Abstract
This study was conducted to determine the most efficient method to produce vitamin D in mushrooms using UV radiation. For this purpose, mushrooms were irradiated with UV-B and UV-C lamps from their caps, stems, both caps and stems (oblique), and sliced surface at doses of 12.5 kJ m−2 and 3.6 kJ m−2, respectively. Then, they were treated by UV-B at 27 °C, 35 °C, and 43 °C. In the next steps, samples were placed in 30 cm and 50 cm distances from the UV source. Afterward, they were irradiated from 15 to 120 min at an intensity of 3.5 W m−2. In the stability tests, samples were stored at 25 °C, frozen, refrigerated and were cooked and their vitamin D2 content was re-analyzed using HPLC. All experiments were repeated three times. In the sliced group treated with UV-B, vitamin D2 content 14.43 μg gr−1 was significantly higher than other groups. The internal temperature of 27 °C was found as optimum temperature with the production of 3.81 μg gr−1 vitamin D. It was revealed that increasing the distance from the UV source had a significant effect on vitamin D production. After 90 min of exposure, the highest amount of vitamin D2 was produced. Data showed that the vitamin D2 content remained almost stable after one day at 25 °C and during the cooking but it decreased about 50% after 7 days of cold storage. The optimal method observed in this study incorporates the use of UV-B lamps, incensement of radiation area in mushrooms and distance reduction from the UV source within 30 cm the internal temperature of 27 °C should be considered as well in the experiment.
Keywords: Vitamin D, Prevention, UV Mushrooms, Agaricus bisporous, Ergocalciferol
Highlights
-
•
UVB lamp is the most effective wavelength in vitamin D2 production in mushrooms.
-
•
The wideness of the exposure area effects on vitamin D2 production in mushrooms.
-
•
27 °C is the optimum temperature for vitamin D2 production in button mushroom.
-
•
Vitamin D in mushrooms is stable after 24 h retention at 25 °C and after cooking.
1. Introduction
Vitamin D is an essential micronutrient that plays a vital role in human health and prevents many diseases such as, cancers, diabetes, cardiovascular and autoimmune diseases. Deficiency of this vitamin is considered as a main global health problem in all ages. Vitamin D has two main forms of D3 and D2. Vitamin D3 is found in animal foods and vitamin D2 is found in plant food sources like mushrooms, fortified foods and supplements. Both types of vitamin D are well-responsible for maintaining the serum level of 25-hydroxy vitamin D in humans (Chen et al., 2015; Phillips et al., 2011). Although vitamin D3 is generally formed under the human skin from 7-dehydrocholesterol because of the sunlight exposure, the production seems to be inefficient due to the seasonal variation and also the air pollution (Elangovan et al., 2017). Considering that only few natural foods contain vitamin D, the best way to receive adequate daily amounts of vitamin D is obtaining it from supplements or fortified foods (Barnkob et al., 2016). Despite the abundance and availability of vitamin D fortified products and supplements, the problem of vitamin D deficiency has not been solved yet. Some possible explanations could be an inadequate level of fortification or poor coverage of these foods by people due to food accessibility, allergic reactions to lanolin in supplements, or food avoidance due to veganism. Therefore, an alternative solution to meet the vitamin D requirement in this population is needed (Calvo & Whiting, 2013; Jover et al., 2002).
Following the global pandemic of COVID-19, the importance of preventing virus infection and the improvement of the immune system has received much attention. Studies have shown that vitamin D can be effective in preventing coronavirus infection in the respiratory tract. It is also demonstrated that the mortality rates of COVID-19 were lower in people with higher serum 25-hydroxy-D levels (Grant et al., 2020; Ilie, Stefanescu, and Smith 2020; Rhodes et al., 2020). Since the vitamin D deficiency is prevalent all around the world and vitamin D rich supplements may not be widely available worldwide in the current state of the pandemic, presenting a natural, rich and cost-effective source of this vitamin in family food basket is consequential for promoting community health and decreasing morbidity and mortality rate of COVID-19 (Buttriss and Lanham-New, 2020; Panarese & Shahini, 2020).
Mushrooms, similar to human skin, can produce vitamin D when exposed to UV radiation . During this process, the ergosterol content of the surface of the mushroom is converted to ergocalciferol (vitamin D2) within a series of photochemical and thermal reactions (Mau et al., 1998). The amount of vitamin D that is made in mushrooms is considerably higher than the content of vitamin D in fortified products of the food industry. For example, if 100 g of fortified milk presents approximately 37 IU vitamin D, there could be an average amount of 19,000 IU of vitamin D per 100 g of mushrooms which faced to UV-B with dose of 15 kJ m−2 (Aborhyem et al., 2020; Urbain et al., 2011).
Several radiation protocols have been reported by different treating methods. Multiple studies have shown disagreements regarding the effect of UV exposure to the different parts of mushrooms. Some of them reported vitamin D2 production was higher in the stem and others stated it was more generated in the caps. Likewise, in the matter of radiation temperature, investigations have stated various results from 25 to 45 °C as the optimum temperature. Concerning the duration of UV exposure, researchers have diversely claimed that vitamin D2 formation was completed within 1 h and also 2 h in different types of mushrooms (Guan et al., 2016; Huang et al., 2015; Jasinghe and Perera 2005, 2006; Kalaras et al., 2012; Koyyalamudi et al., 2009; Lee & Aan, 2016; Teichmann et al., 2007; Wu and Ahn 2014b). Since, various factors could affect the production of vitamin D in mushrooms, more studies are needed to determine the most efficient protocol of UV light exposure on vitamin D production in white button mushrooms.
Many experimental studies have shown that white button mushrooms are rich in ergosterol and thus it could produce a high amount of vitamin D2 by UV irradiation (Jasinghe & Perera, 2005). Since these mushrooms are very popular and accessible, if an efficient protocol is found for mushroom production, vitamin D mushrooms could help to decrease the rate of vitamin D deficiency effectively. In this study, for the first time, this method is examined in the Middle East region. The objective of this study is to determine the most effective protocol of vitamin D production in white button mushrooms (A.bis) in terms of optimum wavelength, mushrooms orientation, ambient temperature, distance from UV source and duration of exposure by using UV irradiation and measuring its stability.
2. Materials and methods
2.1. Materials
Six sheets of PVC with the thickness of 16 mm were purchased from PVC-Tehran (Iran) for the radiant chamber designation, The UV lamps used in this study included a 30 W UVB-89 cm with the output peak wavelength of 312 nm and a 30 W UVC-89 cm with the output peak wavelength of 254 nm, were provided form Vilber (France). UV-proof Plexiglas (Evonic, Germany) was utilized in the frame of radiant chamber. 34 kg of white button mushrooms with 3–4 cm caps diameter were provided from Armita producer (Iran). UV light meter, Lutron (made in Taiwan) was used to measure the dose and the intensity of UV radiation. Standards of Ergocalciferol (D2) and cholecalciferol (D3) (Sigma-Aldrich, Germany), potassium hydroxide (85% pure, Merck Chemicals, Germany), C18 HPLC column (Sigma-Aldrich C18, 2.7 μm, 15 cm × 4.6 mm, Germany) were used in HPLC analyze of vitamin D2. Furthermore, methanol (HPLC grade), acetonitrile (HPLC grade), n-pentane, ethanol (95% pure), sodium ascorbate and sodium hydroxide were purchased from Fisher Chemical (Canada).
2.2. Methods
2.2.1. Design of radiant chamber
The radiant chamber was designed by the authors. According to the picture 1 the dimensions of the chamber are 100 cm × 65 cm × 55 cm. Since UV does not pass through the PVC, all six surfaces of the chamber were covered by PVC sheets. The lamps were attached to a holder on the top center of the chamber, which has three removable internal stainless steel trays (52 cm × 32 cm). The chamber allows adjusting the distance between the tray and lamp at 30, 40, and 50 cm. The door of the chamber contained a transparent UV-proof Plexiglas shield that allows observing inside of the chamber. Since aluminum has more than 90% UV reflectance (Fearon et al., 2007, pp. 379–390), it was used to cover the inner walls of the chamber to reach the maximum level of UV reflection. The chamber was equipped with an industrial ventilator on the top side to prevent excessive temperature rise while radiation. A small regular light bulb was installed on the inside wall of the chamber to observe the color change of mushrooms. A digital thermometer was installed on the outer wall of the chamber to measure the internal temperature of the chamber during radiation. The chamber had three separate switches for visible light, ventilator, and UV lamp.
Fig. 8.
Schematic image of radiant chamber.
2.2.2. Mushrooms initial preparation
White button Mushrooms (A.bis) were purchased from growers in the day of harvest. Then, they were transferred from the farm to the laboratory in the sealed boxes to prevent any light exposure before the UV irradiation process. Each step of the experiment took approximately a week and all mushrooms were treated by UV approximately 5 h after the harvest. To prevent changing their moisture content, the mushrooms were not washed; however, any dirt or residue on the surface of the mushrooms that prevent UV reaching was removed. In each category of treatment, 500 ± 10 g of mushrooms were used. All experiments were carried out in triplicate. After the irradiations, each group of mushrooms was individually mixed using a blender for 2 min, three times and ground to become a homogeneous substance to minimize sampling errors in the extraction procedure. Each sample was packaged in small plastic bags and labeled with encrypted code to blind all analyses.
2.2.3. Mushrooms orientation test with UV-B and UV-C irradiation
In the first step, on the day of harvest mushrooms were purchased from the producer and were divided into four separate groups. In Group I, the mushrooms were exposed to the UV only from their cap's side while positioned horizontally. In Group II, mushrooms were placed upside-down while their stems and gills faced to UV lamp. In Group III, mushrooms were laid oblique on the trays while most of their caps area and one side of their stems were exposed to UV. In Group IV, sliced mushrooms were exposed to the UV which sliced manually with a thickness of approximately 3–4 mm. To compare the effect of different wavelengths on the conversion of ergosterol to D2, this stage of the study was performed with both UV-B and UV-C lamps. The mushroom's caps were about 3–4 cm in diameter and they were all placed in a single layer at 2 cm apart from each other. The total tray's area was 0.49 m2. This study was done within 30 cm away from the light source at intensities of 1.8 W m−2 and 3.5 W m−2 with UV-C and UV-B lamps. The irradiation intensities and doses were measured by UV light meter. The average internal temperature during the experiment was 27 °C and the exposure time was 1 h. This period was selected according to previous studies, which claimed that the conversion of ergosterol to ergocalciferol could be completed within 1 h (Jasinghe & Perera, 2006). All four experiments were performed simultaneously in the radiation chamber and under the completely similar irradiation processes with both lamps, separately.
2.2.4. Temperature test
At this stage of the experiment, the mushrooms were divided into three groups based on the results of previous studies (Jasinghe & Perera, 2005; Ko et al., 2008; Lee & Aan, 2016; Wu and Ahn 2014b). The first group irradiated at 27 °C average internal temperature, the second group at 35 °C average temperature, and the third group irradiated at 43 °C average ambient temperature. In the first group, the ventilator was used to prevent excessive temperature rise. In the second group, in order to reach higher temperatures, the tube was switched on to warm up for 30 min and in the third group, heating candles were used to reach the internal temperature of 43 °C. Considering the previous stage, Mushrooms were laid oblique on trays into the irradiation chamber. The mushroom's caps were about 3–4 cm in diameter and they were placed in 2 cm apart from each other. This experiment was carried out within 30 cm distance from the UV-B lamp and exposure time was one hour.
2.5. Distance test
To determine the effect of different distancing from UV source, the mushrooms were placed at two different distances from the UV lamp (i.e., 30 cm and 50 cm) with the calculated intensity of 3.5 W m−2 and 1.9 W m−2, respectively. Considering the last two steps this phase was irradiated at 27 °C and oblique orientation with a UV-B lamp within 1 h. The mushroom's caps size was 3–4 cm in diameter and they were placed in trays inside the chamber 2 cm apart from each other to not prevent delivery of UV.
2.2.6. Duration of UV exposure test
The mushrooms were divided into six groups and each group was irradiated respectively with UV-B lamps for 15, 30, 45, 60, 90, and 120 min. The calculated UV doses for these periods were 3.1, 6.3, 9.4, 12.6, 18.9, and 25.2 kJ m−2, respectively. Based on the results of the last three stages, this irradiation phase was performed at 27 °C and oblique orientation and distance from UV-B light was 30 cm at the intensity of 3.5 W m−2. The mushroom caps were 3–4 cm in diameter and they were placed 2 cm apart.
2.2.7. Stability of produced vitamin D tests
To perform the stability test of the UV-B treated mushrooms, they were purchased on the day of harvest and were irradiated at 30 cm distance from the UV lamp. In this process, the temperature of the chamber was 27 °C and the duration of exposure was 60 min at the dose of 12.5 kJ m−2. After the irradiation process, mushrooms were randomly divided into seven groups. Group 1 was immediately prepared for extraction and HPLC analysis. Group 2 was kept at 25 °C (room temperature) for 24 h. Group 3 was kept at 4 °C for a week in the fridge. Group 4 was kept at −14 °C for a week in the ordinary freezer. Group 5 was kept at −74 °C for a week in the laboratory ultra-freezer. Group 6 was fully roasted for 10 min after the irradiation process. Finally, Group 7 was fully boiled for 14 min after irradiation.
2.2.8. Analysis of vitamin D2 in UV irradiated mushrooms
Vitamin D2 was extracted and analyzed according to the method developed by Mau et al. (1998) with slight modification as follows (Mau et al., 1998). Freeze-dried mushrooms sample (0.5 g) was accurately weighed into a 250 mL round bottom flask. Then, it was mixed with 4 mL of sodium ascorbate solution (17.5 g in 100 mL of 1 mol L−1 NaOH), 50 mL of 95% ethanol, 10 mL of 50 g 100 mL−1 KOH, and 50 μg of cholecalciferol dissolved in 1 mL methanol. Cholecalciferol was used as the internal standard to apply the recovery due to its structural similarity with vitamin D2. In the next step, the mixture was saponified for 1 h under reflux at 80 °C. The mixture was immediately cooled to the room temperature and transferred into a separating funnel. The mixture was extracted with 15 mL de-ionized water, followed by 15 mL ethanol and then with a three-stage N-pentane of volumes 50, 50, and 20 mL, in the order of their appearance. The pooled organic layers were washed three times by 50 mL of 3 g 100 mL−1 KOH in 5% ethanol. Next, they were washed by deionized water until neutralized. The organic layer was transferred into a round bottom flask, rotary evaporated to dryness at 40 °C, and immediately re-dissolved in 5 mL ethanol for HPLC analysis. The samples were filtered through 0.45 μm filter units. A volume of 20-μl filtered sample was injected into the Waters 600E HPLC system equipped with a Waters 486 tenable absorbance UV detector. Afterward, it was eluted through a reversed phase C18 column (using acetonitrile/methanol (75:25) as the mobile phase at a flow rate of 2.3 mL min−1. The UV detection of the eluate was performed at 282 nm. The vitamins D2 and D3 were qualitatively analyzed by comparing the retention times of standards obtained and quantification was done using a calibration curve. The results are expressed on μg per each grams of mushrooms fresh body weight.
2.9. Statistical analysis
The data were expressed as means ± SD. When appropriate, a comparison was made between treatment groups using the one-way analysis of variance (ANOVA). Next, pairwise comparisons were used to determine the significance of any differences among the groups and independent t-test. A P-value of <0.05 was considered to be significant. All analyses were performed using the SPSS 25.0 statistical software.
3. Results
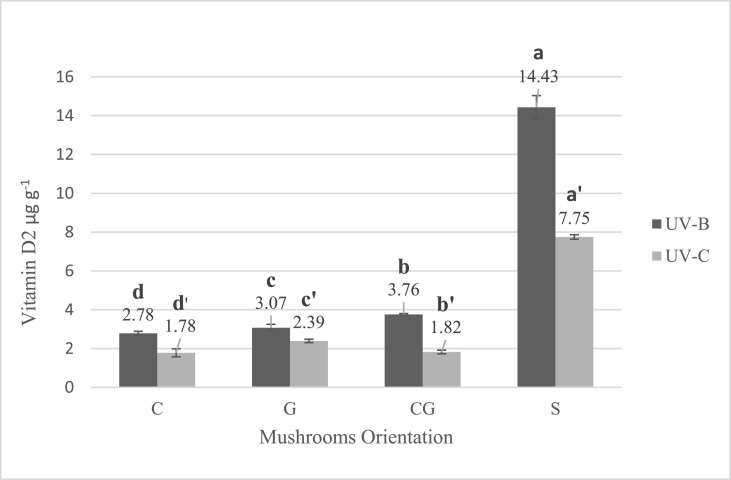
The concentration of vitamin D2 in all orientation groups was analyzed by HPLC (Fig. 1 ). Before the radiation processes, the amount of vitamin D2 in the control group was 0.004. After 1 h of radiation, at the intensity of 3.5 W m−2 in the first category, which was irradiated from the side of their caps the vitamin D2 content , was 2.78. In the second category, which faced from their stems to the UV-B lamp, it was 3.00. In the third category, which was laid oblique on the trays, it was 3.76. In the sliced group, 14.43 of vitamin D2 was produced. In the UV-C group, the obtained results were 1.78, 2.39, 1.82, and 7.75, for categories 1 to 4, respectively.
Fig. 1.
Effect of mushrooms orientation during radiation on vitamin D2 production (error bars represent standard deviation). Means assigned with different letters indicate significant differences (p < 0.05).
C: caps faced UV, G: gills faced UV, CG: caps and gills faced UV (oblique), and S: sliced mushrooms.
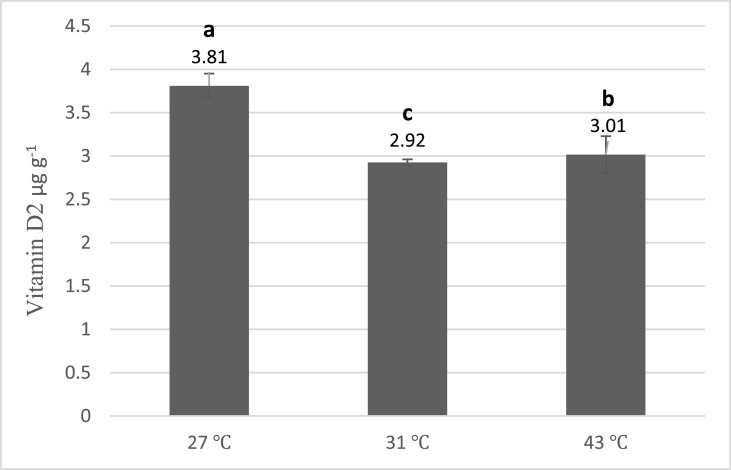
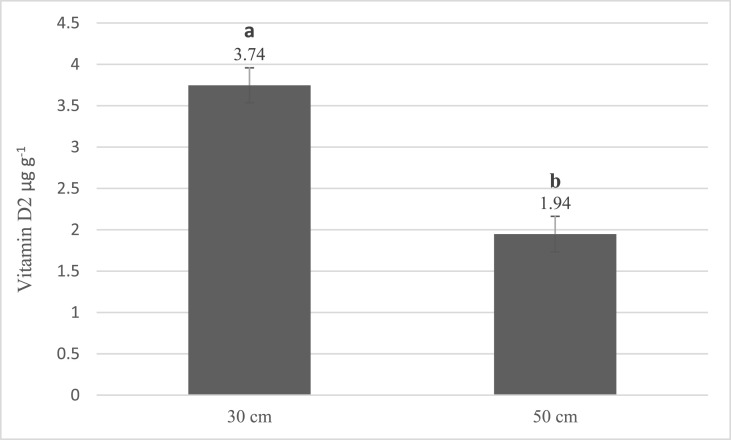
The data of vitamin D2 concentration in processes with different temperatures are given in Fig. 2 . According to the results, among these three groups, 27 ± 1.3°C (which produced 3.81 vitamin D) is an appropriate temperature for converting ergosterol to ergocalciferol (p < 0.05). In the distance test (Fig. 3 ), between 30 and 50 cm distance from the UV source, a steep descending slope occurred in vitamin D production. At the intensity of 3.5 W m−2 in 30 cm distance from UV source, 3.74 of vitamin D2 was produced. Finally, in 50 cm away from UV with 1.9 W m−2 intensity, 1.94 vitamin D2 was produced (p < 0.05).
Fig. 2.
Effect of different temperatures on vitamin D2 production by UV-B radiation (error bars represent standard deviation). Means assigned with different letters indicate significant differences (p < 0.05).
Fig. 3.
Effect of distance from UV source of vitamin D2 production (error bars represent standard deviation). Means assigned with different letters indicate significant differences (p < 0.05).
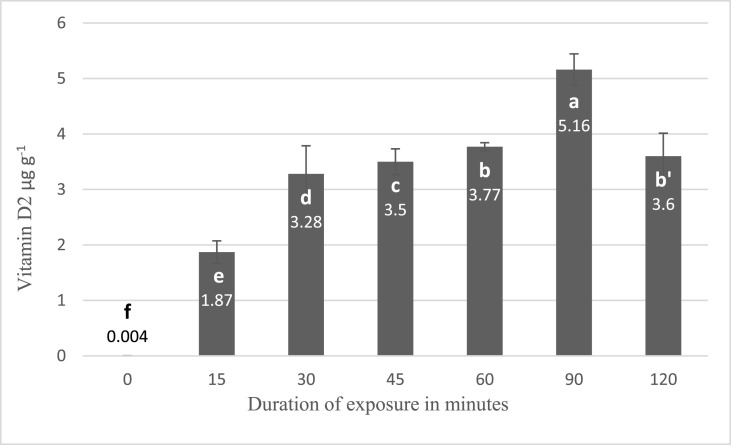
Fig. 4 presents the effect of different duration of exposure on vitamin D production in A.bis mushrooms treated by UV-B lamps. The intensity of lamps was 3.5 W m−2 during 15, 30, 45, 60, 90, and 120 min and had a 30 cm distance from UV lamp. The findings show that vitamin D production in mushrooms increased by longer duration of exposure up to 90 min, where the highest amount of vitamin D2 was produced (p < 0.05). However, it was reduced from 5.10 to 3.60 after 120 min exposure due to the excessive radiation (p < 0.05).
Fig. 4.
Effect of duration of UV-B exposure on vitamin D2 production (error bars represent standard deviation). Means assigned with different letters indicate significant differences (p < 0.05).
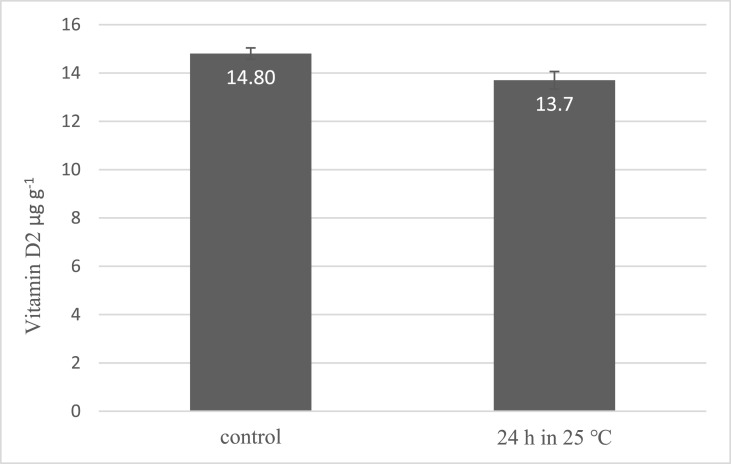
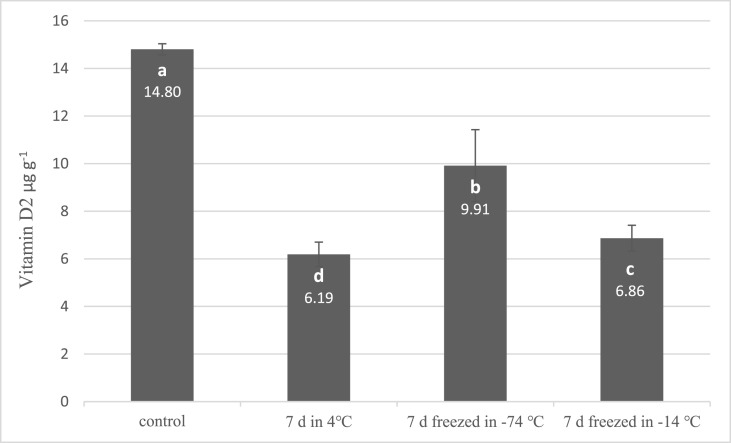
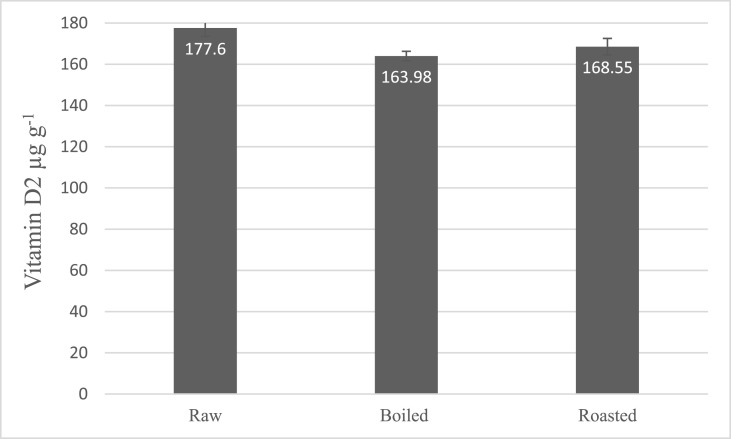
In the stability tests (Fig. 5 ), the vitamin D2 content of A.bis sliced mushrooms treated with 12.5 kJ m−2 UV-B light after 24 h retention in room temperature was slightly decreased (6% reduction) from 14.80 to 13.70 (non-significantly, p > 0.05). Fig. 6 shows that the vitamin D2 content was decreased significantly after 7 d of cold storage in all three temperature groups (p < 0.05). The stability of produced Vitamin D in mushrooms was reported after cooking processes on a dry weight basis due to the difference in the moisture content of cooked and raw mushrooms. Cooking by both boiling and roasting methods does not significantly reduce the vitamin D content which produced in mushrooms. The vitamin D2 retention was measured 92% ± 1.2% in boiled mushrooms and 94% ± 2.3% in roasted mushrooms (Fig. 7 ).
Fig. 5.
Stability of produced vitamin D2 at room temperature (error bars represent standard deviation). Data shows that there is no significant reduction in produced vitamin D2 after a day in 25 °C (P > 0.05).
Fig. 6.
Stability of produced vitamin D2 in cold storage (error bars represent standard deviation). Means assigned with different letters indicate significant differences (p < 0.05).
Fig. 7.
Fig. 7. Stability of produced vitamin D2 after cooking processes (error bars represent standard deviation). Data shows that there is no significant reduction in produced vitamin D2 after cooking processes (P > 0.05).
4. Discussion
4.1. Effect of mushrooms orientation during radiation on the conversion of ergosterol to vitamin D2
The first step of the preliminary experiment was to determine an appropriate orientation for vitamin D2 synthesis in A.bis mushrooms. The moisture content of mushrooms in all groups and all stages were measured to be about 89% ± 2%. As expected, the amount of vitamin D2 in the sliced groups was significantly higher than the other groups in both UV-B and UV-C radiations (p < 0.05). One explanation for this result could be the higher area of exposure in the sliced group in comparison to the whole body mushrooms. According to the results of this phase of the study, it is clear that if sliced mushrooms were exposed for much lower time to the UV, it could easily achieve the RDA of vitamin D in each serving of mushrooms in all age groups. However, in the study area, sliced mushrooms are not popular and people prefer buying whole mushrooms. Besides, since not all growers present sliced mushrooms, it seems that if we intend to provide a new source of vitamin D in the family food basket, it is better to present it in the most popular form regarding the economic aspects for growers. Since the ultimate objective of this study was to achieve a proper protocol for producing vitamin D mushrooms for commercialization, the mushrooms radiated with oblique orientation seem to be a good choice to use for mass production concerning attainability in farms and industrialization. It also should be considered that which orientation is more suitable for growers to expose mushrooms to the UV after harvest in mass production. Our results showed that the oblique group produced vitamin D2 efficiently and significantly higher than the cap and stem faced groups (p < 0.05). Therefore, an oblique orientation that both caps and stems were faced up to the UV source was chosen for the rest of the experiment. Furthermore, if consumers throw out the stems during the washing or preparation process, the caps still provide high amounts of vitamin D.
Jasinghe and Perera (2005) reported that the conversion of ergosterol to vitamin D2 was four times higher when gills were exposed to the UV; however, we did not meet any significant difference in both UV-B and UV-C exposures between caps faced and gills faced groups. This discrepancy might be due to the usage of UV-A lamps in their study instead of UV-B and UV-C. One year later, these researchers also investigated the effect of the orientation of exposure in shiitake mushrooms and reported that when mushrooms faced from their gill side to the UV for 2 h, the production of vitamin D2 became more than three times higher compared to the caps side group. These inconsistent outcomes may be appeared due to the fact that shiitake mushrooms and white button mushrooms have different properties. These results are supported by Ko et al. (2008) on shiitake and white button mushrooms, but the difference between cap faced UV and gill faced was observed lower in white button mushrooms than in shiitake. Guan et al. (2016) using UV-C lamps showed that vitamin D2 content was higher in caps than stems in white button mushrooms after irradiation dose-dependently, which this result is consistent with our findings (Guan et al., 2016; Jasinghe and Perera 2005, 2006; Ko et al., 2008). Although it was claimed that the concentration of ergosterol was higher in mushroom's gills than other parts, the area of exposure in the cap-faced group was higher as well as sliced mushrooms. Our results support that the wideness of the exposure area has an important influence on vitamin D2 production rather than the effect of ergosterol distribution in the mushrooms body.
4.2. Effect of treatment with different UV wavelength on the conversion of ergosterol to vitamin D2
Ergosterol absorbs UV radiation between 240 and 320 nm (Holick, 1989). In Fig. 1, it is clear that UV-B lamp with the output peak wavelength of 312 nm and intensity of 3.5 W m−2 was significantly more efficient in converting the ergosterol to ergocalciferol than UV-C with the output peak wavelength of 254 nm and intensity of 1.8 W m−2 (p < 0.05). Similar results also were reported by Jasinghe and Perera (2005) and ; Mau et al. (1998) with different intensities of irradiation and output peak wavelengths in the range of UV-B and UV-C. These findings may also showed up due to a lower intensity of radiation in the UV-C group.
4.3. Effect of different internal temperature during radiation on the conversion of ergosterol to vitamin D2 in mushrooms
According to Fig. 2, it is concluded that the average temperature of 27 ± 1.3 °C was more effective than other groups in ergosterol conversion. It seems that temperatures higher than 43 °C may not be the optimum, moreover, it could affect the quality characteristics of mushrooms such as their color and texture. Nevertheless, further studies are needed to evaluate different temperatures near room temperature. Previous studies have reported different temperatures as optimum for converting ergosterol to vitamin D2 in different types of mushrooms. Temperatures lower than room temperature were also investigated and provided poorer results (Mau et al., 1998). It is of note that temperatures higher than 35 °C had not been tested before in A.bis mushrooms.
Contrary to our results, Jasinghe and Perera (2005) reported 35 °C as the optimum temperature for converting ergosterol to vitamin D2 in mushrooms. However, they also investigated the ambient temperature of 27 °C in shiitake mushrooms, but they did not reveal this temperature as optimum (Jasinghe & Perera, 2005).. Wu et al. (2014) examined the effect of ambient temperature in the range from 15 °C to 45 °C in oyster mushrooms. They found out that the vitamin D2 content increased while the ambient temperature rises to 35 °C in oyster mushrooms. They represented 28.16 °C as the optimum temperature for their experiment, this finding is very close to our result on the optimum radiation temperature (Wu & Ahn, 2014a). Lee (2016) et al. used the Response Surface Methodology (RSM) to determine the optimum level of ergosterol to ergocalciferol transformation in A.bis powder by UV-B radiation. They reported that 26.33 °C is the optimum temperature for this experiment. This temperature likewise, almost similar to our results (Lee & Aan, 2016). Although the ambient temperature during irradiation plays an important role in vitamin D2 production, not all studies reported this factor in their results.
4.4. Effect of different distances from UV source on the conversion of ergosterol to vitamin D2
It is deduced that the distance from UV source is a major factor of UV reaching and highly affects the production of vitamin D2. This finding also was reported by other researchers for different irradiation conditions and various distances from UV source from 3.18 cm up to 137 cm on several types of mushrooms (Morales et al., 2017; Ozzard et al., 2008; Urbain et al., 2016). Results indicated that the transformation of ergosterol into ergocalciferol was enhanced when samples were placed closer to the UV source. For instance, vitamin D2 production within 4 cm away from the UV lamp source was 5 times higher than 24 cm distance from the UV lamp in shiitake mushrooms (Morales et al., 2017). Although, according to the earlier results, it was obvious that decreasing the distance from the UV source could increases the vitamin D2 generation in mushrooms, it was preferred to take this factor into account and evaluate the proportion of this effect in the experimental design.
Koyyalamudi et al. (2009) examined the effect of distance from UV-C light on vitamin D2 production at 30, 40, and 50 cm and mean intensities of 4.03, 3.16, and 2.5 W m−2, respectively, in A.bis mushrooms. They also achieved similar results, but they reported that in 50 cm distance from UV source, less discoloration occurs compared to 30 and 40 cm intervals (Koyyalamudi et al., 2009). Since the interval between UV lamps and samples could affect the quality and nutritional properties of mushrooms, further studies are needed to evaluate the sensory and qualitative characteristics of UV-treated mushrooms at different distances from the light source.
4.5. Effect of duration of exposure on vitamin D2 production in mushrooms
Vitamin D2 content in treated mushrooms was increased significantly with longer exposure time and different increment rates until 90 min of exposure (p < 0.05). It is concluded that the transformation of ergosterol to ergocalciferol was completed between 60 and 120 min of radiation. After this maximization, photodegradation of vitamin D appeared due to extra irradiation. As a result, it reduced vitamin D2 content from 5.10 at 90 min of exposure to 3.60 after 120 min, which is a 30% reduction (p < 0.05). Jasinghe and Perera (2006) claimed that the conversion of ergosterol to vitamin D2 is completed within 1 h when each side of shiitake mushrooms was exposed to the UV simultaneously. In the case of the mushrooms which faced to the UV from their caps or from their gills, they also declared that the ergocalciferol formation was completed within 2 h in both groups. This dissimilarity is attributed to radiating not all sides of mushrooms concurrently in our experiment or the different exposure surface the mushrooms body and the usage of shiitake mushrooms instead of A.bis mushrooms (Jasinghe & Perera, 2006).
Pulsed UV (PUV) technology is an efficient method for enriching mushrooms with vitamin D. PUV delivers a wide range of 100–800 nm wavelength as a high amount of intensity in just one pulse. Studies in this technique showed that the PUV method could be more effective than continuous UV radiation in the production of vitamin D2. For example, Kelaras et al. (2012) demonstrated that they reached a maximum level of vitamin D2 after 12 pulses treatment in just a few seconds. Although this approach is effective and safe, the high cost of PUV instruments could be a limiting factor for use in mass production by growers, especially in developing countries (Kalaras et al., 2012; Koyyalamudi et al., 2011).
4.6. Stability of vitamin D2 produced in the A.bis mushrooms by UV irradiation under different storage condition and cooking processes
To determine the stability of produced vitamin D2 in mushrooms, it was compared using different storage methods rather than comparing the effect of storage time on vitamin D2 content. Since mushrooms are classified as perishable foods, they have a limited shelf life whether at room temperature or in the fridge. Therefore, the stability test was conducted after 24 h retention in room temperature, 7 d in cold storages, and after cooking processes.
In a study by Ložnjak and Jakobsen (2018) on the retention of bio-fortified white button mushrooms after household cooking, the vitamin D2 retention was evaluated in fried mushrooms between 81 and 88%, but in boiled mushrooms, the true retention was measured 62%. However, we did not meet a significant difference between the two methods of roasting and boiling, the retention of our bio-fortified mushrooms was 92% and 94%, clearly more than the described study. In another study by Matilla et al. (1999), vitamin D stability was measured in two types of wild mushrooms after frying. In the first type (C. Cibarius) the ergocalciferol retention was reported 86.5% and in the second type (C. Tubaeformis) the retention was calculated 99%; these results were more similar to our experiment. Jakobsen and Knuthsen (2014) also demonstrated a retention of 73% and 89% of vitamin D2 in different type of bread (Jakobsen & Knuthsen, 2014; Ložnjak & Jakobsen, 2018; Mattila et al., 1999). Overall, it is concluded that vitamin D2 content of irradiated mushrooms is almost stable during the cooking processes.
Contrary to our result, Koyyalamudi et al. (2009) reported that vitamin D2 produced in A.bis mushrooms under the UV-C irradiation is stable after 8 d of shelf life at the room temperature and at 4 °C. The decreasing trend at cold storage temperatures observed in our study was similar to the results of Robert et al. (2008) by the first-order kinetics. According to these authors, vitamin D2 content of UV-B treated mushrooms with doses of 1 kJ m−2 and 1.5 kJ m−2 reduced after 4 d in the fridge (Koyyalamudi et al., 2009; Roberts et al., 2008).
5. Conclusion
It could be concluded that between the two wavelengths of UV-B and UV-C, UV-B produced a greater level of vitamin D2 with an output peak of 312 nm in mushrooms. In the orientation test, it was demonstrated that the sliced group and oblique group generated a higher amount of vitamin D with both UVB and UVC lamps, which indicates the positive effect of widening the exposure area. The findings of this article revealed that the optimum temperature of vitamin D2 formation is 27 °C. The reduction of the distance to 30 cm with the intensity of 3.5 W m−2, generated a greater level of vitamin D2, therefore, it could be deduced that the intensity of radiation considerably effects on vitamin D2 formation. The maximum ergosterol conversion to vitamin D2 was observed after 90 min of exposure and then photodegradation occurred. Vitamin D2 which was produced in mushrooms was remained stable after 24 h.at room temperature and after the cooking processes. However, it was reduced about 50% after 7 days of cold storage in the fridge and freezer; the observed reduction has a positive relationship with the storage temperature.
Data access and responsibility
The principal investigator, Sarina Salemi, had full access to the entire data of the study and took responsibility for the integrity of data and the accuracy of the data analysis.
CRediT authorship contribution statement
Sarina Salemi: Conceptualization, Methodology, Validation, Investigation, Resources, Writing - original draft, Project administration, Funding acquisition. Ahmad Saedisomeolia: Supervision, Project administration, Writing - review & editing, Conceptualization, Validation. Fateme Azimi: Investigation, Methodology, Resources. Sareh Zolfigol: Investigation. Ezeddin Mohajerani: Resources, Conceptualization, Methodology. Mehrdad Mohammadi: Resources, Conceptualization, Methodology. Mehdi Yaseri: Formal analysis.
Declaration of competing interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Acknowledgment
This study was supported by National Institute for Medical Research Development (NIMAD) with the Grant numbers of 987587. We would also like to thank Mr. M Barangi from Kesht v Sanat Pishgaman Taleghan Company for providing the required mushrooms for this study. We would also like to show our gratitude to the Dr. R Chahardoli, from biochemistry laboratory of Tehran University of Medical Science, for sharing their pearls of wisdom with us during the laboratory phase of this research, and we also thank Dr. A Mohseni-Kia from International Goods Inspection Company, who provided insight and expertise that greatly assisted the research, although any errors are our own and should not tarnish the reputations of these persons.
References
- Aborhyem Samar, Hamza Dina, Agamy Neveen. 'Vitamin D content in some food items sold in Egyptian markets'. Canadian Journal of Clinical Nutrition. 2020;8:62–77. [Google Scholar]
- Barnkob Line Lundbæk, Argyraki Aikaterini, Petersen Paul Michael, Jakobsen Jette. 'Investigation of the effect of UV-LED exposure conditions on the production of vitamin D in pig skin. Food Chemistry. 2016;212:386–391. doi: 10.1016/j.foodchem.2016.05.155. [DOI] [PubMed] [Google Scholar]
- Buttriss J.L., Lanham-New S.A. Is a vitamin D fortification strategy needed? Nutrition Bulletin. 2020;45:115. doi: 10.1111/nbu.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo Mona S., Whiting Susan J. 'Survey of current vitamin D food fortification practices in the United States and Canada'. The Journal of Steroid Biochemistry and Molecular Biology. 2013;136:211–213. doi: 10.1016/j.jsbmb.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Chen Shin-Yu, Hui-Tzu Yu, Kao Ju-Po, Yang Chung-Chun, Chiang Shen-Shih, Mishchuk Darya O., Mau Jeng-Leun, Carolyn M., Slupsky 'Consumption of vitamin D2 enhanced mushrooms is associated with improved bone health. The Journal of Nutritional Biochemistry. 2015;26:696–703. doi: 10.1016/j.jnutbio.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Elangovan Harendran, Chahal Sarinder, Gunton Jenny E. 'Vitamin D in liver disease: Current evidence and potential directions. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2017;1863:907–916. doi: 10.1016/j.bbadis.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Fearon E., Sato T., Wellburn D., Watkins K.G., Dearden G. 2007. Thermal effects of substrate materials used in the laser curing of particulate silver inks. (Laser assisted net shape engineering). [Google Scholar]
- Grant William B., Henry Lahore, McDonnell Sharon L., Baggerly Carole A., French Christine B., Jennifer L Aliano, Bhattoa Harjit P. 'Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Wenqiang, Zhang Jie, Yan Ruixiang, Shao Suqin, Zhou Ting, Lei Jing, Wang Zhidong. 'Effects of UV-C treatment and cold storage on ergosterol and vitamin D2 contents in different parts of white and brown mushroom (Agaricus bisporus) Food Chemistry. 2016;210:129–134. doi: 10.1016/j.foodchem.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Holick Michael F. 'Phylogenetic and evolutionary aspects of vitamin D from phytoplankton to humans. Vertebrate Endocrinology: Fundamentals and Biomedical Implications. 1989;3:7–43. [Google Scholar]
- Huang Shih-Jeng, Lin Chun-Ping, Tsai Shu-Yao. 'Vitamin D2 content and antioxidant properties of fruit body and mycelia of edible mushrooms by UV-B irradiation. Journal of Food Composition and Analysis. 2015;42:38–45. [Google Scholar]
- Ilie Petre Cristian, Simina Stefanescu, Lee Smith. 'The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clinical and Experimental Research. 2020:1–4. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen Jette, Knuthsen Pia. 'Stability of vitamin D in foodstuffs during cooking. Food Chemistry. 2014;148:170–175. doi: 10.1016/j.foodchem.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Jasinghe Viraj J., Perera Conrad O. 'Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chemistry. 2005;92:541–546. [Google Scholar]
- Jasinghe Viraj J., Perera Conrad O. 'Ultraviolet irradiation: The generator of vitamin D2 in edible mushrooms. Food Chemistry. 2006;95:638–643. [Google Scholar]
- Jover Eric, Moldovan Zaharie, Bayona Josep Maria. 'Complete characterisation of lanolin steryl esters by sub-ambient pressure gas chromatography–mass spectrometry in the electron impact and chemical ionisation modes. Journal of Chromatography A. 2002;970:249–258. doi: 10.1016/s0021-9673(02)00539-3. [DOI] [PubMed] [Google Scholar]
- Kalaras Michael D., Beelman Robert B., Elias Ryan J. 'Effects of postharvest pulsed UV light treatment of white button mushrooms (Agaricus bisporus) on vitamin D2 content and quality attributes. Journal of Agricultural and Food Chemistry. 2012;60:220–225. doi: 10.1021/jf203825e. [DOI] [PubMed] [Google Scholar]
- Ko J.A., Lee B.H., Lee J.S., Park Hyun Jin. 'Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus)'. Journal of Agricultural and Food Chemistry. 2008;56:3671–3674. doi: 10.1021/jf073398s. [DOI] [PubMed] [Google Scholar]
- Koyyalamudi Sundar Rao, Jeong Sang-Chul, Pang Gerald, Anthony Teal, Biggs Tony. 'Concentration of vitamin D2 in white button mushrooms (Agaricus bisporus) exposed to pulsed UV light. Journal of Food Composition and Analysis. 2011;24:976–979. [Google Scholar]
- Koyyalamudi Sundar Rao, Jeong Sang-Chul, Song Chi-Hyun, Cho Kai Yip, Pang Gerald. 'Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. Journal of Agricultural and Food Chemistry. 2009;57:3351–3355. doi: 10.1021/jf803908q. [DOI] [PubMed] [Google Scholar]
- Lee Nam Keun, Aan Byung-Yong. 'Optimization of ergosterol to vitamin D 2 synthesis in Agaricus bisporus powder using ultraviolet-B radiation. Food Science and Biotechnology. 2016;25:1627–1631. doi: 10.1007/s10068-016-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ložnjak Petra, Jakobsen Jette. 'Stability of vitamin D3 and vitamin D2 in oil, fish and mushrooms after household cooking. Food Chemistry. 2018;254:144–149. doi: 10.1016/j.foodchem.2018.01.182. [DOI] [PubMed] [Google Scholar]
- Mattila Pirjo, Ronkainen Riitta, Lehikoinen Kaisa, Piironen Vieno. 'Effect of household cooking on the vitamin D content in fish, eggs, and wild mushrooms. Journal of Food Composition and Analysis. 1999;12:153–160. [Google Scholar]
- Mau Jeng-Leun, Chen Pei-Ru, Yang Joan-Hwa. 'Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. Journal of Agricultural and Food Chemistry. 1998;46:5269–5272. [Google Scholar]
- Morales Diego, Gil-Ramirez Alicia, Smiderle Fhernanda R., Adriana J., Piris, Ruiz-Rodriguez Alejandro, Soler-Rivas Cristina. 'Vitamin D-enriched extracts obtained from shiitake mushrooms (Lentinula edodes) by supercritical fluid extraction and UV-irradiation. Innovative Food Science & Emerging Technologies. 2017;41:330–336. [Google Scholar]
- Ozzard Andrew, Hear Gurdip, Morrison Gavin, Hoskin Mike. 'Vitamin D deficiency treated by consuming UVB-irradiated mushrooms. British Journal of General Practice. 2008;58:644–645. doi: 10.3399/bjgp08X341959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panarese Alba, Shahini Endrit. 'Covid‐19, and vitamin D'. Alimentary Pharmacology & Therapeutics. 2020;51:993. doi: 10.1111/apt.15752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips Katherine M., Ruggio David M., Horst Ronald L., Minor Bart, Ryan R Simon, Feeney Mary Jo, Byrdwell William C., Haytowitz David B. 'Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States'. Journal of Agricultural and Food Chemistry. 2011;59:7841–7853. doi: 10.1021/jf104246z. [DOI] [PubMed] [Google Scholar]
- Rhodes Jonathan M., Subramanian Sreedhar, Laird Eamon, Kenny Rose Anne. 2020. 'low population mortality from COVID‐19 in countries south of latitude 35 degrees North–supports vitamin D as a factor determining severity', Alimentary Pharmacology & Therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts John S., Teichert Arnaud, McHugh Tara H. 'Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. Journal of Agricultural and Food Chemistry. 2008;56:4541–4544. doi: 10.1021/jf0732511. [DOI] [PubMed] [Google Scholar]
- Teichmann Anja, Dutta Paresh C., Anders Staffas, Jägerstad Margaretha. 'Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation. LWT-Food Science and Technology. 2007;40:815–822. [Google Scholar]
- Urbain Paul, Singler Fabian, Gabriele Ihorst, Biesalski Hans-Konrad, Bertz Hartmut. 'Bioavailability of vitamin D 2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: A randomized controlled trial. European Journal of Clinical Nutrition. 2011;65:965–971. doi: 10.1038/ejcn.2011.53. [DOI] [PubMed] [Google Scholar]
- Urbain Paul, Valverde Juan, Jakobsen Jette. 'Impact on vitamin D 2, vitamin D 4 and agaritine in Agaricus bisporus mushrooms after artificial and natural solar UV light exposure. Plant Foods for Human Nutrition. 2016;71:314–321. doi: 10.1007/s11130-016-0562-5. [DOI] [PubMed] [Google Scholar]
- Wu Wei-Jie, Ahn Byung-Yong. 'Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) using response surface methodology. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095359. [DOI] [PMC free article] [PubMed] [Google Scholar]