pressure of the pleural cavity induce increased transpulmonary pressure, which, together with shear stress and changes of lung structure and function, contributes to the pathology of pneumothorax in patients with ARDS (Fig. 3 ).
Fig. 3.
Schematic illustration for the mechanism of pneumothorax in critically ill patients with COVID-19.
Furthermore, the incidence of pneumothorax is related to the duration of ARDS. The pneumothorax of the 5 critically ill COVID-19 patients occurred 2 weeks after the beginning of dyspnea. Gattinoni L et al. reported an incidence of pneumothorax of 87% in patients with late ARDS (more than 2 weeks), which is much higher than the 30% incidence in patients with early ARDS (less than 1 week).5 The aforementioned complicating factors increase the incidence of pneumothorax in critically ill COVID-19 with ARDS. The evaluation of probability of the risk in the 5 patients is listed in Table 3 .
Table 3.
Evaluation of risks related to pneumothorax in the 5 patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Increased alveolar pressure | Possible | Probable | Irrelevant | Possible | Possible |
| Increased negative pressure of pleural cavity* | Probable | Possible | Probable | Probable | Probable |
| Alveolar shear stress | Probable | Probable | Probable | Probable | Probable |
| Changes of lung structure and function | Probable | Probable | Probable | Probable | Probable |
severe cough, forced inhaled or paradoxical thoracoabdominal motion
Management after the occurrence of pneumothoarx in patients with ARDS
The literature on the treatment of ARDS with pneumothorax is very limited. Treatment options vary from observation, traditional tube thoracostomy, pleurodesis, open thoracotomy to thoracoscopic surgery. Observation was used in 2 patients with small-amount pneumothorax, and traditional tube thoracostomy was performed in the other 3 patients with large pneumothorax and severe dysnea. Persistent air leaks exist in 2% of patients with ARDS, increasing the mortality rate by 26%.24 In our study, air leaks persisted in 2 of the 4 dead patients until their death. Martínez-Escobar et al. reported that treatment of persistent air leak with pleurodesis using autologous blood in ARDS patients with pneumothorax reduced ICU stay and weaning time and lowered mortality.24 Patients with ARDS and pneumothorax are usually critically ill and unsuitable for thoracotomy or thoracoscopic surgery. Wagner et al. found that thoracotomy performed for ARDS with pneumothoraces increased mortality of patients to 56% vs 32%,25 while Huang et al. reported successful management of a critically ill patient with H7N9 infection, ARDS and refractory pneumothorax through the combination of ECMO and video-assisted thoracic surgery of pulmonary bullae resection.25 In general, the current literature on thoracotomy or thoracoscopic surgeries for ARDS complicated by pneumothorax is extremely limited, and further researches are need. However, with the development of interventional pulmonology, new treatment methods have been provided for these critically ill patients. Endobronchial one-way valve facilitates the weaning process from ECMO for a patient with ARDS and persistent air leak.26 Case 1 encountered refractory hypoxemia and acidosis after pneumothorax and was treated by VV-ECMO. As a rescue therapy of severe ARDS and pneumothorax caused by COVID-19, VV-ECMO provides sufficient support of oxygen, allowing the lungs to rest, and earning more time for the treatment of pneumothorax and the elimination of the virus.
The prevention and prompt recognition of pneumothorax in COVID-19 patients with ARDS
Pneumothorax is a frequent complication of critically ill COVID-19 patients and related to poor prognosis, and therefore the prevention and prompt recognition of pneumothorax are particularly important. Protective ventilation—the prevision of Pplat less than 30cmH2O and VT normalized to predicted body weight—reduces the alveolar pressure, overdistension alveolus, and strongly risk of pneumothorax and persistent air leak in patient s with ARDS.27 In addition, the use of neuromuscular blockers reduces the negative pressure in pleural cavity, shear stress and changes of lung structure, and then is associated with lower incidence of pneumothorax in patients with severe ARDS (4% vs 11.7%),15 which was also confirmed in a systematic evaluation. Cases 1 and 4 developed pneumothorax while reducing the dose of neuromuscular blocker, indicating the need of close monitor for RR, paradoxical thoracoabdominal motion or other signs of forced inhalation. Furthermore, antivirus and prone ventilation may facilitate the recovery and recruitment of lung before stopping using neuromuscular blocker. But the use of recruitment maneuver requires careful consideration, given the circumstance of case 2 and previous literatures.16 Although protective ventilation strategies and neuromuscular blocker have been widely applied, high incidence of pneumothorax remains in critically ill COVID-19 patients with ARDS treated by mechanical ventilation. Cases 3 suggested prompt use of ECMO in patients with rapidly progressing ARDS to allow the reduction of peak and mean airway pressure, VT, ventilator rate and oxygen concentration, risk of acute lung injury and forced inhalation, which may reduce the incidence of pneumothorax and mortality. Of course, management of cough after ECMO decannulation and weaning from ventilator are also very important. Combes A et al. reported that extracorporeal carbon dioxide removal (ECCO2R) to facilitate ultra-protective ventilation (VT 4mL/kg and Pplat ≤ 25 cmH2O) in patients with moderate ARDS was feasible and safe.28 Hence, timely use of ECMO or ECCO2R combined by ultra-protective ventilation may play an important role in the prevention of pneumothorax in critically ill patients with severe ARDS, particularly in who newly developed emphysema, fibrosis or pulmonary cysts in chest CT images.
Limitations
There are several problems which remain to be answered by future studies with larger sample size. Firstly, the optimal time and manner for the retreat of neuromuscular blockers remains to be clarified. Secondly, suitable treatment options for COVID-19 patients with persistent air leaks are still unclear. Thirdly, the difficulty to obtain timely CT images for critically ill COVID-19 patients calls for monitoring with more portable means of examination, such as bedside lung ultrasound bedside CT, but the value of these is uncertain. Fourthly, the risks and advantages of recruitment maneuver in critically ill COVID-19 patients with severe ARDS should be further studied. Fifthly, early use of ECMO or ECCO2R needs to be weighed against rescue therapy on the effect of lower incidence of pneumothorax and mortality of critically ill COVID-19 with ARDS.
Conclusions
In summary, pneumothorax remains a frequent and fatal complication of COVID-19 patients with ARDS despite the use of protective ventilation strategies. Our findings indicate that pneumothorax is most likely to occur 2 weeks after the beginning of dyspnea and associated with many factors, including the reduction of neuromuscular blocking agents, recruitment maneuver, severely coughing, changes of lung structure and function during ARDS over time. Apart from traditional protective ventilation and use of neuromuscular blockers, timely treatment of ECMO or ECCO2R combined by ultra-protective ventilation may provide new option for the prevention of pneumothorax in critically ill COVID-19 patients with severe ARDS, especially in whom with newly developed emphysema, fibrosis or pulmonary cysts in CT images. Although our study is limited by the small sample size and retrospective nature, we believe the findings reported is important for reducing the incidence of pneumothorax and mortality, and improving the prognosis for critically ill COVID-19 patients.
Authorship
XH W, SL G made substantial contributions to the study concept and design. XH W was in charge of the manuscript draft. XL H, X W, LX Z, and JH Z collected and confirmed data accuracy. J D applied for the ethical approval, XZ L participated in drafting of the manuscript. XL H, HM M, and SL G were the clinical experts in charge of the treatment of the patients. All authors made substantial revisions to the manuscript.
Declaration of Competing Interest
The authors have no potential conflict of interest to disclose.
Acknowledgments
Acknowledgments
Not applicable.
Funding
This work was supported by: (1) Chongqing Education Board “new coronavirus infection and prevention” emergency scientific research project (KYYJ202006); (2) Chongqing Science and Technology Bureau “new coronavirus pneumonia epidemic emergency science and technology special” the fourth batch of projects. (3) Famous teacher project of Chongqing talent plan. (4) Chongqing youth and middle-aged medical high-end reserve talent project: respiratory internal medicine.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hrtlng.2020.10.002.
Appendix. Supplementary materials
Data for reference
The data could be available from the corresponding author upon reasonable request after the paper published.
References
- 1.WHO. Coronavirus disease (COVID-19) Pandemic. Vol 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. http://doi/org/10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xun Li, L. W, S. Y, Shen Z.G. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. medRxiv preprint. 2020. http://doi/org/10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed]
- 4.Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. http://doi/org/10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L., Bombino M., Pelosi P. Lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA. 1994;271(22):1772–1779. http://doi/org/10.1001/jama.1994.03510460064035 [PubMed] [Google Scholar]
- 6.Woodring J.H. Pulmonary interstitial emphysema in the adult respiratory distress syndrome. Crit Care Med. 1985;13(10):786–791. doi: 10.1097/00003246-198510000-00003. http://doi/org/10.1097/00003246-198510000-00003 [DOI] [PubMed] [Google Scholar]
- 7.Sihoe A.D., Wong R.H., Lee A.T. Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest. 2004;125(6):2345–2351. doi: 10.1378/chest.125.6.2345. http://doi/org/10.1378/chest.125.6.2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. http://doi/org/10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin L., Li T.S. Interpretation of "Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) infection by the National Health Commission (Trial Version 5)". Zhonghua Yi Xue Za Zhi. 2020;100(0):E1. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. http://doi/org/10.3760/cma.j.issn.0376-2491.2020.0001 [DOI] [PubMed] [Google Scholar]
- 10.Ranieri V.M., Rubenfeld G.D., Thompson B.T. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Briel M., Meade M., Mercat A. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. http://doi/org/10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 12.Terzi E., Zarogoulidis K., Kougioumtzi I. Acute respiratory distress syndrome and pneumothorax. J Thorac Dis. 2014;6(Suppl 4):S435–S442. doi: 10.3978/j.issn.2072-1439.2014.08.34. http://doi/org/10.3978/j.issn.2072-1439.2014.08.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camporota L., Hart N. Lung protective ventilation. BMJ. 2012;344:e2491. doi: 10.1136/bmj.e2491. http://doi/org/10.1136/bmj.e2491 [DOI] [PubMed] [Google Scholar]
- 14.Gao Z.W., Zhao H.M., Sun Q.S., Sun H., Huang Y.Z., Zheng P. Systematic evaluation of neuromuscular blocking agents on prognosis of patients with moderate to severe acute respiratory distress syndrome. Zhonghua Yi Xue Za Zhi. 2019;99(48):3819–3825. doi: 10.3760/cma.j.issn.0376-2491.2019.48.012. http://doi/org/10.3760/cma.j.issn.0376-2491.2019.48.012 [DOI] [PubMed] [Google Scholar]
- 15.Papazian L., Forel J.M., Gacouin A. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. http://doi/org/10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 16.Morais C.C., De Santis S.R., Filho J.R. Monitoring of pneumothorax appearance with electrical impedance tomography during recruitment maneuvers. Am J Respir Crit Care Med. 2017;195(8):1070–1073. doi: 10.1164/rccm.201609-1780LE. http://doi/org/10.1164/rccm.201609-1780LE [DOI] [PubMed] [Google Scholar]
- 17.Zhang S., Qin R. Production and law of variation of the pleural cavity intrinsic pressure and the pressure of alveolar wall during respiratory process. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012;29(2):264–266. http://doi/org/10.1056/NEJMoa1005372 [PubMed] [Google Scholar]
- 18.Finfer S., Rocker G. Alveolar overdistension is an important mechanism of persistent lung damage following severe protracted ARDS. Anaesth Intensive Care. 1996;24(5):569–573. doi: 10.1177/0310057X9602400511. http://doi/org/10.1177/0310057X9602400511 [DOI] [PubMed] [Google Scholar]
- 19.Parker J.C., Hernandez L.A., Peevy K.J. Mechanisms of ventilator-induced lung injury. Crit Care Med. 1993;21(1):131–143. doi: 10.1097/00003246-199301000-00024. http://doi/org/10.1097/00003246-199301000-00024 [DOI] [PubMed] [Google Scholar]
- 20.Henderson W.R., Chen L., Amato M., Brochard L.J. Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(7):822–833. doi: 10.1164/rccm.201612-2495CI. http://doi/org/10.1164/rccm.201612-2495CI [DOI] [PubMed] [Google Scholar]
- 21.Woodside K.J., VanSonnenberg E., Chon K.S., Loran D.B., Tocino I.M., Zwischenberger J.B. Pneumothorax in patients with acute respiratory distress syndrome: pathophysiology, detection, and treatment. J Intensive Care Med. 2003;18(1):9–20. doi: 10.1177/0885066602239120. http://doi/org/10.1177/0885066602239120 [DOI] [PubMed] [Google Scholar]
- 22.Liu Xi, W R., Q G. A report on the general observation of the necropsy of COVID-19. Journal of Forensic Medicine. 2020;36(1):1–3. http://doi/org/10.12116/j.issn.1004-5619.2020.01.00 [Google Scholar]
- 23.Joynt G.M., Antonio G.E., Lam P. Late-stage adult respiratory distress syndrome caused by severe acute respiratory syndrome: abnormal findings at thin-section CT. Radiology. 2004;230(2):339–346. doi: 10.1148/radiol.2303030894. http://doi/org/10.1148/radiol.2303030894 [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Escobar S., Ruiz-Bailen M., Lorente-Acosta M.J., Vicente-Rull J.R., Martinez-Coronel J.F., Rodriguez-Cuartero A. Pleurodesis using autologous blood: a new concept in the management of persistent air leak in acute respiratory distress syndrome. J Crit Care. 2006;21(2):209–216. doi: 10.1016/j.jcrc.2005.10.003. http://doi/org/10.1016/j.jcrc.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Wagner P.K., K M., S C. Extracorporeal gas exchange in adult respiratory distress syndrome: associated morbidity and its surgical treatment. Br J Surg. 1990;77(12):1395–1398. doi: 10.1002/bjs.1800771224. http://doi/org/10.1002/bjs.1800771224 [DOI] [PubMed] [Google Scholar]
- 26.Huang J., Li H., Chen S., Lan C., Lin Q., Weng H. First successful combination of extracorporeal membrane oxygenation (ECMO) with video-assisted thoracic surgery (VATS) of pulmonary bullae resection in the management of refractory pneumothorax in a critically ill patient with H7N9 pneumonia and acute respiratory distress syndrome: a case report. Medicine. 2019;98(20):e15661. doi: 10.1097/MD.0000000000015661. http://doi/org/10.1002/bjs.1800771224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghiani A., Hansen M., Tsitouras K., Neurohr C. Endobronchial one-way valve therapy facilitates weaning from extracorporeal membrane oxygenation in a patient with ARDS and persistent air leak. Case Rep Crit Care. 2018;2018 doi: 10.1155/2018/9736217. http://doi/org/10.1155/2018/9736217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combes A., F V., P T., Investigators. ESOI Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. 2019;45(5):592–600. doi: 10.1007/s00134-019-05567-4. http://doi/org/10.1007/s00134-019-05567-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data could be available from the corresponding author upon reasonable request after the paper published.