Abstract
Background:
Inguinal lymphadenectomy (I-LND) remains underutilized in patients with invasive penile cancer. Using a large national cancer registry, we assessed temporal trends in I-LND utilization and evaluated the impact of I-LND on the survival in patients in whom I-LND is an absolute indication per NCCN guidelines (T1b-4 N0/x-1).
Methods:
The National Cancer Database (NCDB) was queried for all non-metastatic penile patients with T1b-4 N0/X-N1 squamous cell carcinoma of the penis from 2004–2014. Adjusting for patient, demographic, and clinicopathologic characteristics, multivariable logistic regression models were used to examine the association between available covariates and receipt of I-LND. Cox-proportional hazards regression analyses were then used to assess the impact of clinical and pathological variables on overall survival. A propensity score weighted analysis was performed to assess the effect of I-LND in overall survival.
Results:
A total of 2224 patients met criteria for analysis, of which 606 (27.2%) underwent I-LND. Following adjustment, I-LND utilization was more likely in younger patients, those presenting with palpable adenopathy (cN1), treated in an academic facility, and those with a more contemporary diagnosis. On survival analysis, controlling for all known and measured confounders, I-LND receipt was associated with improved OS (HR 0.79 [CI 0.74–0.84], p < 0.001).
Conclusion:
In hospitals reporting to NCDB, the overall rate of I-LND for patients with invasive penile cancer was only 27.2%. Receipt if I-LND was associated with an increase in OS, justifying the use I-LND utilization as an important quality metric for performance reporting in patients with invasive penile cancer.
Keywords: Penile Cancer, Inguinal Lymphadenectomy, Utilization, Overall Survival, NCDB
Introduction:
Squamous cell carcinoma (SCC) of the penis is a rare condition diagnosed in approximately 1 in 100,000 men and accounts for less than 1% of cancers diagnosed in men in the US 1. Although rare, the disease carries significant morbidity and mortality with a 5-year cancer specific survival of approximately 50%. Given its rarity, the management of penile cancer has been based on small retrospective studies and consequently there is significant variation in the treatment of the disease.
Inguinal lymphadenectomy (I-LND) remains the most important staging and therapeutic intervention that can be performed in patients with invasive penile cancer2,3. Despite this, a recent population studies showed that in the US only 19% to 37% of patients with high grade or invasive penile disease undergo lymphadenectomy as recommended by the NCCN guidelines4,5. A major factor limiting the use of inguinal lymphadenectomy is the high risk of post-operative complications associated with the procedure6. Recent retrospective reports have shown the importance I-LND for patients with microscopic or low volume nodal disease; prompting the NCCN7 recommendation that I-LND be offered to any patient with high grade or invasive penile cancer (pT1b-T4), presenting with no or unilateral/low volume adenopathy (cN0-N1)7. For those with bilateral (cN2) or bulky (cN3) adenopathy the recommendation is for neoadjuvant chemotherapy followed by I-LND in select candidates7.
In this study, we aim to assess the utilization of inguinal lymphadenectomy (I-LND) in patients in whom I-LND is absolutely indicated per the NCCN guidelines ( high grade or invasive penile cancer (T1b-4) with minimal or no nodal involvement (N0/x-N1)), and assess the association between receipt of I-LND and overall survival using the National Base Cancer Database (NCDB).
Methods:
Data Source
The NCDB, a program of the ACS® CoC (Commission on Cancer) and the American Cancer Society, is a national cancer registry established in 1989 that serves as a comprehensive clinical surveillance resource for cancer care in the United States. The NCDB compiles data from more than 1,500 commission accredited cancer programs in the United States and Puerto Rico, and captures approximately 70% of all newly diagnosed cancer cases 8.
Definitions:
Cohort and primary outcome
Patients with penile squamous cell carcinoma were identified in the NCDB based on ICD-O-3 site and histology codes. Cases were selected based on squamous cell histology (8070 and 8071). Our study cohort included patients diagnosed between 2004 and 2014. Patients were selected based on invasive pathology defined as T1b-4, lack or unilateral low volume inguinal adenopathy (N0/x-N1), known surgical excision (partial or radical penectomy) and known lymph node procedure. Patients who died within 30 days of diagnosis, had clinical N2–3 nodal involvement or metastatic disease on presentation, did not receive treatment at the reporting hospital, or had a history of a prior malignancy were excluded from the study (Figure 1). In cases where pathological stage was not available clinical stage was used. To further limit any coding errors, only patients with a reported nodal count were included in the lymphadenectomy group, as well as excluding any patient with a lymph node count from the non-lymphadenectomy group. Our primary outcome measures were receipt of inguinal lymphadenectomy and overall survival.
Figure 1.
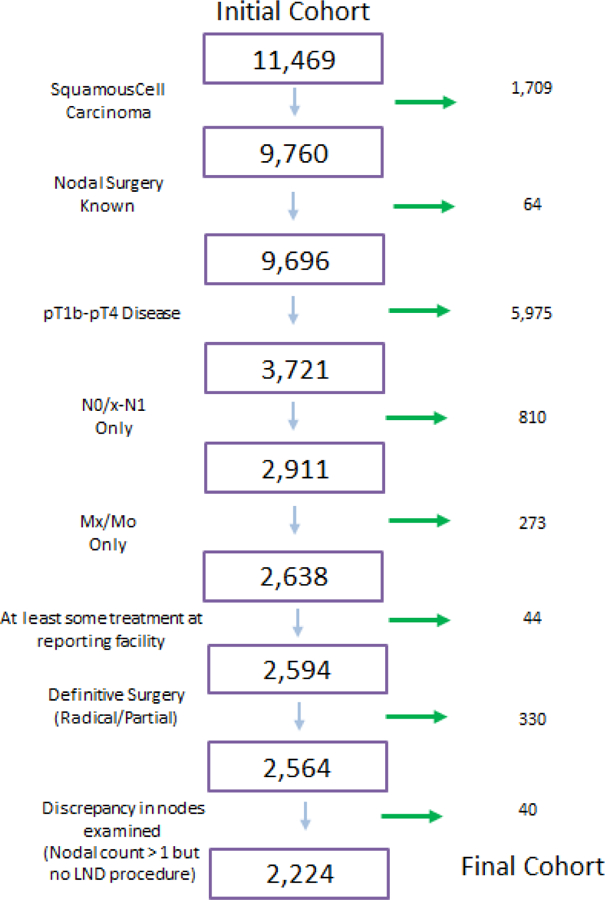
Consort Diagram Depicting Inclusion Criteria for the analyzed cohort
Covariates
Patients were evaluated using clinical, demographic, and pathologic characteristics available in the NCDB. Variables included patient characteristics such as year of diagnosis, age, race, Charlson-Deyo comorbidity classification 9, insurance status, median household income, and demographic location. Disease characteristics included pathological stage and grade, clinical and pathological nodal stage, and receipt of I-LND. Hospital characteristics included facility type and location.
Statistical Analysis
Temporal trends in the use of I-LND were assessed from 2004–2014 using Cochran-Armitage trend tests. We tested the association between I-LND and patient/tumor characteristics using Chi-squared tests. We then used multivariate logistic regression to simultaneously examine the association between all variables with the receipt of I-LND, accounting for within-hospital correlation with robust standard errors. Cox-proportional hazards regression analyses were then used to assess the impact of clinical and pathological variables on overall survival. We then conducted a propensity score weighted analysis 10 to assess the effect of I-LND in overall survival (OS). We estimated the propensity score via logistic regression, including all pre-treatment covariates, and using spline terms for age and year of diagnosis. Patients outside of the area of common support were dropped from the analysis, and we assessed balance after applying inverse probability weighting to the final cohort. We then used weighted Kaplan-Meier curves and a weighted Cox regression model to test the association between I-LND and survival. All statistical analyses were done with SAS version 9.4 and R version 3.33, with p <0.05 considered statistically significant.
Results:
We identified 2,224 men that met study inclusion criteria (Table 1). The cohort consistent mostly of older (median age 69), Caucasian (85.3%) men with a low comorbidity index (CCI 0: 65.7%). The majority of patients were treated in a comprehensive community (35.5%) or Academic/Research (45.9%) facility.
Table 1.
Sociodemographic and Clinical Characteristics of 2224 patients with pT1b-4 cN0/x-N1 Penile Squamous Cell Carcinoma
| Patient Characteristic | All Patients | (%) | I-LND | (%) | I-LND | (%) | p-value |
|---|---|---|---|---|---|---|---|
| Performed | Not Performed | ||||||
| Age | <0.001 | ||||||
| <50 | 233 | 10.5 | 99 | 42.5 | 134 | 57.5 | |
| 51–60 | 369 | 16.6 | 152 | 41.2 | 217 | 58.8 | |
| 61–70 | 539 | 24.2 | 171 | 31.7 | 368 | 68.3 | |
| 71+ | 1083 | 48.7 | 184 | 17.0 | 899 | 83.0 | |
| Race | 0.986 | ||||||
| White | 1898 | 85.3 | 516 | 27.2 | 1382 | 72.8 | |
| Black | 231 | 10.4 | 64 | 27.7 | 167 | 72.3 | |
| Other/Unknown | 95 | 4.3 | 26 | 27.4 | 69 | 72.6 | |
| Hispanic | 0.014 | ||||||
| No | 1846 | 83.0 | 487 | 26.4 | 1359 | 73.6 | |
| Yes | 259 | 11.6 | 90 | 34.7 | 169 | 65.3 | |
| Unknown | 119 | 5.4 | 29 | 24.4 | 90 | 75.6 | |
| Charlson-Denyo Score | 0.009 | ||||||
| 0 | 1461 | 65.7 | 416 | 28.5 | 1045 | 71.5 | |
| 1 | 564 | 25.4 | 154 | 27.3 | 410 | 72.7 | |
| 2 | 199 | 8.9 | 36 | 18.1 | 163 | 81.9 | |
| Insurance Status | <0.001 | ||||||
| Not Insured | 162 | 7.3 | 60 | 37.0 | 102 | 63.0 | |
| Private Insurance | 553 | 24.9 | 193 | 34.9 | 360 | 65.1 | |
| Medicaid | 156 | 7.0 | 51 | 32.7 | 105 | 67.3 | |
| Medicare | 1268 | 57.0 | 268 | 21.1 | 1000 | 78.9 | |
| Other Government | 27 | 1.2 | 10 | 37.0 | 17 | 63.0 | |
| Unknown | 58 | 2.6 | 24 | 41.4 | 34 | 58.6 | |
| Median Income | 0.489 | ||||||
| < 30,000 | 497 | 22.3 | 133 | 26.8 | 364 | 73.2 | |
| 30,000 – 34,999 | 625 | 28.1 | 173 | 27.7 | 452 | 72.3 | |
| 35,000 – 45,999 | 563 | 25.3 | 156 | 27.7 | 407 | 72.3 | |
| 46,000 + | 513 | 23.1 | 141 | 27.5 | 372 | 72.5 | |
| Unknown | 26 | 1.2 | 3 | 11.5 | 23 | 88.5 | |
| High School Graduate | 0.166 | ||||||
| 29% or more | 526 | 23.7 | 159 | 30.2 | 367 | 69.8 | |
| 20% - 28.9% | 664 | 29.9 | 171 | 25.8 | 493 | 74.2 | |
| 14% – 19.9% | 626 | 28.1 | 175 | 28.0 | 451 | 72.0 | |
| < 14% | 384 | 17.3 | 98 | 25.5 | 286 | 74.5 | |
| Unknown | 24 | 1.1 | 3 | 12.5 | 21 | 87.5 | |
| Demographic Location | 0.036 | ||||||
| Large Metropolitan | 955 | 42.9 | 286 | 29.9 | 669 | 70.1 | |
| Small Metropolitan | 690 | 31.0 | 166 | 24.1 | 524 | 75.9 | |
| Suburban | 307 | 13.8 | 87 | 28.3 | 220 | 71.7 | |
| Rural | 200 | 9.0 | 54 | 27.0 | 146 | 73.0 | |
| Unknown | 72 | 3.2 | 13 | 18.1 | 59 | 81.9 | |
| Facility Type* | <0.001 | ||||||
| Community Cancer | 194 | 8.7 | 24 | 12.4 | 170 | 87.6 | |
| Comprehensive Community | 762 | 34.3 | 113 | 14.8 | 649 | 85.2 | |
| Academic/Research | 984 | 44.2 | 389 | 39.5 | 595 | 60.5 | |
| Integrated Cancer Network | 206 | 9.3 | 42 | 20.4 | 164 | 79.6 | |
| Facility Location by Region* | 0.269 | ||||||
| New England | 149 | 6.7 | 26 | 17.4 | 123 | 82.6 | |
| Mid Atlantic | 307 | 13.8 | 82 | 26.7 | 225 | 73.3 | |
| South Atlantic | 486 | 21.9 | 127 | 26.1 | 359 | 73.9 | |
| East North Central | 380 | 17.1 | 103 | 27.1 | 277 | 72.9 | |
| East South Central | 199 | 8.9 | 61 | 30.7 | 138 | 69.3 | |
| West North Central | 167 | 7.5 | 44 | 26.3 | 123 | 73.7 | |
| West South Central | 188 | 8.5 | 54 | 28.7 | 134 | 71.3 | |
| Mountain | 87 | 3.9 | 19 | 21.8 | 68 | 78.2 | |
| Pacific | 183 | 8.2 | 52 | 28.4 | 131 | 71.6 | |
| Pathological T-Stage | 0.070 | ||||||
| T1b | 122 | 5.5 | 21 | 17.2 | 101 | 82.8 | |
| T2 | 1354 | 60.9 | 371 | 27.4 | 983 | 72.6 | |
| T3 | 703 | 31.6 | 200 | 28.4 | 503 | 71.6 | |
| T4 | 45 | 2.0 | 14 | 31.1 | 31 | 68.9 | |
| Tumor Grade | 0.364 | ||||||
| 1 & 2 | 1520 | 68.3 | 428 | 28.2 | 1092 | 71.8 | |
| 3 & 4 | 619 | 27.8 | 156 | 25.2 | 463 | 74.8 | |
| Unknown | 85 | 3.8 | 22 | 25.9 | 63 | 74.1 | |
| Clinical Nodal Stage | <0.001 | ||||||
| N0/x | 2094 | 94.2 | 518 | 24.7 | 1576 | 75.3 | |
| N1 | 130 | 5.8 | 88 | 67.7 | 42 | 32.3 | |
| Year of Diagnosis | 0.001 | ||||||
| 2004 | 129 | 5.8 | 30 | 23.3 | 99 | 76.7 | |
| 2005 | 151 | 6.8 | 33 | 21.9 | 118 | 78.1 | |
| 2006 | 165 | 7.4 | 38 | 23.0 | 127 | 77.0 | |
| 2007 | 188 | 8.5 | 46 | 24.5 | 142 | 75.5 | |
| 2008 | 196 | 8.8 | 42 | 21.4 | 154 | 78.6 | |
| 2009 | 196 | 8.8 | 57 | 29.1 | 139 | 70.9 | |
| 2010 | 215 | 9.7 | 63 | 29.3 | 152 | 70.7 | |
| 2011 | 226 | 10.2 | 60 | 26.5 | 166 | 73.5 | |
| 2012 | 228 | 10.3 | 76 | 33.3 | 152 | 66.7 | |
| 2013 | 248 | 11.2 | 67 | 27.0 | 181 | 73.0 | |
| 2014 | 282 | 12.7 | 94 | 33.3 | 188 | 66.7 | |
Missing information in 78 patients
606 (27.2%) men underwent lymphadenectomy over the study period (2004–2014). I-LND utilization showed a steady increased over the study period (Figure 2), with 33.6% of eligible patients receiving I-LND in 2014 compared to 23.3% in 2004 (p<0.001). When comparing patients who underwent an I-LND and those who did not, significant differences were seen with respect to age (<0.001), Hispanic ethnicity (p=0.014), Charlson-Deyo score (p=0.009), insurance status (p<0.001), Demographic location (p=0.036), treatment facility (p<0.001), and nodal status (p<0.001), as shown in Table 1.
Figure 2.
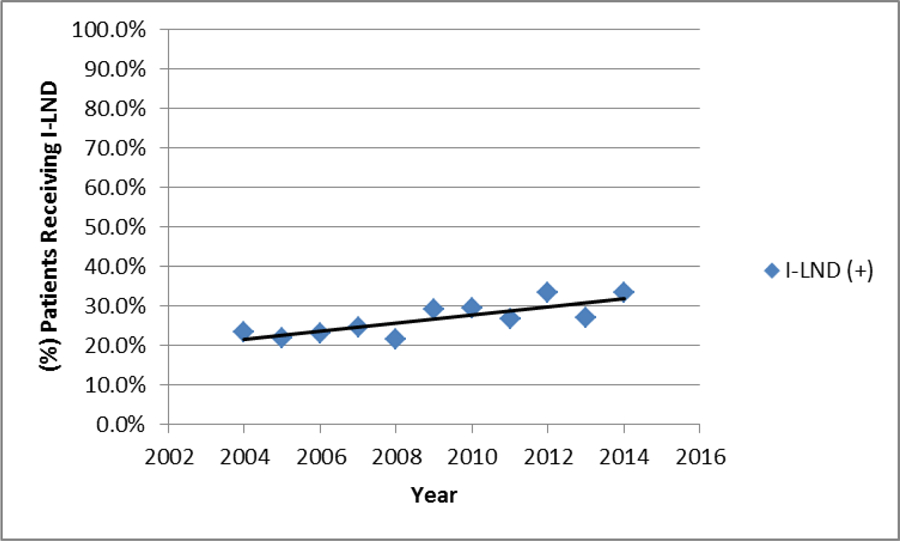
Graph depicting the percentage of patient with pTlb-4 cNO/x recieving Inguinal Lymaphenectomy (l-LND)
Following adjustment, I-LND was more likely to be performed in patients treated in an Academic/Research facility (OR 4.22 [CI 2.61–6.81], p < 0.001), presenting with palpable unilateral inguinal adenopathy (cN1) (OR 5.59 [CI 3.71–8.42], p < 0.001), and in those with a more contemporary diagnosis (OR 1.06 [CI 1.02–1.10], p< 0.001). In comparison, utilization of I-LND was less likely to occur with increased age (p <0.001), especially for those ≥ 71 years old (OR 0.41 [CI 0.27–0.62], p < 0.001) and those presenting with T1b pathology (OR 0.41 [CI 0.24–.071], p = 0.002). A similar but not statistically significant trend (p = 0.091) was seen in regards to worsening comorbidity, with those presenting with severe comorbidity (CCI-2) being less likely to receive I-LND than CCI-0 (OR 0.64 [CI 0.42–.098), p = 0.040), shown in Table 2.
Table 2.
Multivariate Regression Analysis for Receipt of Lymphadenectomy
| Patient Characteristics | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Age | <0.001 | |||
| <50 | Ref | Ref | Ref | Ref |
| 51–60 | 1.15 | 0.78 | 1.71 | 0.470 |
| 61–70 | 0.99 | 0.65 | 1.51 | 0.972 |
| 71+ | 0.43 | 0.29 | 0.65 | <0.001 |
| Race | 0.076 | |||
| White | Ref | Ref | Ref | Ref |
| Black | 0.93 | 0.65 | 1.32 | 0.687 |
| Unknown/Other | 0.59 | 0.37 | 0.94 | 0.025 |
| Hispanic | 0.962 | |||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.05 | 0.74 | 1.50 | 0.788 |
| Unknown | 1.02 | 0.63 | 1.64 | 0.940 |
| Charlson-Denyo Score | 0.091 | |||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 0.97 | 0.79 | 1.19 | 0.765 |
| 2 | 0.64 | 0.42 | 0.98 | 0.040 |
| Insurance Status | 0.818 | |||
| Private Insurance | Ref | Ref | Ref | Ref |
| Not Insured | 0.91 | 0.61 | 1.36 | 0.657 |
| Medicaid | 0.78 | 0.51 | 1.19 | 0.246 |
| Medicare | 0.93 | 0.69 | 1.25 | 0.645 |
| Other Government | 0.94 | 0.43 | 2.05 | 0.876 |
| Unknown | 1.26 | 0.64 | 2.48 | 0.512 |
| Median Income | 0.655 | |||
| < 30,000 | Ref | Ref | Ref | Ref |
| 30,000 – 34,999 | 1.15 | 0.83 | 1.59 | 0.414 |
| 35,000 – 45,999 | 1.19 | 0.85 | 1.66 | 0.318 |
| 46,000 + | 1.31 | 0.85 | 2.03 | 0.218 |
| High School Graduate | 0.474 | |||
| 29% or more | Ref | Ref | Ref | Ref |
| 20% – 28.9% | 0.87 | 0.63 | 1.21 | 0.411 |
| 14% – 19.9% | 1.05 | 0.74 | 1.49 | 0.795 |
| <14% | 0.84 | 0.52 | 1.36 | 0.483 |
| Demographic Location | 0.489 | |||
| Rural | Ref | Ref | Ref | Ref |
| Large Metropolitan | 1.10 | 0.68 | 1.78 | 0.689 |
| Small Metropolitan | 0.98 | 0.64 | 1.51 | 0.939 |
| Suburban | 1.07 | 0.64 | 1.79 | 0.784 |
| Unknown | 0.56 | 0.21 | 1.49 | 0.249 |
| Facility Type | <0.001 | |||
| Community Cancer | Ref | Ref | Ref | Ref |
| Comprehensive Community | 1.23 | 0.75 | 2.02 | 0.403 |
| Academic/Research | 4.04 | 2.53 | 6.45 | <0.001 |
| Integrated Cancer Network | 1.75 | 0.93 | 3.27 | 0.082 |
| Facility Location | 0.384 | |||
| New England | Ref | Ref | Ref | Ref |
| Mid Atlantic | 1.19 | 0.68 | 2.10 | 0.538 |
| South Atlantic | 1.58 | 0.90 | 2.77 | 0.110 |
| East North Central | 1.14 | 0.63 | 2.08 | 0.671 |
| East South Central | 2.21 | 1.10 | 4.43 | 0.025 |
| West North Central | 1.16 | 0.57 | 2.35 | 0.684 |
| West South Central | 1.38 | 0.72 | 2.65 | 0.329 |
| Mountain | 1.23 | 0.54 | 2.79 | 0.623 |
| Pacific | 1.63 | 0.90 | 2.97 | 0.107 |
| Pathological T-Stage | 0.005 | |||
| T2 | Ref | Ref | Ref | Ref |
| T1b | 0.41 | 0.24 | 0.71 | 0.002 |
| T3 | 0.93 | 0.75 | 1.15 | 0.500 |
| T4 | 0.65 | 0.33 | 1.28 | 0.211 |
| Tumor Grade | 0.421 | |||
| 1 & 2 | Ref | Ref | Ref | Ref |
| 3 & 4 | 1.11 | 0.87 | 1.40 | 0.399 |
| Unknown | 0.79 | 0.48 | 1.31 | 0.363 |
| Clinical N-Stage | <0.001 | |||
| N0 | Ref | Ref | Ref | Ref |
| N1 | 5.59 | 3.71 | 8.42 | <0.001 |
| Year of Diagnosis | 0.002 | |||
| Trend from 2004–2014 | 1.06 | 1.02 | 1.11 | 0.001 |
On survival analyses, increasing pathological stage (pT3: HR 1.22 [CI 1.06–1.40], p=0.006; pT4: HR 2.32 [CI 1.51–3.55], p<0.001), pN1 disease (HR 1.49 [CI 1.16–1.92], p=0.011) high grade disease (HR 1.46 [CI 1.27–1.69]. p < 0.001), increasing age (≥ 71) (HR 1.72 [CI 1.20–2.46], p = 0.003) and worsening comorbidity (CCI-1: HR 1.43 [CI 1.22–1.68] CCI-2: HR 1.91 [CI 1.52–2.39], p < 0.001 for both) were all associated with decreased survival. Receipt of I-LND (HR 0.59 [CI 0.48–0.72], p < 0.001) and Hispanic ethnicity (HR 0.68 [CI 0.51–0.92], p = 0.039) were both associated with improved survival, as shown in Table 3. Following propensity score adjustment, receipt of I-LND continued to show a survival benefit in the study cohort (HR 0.79 [CI 0.74–0.84], p < 0.001), as shown in Figure 3.
Table 3.
Cox Multivariate Regression Analysis for Overall Survival
| Patient Characteristics | HR | 95% CI | p-value | |
|---|---|---|---|---|
| Age | <0.001 | |||
| <50 | Ref | Ref | Ref | Ref |
| 51–60 | 0.97 | 0.68 | 1.38 | 0.843 |
| 61–70 | 1.06 | 0.74 | 1.51 | 0.755 |
| 71+ | 1.72 | 1.20 | 2.46 | 0.003 |
| Race | 0.337 | |||
| White | Ref | Ref | Ref | Ref |
| Black | 1.19 | 0.93 | 1.51 | 0.165 |
| Unknown/Other | 1.13 | 0.77 | 1.66 | 0.539 |
| Hispanic | 0.039 | |||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.68 | 0.51 | 0.92 | 0.012 |
| Unknown | 1.03 | 0.79 | 1.34 | 0.835 |
| Charlson-Denyo Score | <0.001 | |||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 1.43 | 1.22 | 1.68 | < 0.001 |
| 2 | 1.91 | 1.52 | 2.39 | < 0.001 |
| Insurance Status | 0.964 | |||
| Private Insurance | Ref | Ref | Ref | Ref |
| Not Insured | 0.97 | 0.68 | 1.38 | 0.860 |
| Medicaid | 1.05 | 0.74 | 1.48 | 0.794 |
| Medicare | 1.06 | 0.87 | 1.29 | 0.558 |
| Other Government | 1.19 | 0.62 | 2.26 | 0.604 |
| Unknown | 0.88 | 0.54 | 1.42 | 0.599 |
| Median Income | 0.746 | |||
| < 30,000 | Ref | Ref | Ref | Ref |
| 30,000 – 34,999 | 0.89 | 0.71 | 1.11 | 0.311 |
| 35,000 – 45,999 | 0.92 | 0.70 | 1.20 | 0.531 |
| 46,000 + | 0.95 | 0.70 | 1.30 | 0.767 |
| High School Graduate | 0.738 | |||
| 29% or more | Ref | Ref | Ref | Ref |
| 20% – 28.9% | 0.89 | 0.71 | 1.10 | 0.274 |
| 14% – 19.9% | 0.90 | 0.70 | 1.16 | 0.415 |
| <14% | 0.93 | 0.69 | 1.25 | 0.627 |
| Demographic Location | 0.712 | |||
| Rural | Ref | Ref | Ref | Ref |
| Large Metropolitan | 0.92 | 0.70 | 1.21 | 0.551 |
| Small Metropolitan | 0.85 | 0.66 | 1.09 | 0.206 |
| Suburban | 0.89 | 0.66 | 1.19 | 0.415 |
| Unknown | 0.90 | 0.59 | 1.37 | 0.627 |
| Facility Type | 0.572 | |||
| Community Cancer | Ref | Ref | Ref | Ref |
| Comprehensive Community | 1.15 | 0.90 | 1.48 | 0.266 |
| Academic/Research | 0.89 | 0.69 | 1.15 | 0.365 |
| Integrated Cancer Network | 1.06 | 0.76 | 1.48 | 0.745 |
| Facility Location | 0.272 | |||
| New England | Ref | Ref | Ref | Ref |
| Mid Atlantic | 1.19 | 0.86 | 1.64 | 0.306 |
| South Atlantic | 1.35 | 0.98 | 1.84 | 0.063 |
| East North Central | 1.46 | 1.06 | 2.00 | 0.019 |
| East South Central | 1.39 | 0.96 | 2.00 | 0.081 |
| West North Central | 1.26 | 0.87 | 1.80 | 0.220 |
| West South Central | 1.13 | 0.77 | 1.67 | 0.533 |
| Mountain | 1.56 | 1.06 | 2.30 | 0.024 |
| Pacific | 1.28 | 0.88 | 1.85 | 0.193 |
| Pathological T-Stage | <0.001 | |||
| T2 | Ref | Ref | Ref | Ref |
| T1b | 0.89 | 0.58 | 1.34 | 0.5649 |
| T3 | 1.22 | 1.06 | 1.40 | 0.0063 |
| T4 | 2.32 | 1.51 | 3.55 | < 0.001 |
| Tumor Grade | <0.001 | |||
| 1 & 2 | Ref | Ref | Ref | Ref |
| 3 & 4 | 1.46 | 1.27 | 1.69 | < 0.001 |
| Unknown | 1.08 | 0.77 | 1.52 | 0.6482 |
| Pathological N-Stage | 0.002 | |||
| N0 | Ref | Ref | Ref | Ref |
| N1 | 1.49 | 1.16 | 1.92 | 0.0018 |
| Inguinal Lymphadenectomy | <0.001 | |||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.59 | 0.48 | 0.72 | < 0.001 |
| Year of Diagnosis | 0.605 | |||
| Trend from 2004–2014 | 1.01 | 0.98 | 1.04 | 0.6054 |
Figure 3.
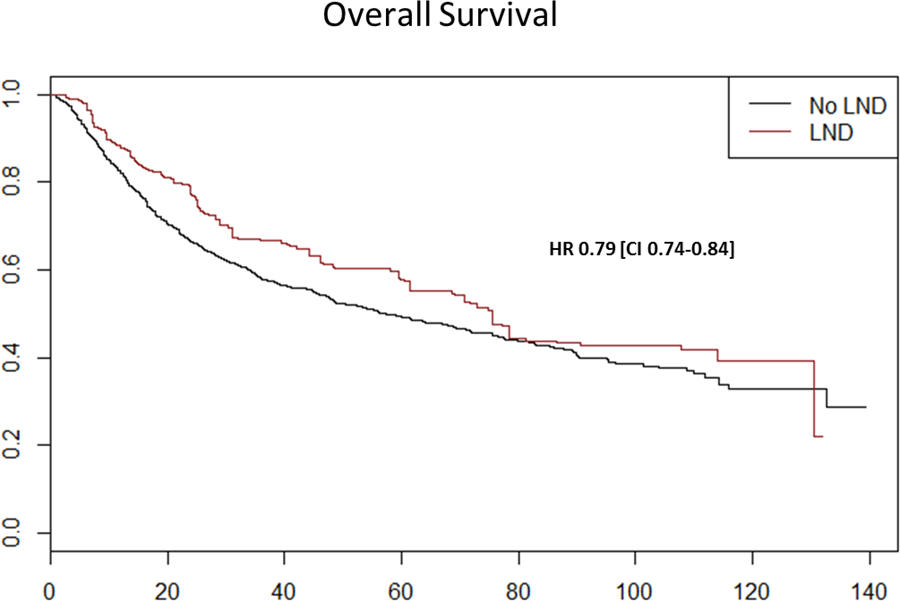
Kaplan-Meier survival curves of overall survival of penile cancer patients as function of receipt of lymphadenectomy after propensity score weighting
Discussion:
The impact of inguinal lymphadenectomy (I-LND) on the survival of patients with invasive penile cancer has been recognized as early as 1986 11. Despite the early evidence and numerous confirming retrospective studies 2,12, I-LND in the US remains underutilized4,5,13. The main hesitancy for the use of I-LND is the significant comorbidity associated with the procedure, which has a reported complication rate of 14%–37% 14,15. Despite strong recommendations in both the NCCN7 and EUA16 guidelines, the use of I-LND in patients with high grade or invasive penile cancer and cN0–1 disease remains controversial, mainly due to fear of overtreatment leading to the use of surveillance strategies and fine needle aspiration17,18 in the hope to improve patient selection. In this observational cohort, consisting of patients with an absolute indication for I-LND per NCCN guidelines (T1b-4 N0/x-1), I-LND was found to have significant impact on survival with a difference in median overall survival of 18.1 months (75.5 months (+) I-LND vs. 57.4 months (−) I-LND).
In our cohort, the rate of lymphadenectomy was calculated at 27.2% over a 10-year period. Although the rate is alarming low, these findings are consistent with those reported by Chippollini et al5 (NCDB) and Johnson et al13 (SEER), who using similar patient cohorts calculated comparable lymphadenectomy rates of 19.6% and 26.5%, respectively. Marginal I-LND utilization is not specific to penile cancer with vulvar cancer19 (20.4%) and lower extremity melanoma20 (39%) reporting similar sub-standard rates. Encouraging, was the increase use I-LND over the study period (Figure 2), which is likely related to less ambiguous recommendations with the introduction of treatment guidelines7,16, emphasis in centralization of care5,21 and the introduction of minimally invasive techniques geared towards improvement of post-operative complications 22
Utilization of I-LND was more likely to occur in younger patients (p < 0.001), those with a more contemporary diagnosis (p = 0.02), and those treated in an Academic/Research facility (p < 0.001). In contrast, I-LND utilization did not increase with worsening pathological stage, and the likelihood of receiving I-LND was decreased for those presenting with T1b lesions (OR 0.41 [CI 0.24–.071], p = 0.002). These findings are worrisome given numerous reports demonstrating the increased incidence of lymphoid metastases associated with lympho-vascular invasion23 and poorly differentiated cancers23, which are features that define T1b lesions per AJCC TNM staging system24. Furthermore, lymphadenectomy was more common for those presenting with clinically palpable adenopathy (cN1:73.9% vs cN0/x: 23.1%, p < 0.001), demonstrating an erroneous risk adapted approach given the far greater benefit seen in those with microscopic nodal disease25. Interestingly, comorbidity index (p = 0.079), median household income (p = 0.841), demographic location (p = 0.656), or education level (p = 0.738) had no effect in on I-LND utilization, which have been preconceived factors related to access to appropriate care in patients with penile cancer, who often present from rural, and underserved areas.
Similar observations were noted by Bilimoria et al20, when lymphadenectomy utilization was assessed in melanoma patients. Using the NCDB database, the authors demonstrated that utilization of lymphadenectomy decreased with advancing age, treatment in community versus academic/research facility and lesion location (lower extremities). As with penile cancer, increasing comorbidity, insurance type, household income, demographic location and race had no effect on lymphadenectomy utilization. Together, the data suggests that the low utilization of I-LND is likely related to physician experience and comfort rather patient factors; emphasizing the need to centralize care for those presenting with rare malignancies21,26. This is supported by a recent report in which the adherence to EAU penile guidelines was studied in an Italian population27; where the care of penile cancer patients is concentrated at eight tertiary care centers. The study showed that overall adherence to EUA guideline recommendations occurred in 66% of the cases, with an I-LND utilization rate of 70%.
Our survival analyses demonstrated that receipt of lymphadenectomy and Hispanic ethnicity were both associated with improved survival; whereas, increasing age, worsening comorbidity, higher pathological stage, high grade disease, and pathological nodal disease were all associated with decreased survival. Following adjustment for known confounders using propensity score weighting, receipt of I-LND continued to have a beneficial effect on overall survival (HR 0.79 [CI 0.74–0.84], p < 0.001). The above findings are of great importance as it validates the findings of several small retrospective reports 2,3,12 on the importance of I-LND in the survival of patients with invasive penile cancer using observational data from a large national cancer registry. Penile cancer remains one of the few disease processes, along with testis cancer 28, in which a modifiable factor such as lymphadenectomy is proven to provide a survival advantage. As such, we must emphasize the use of I-LND as an important quality metric for the management of patients with invasive penile cancer.
Despite its strengths our study is not devoid of limitations. First, large retrospective databases are prone to coding errors, which must be addressed carefully prior to data analysis. We attempted to control for coding errors by excluding patients with a reported lymph node procedure but no documented lymph node count; as well as, any patient with a lymph node count but no documented lymph node procedure. Given the alarming low rate of I-LND utilization calculated from the NCDB database (27.2%), one must wonder if this number represents a true practice representation versus a lack of data capture from the NCDB database. Lymphadenectomy procedures in penile cancer compared to other malignancies may present a coding issue for the NCDB as these procedures are usually performed in staged fashion. The NCDB database only tracks procedures performed in a COC-approved facility and those that pertain to the initial cancer treatment29 ; therefore, a staged lymphadenectomy performed in a non-COC facility or miss-categorize as a recurrence could potentially be left out by the coders leading to the under reporting of I-LND in the database. Despite, the role that miscoding or under reporting may have played in low I-LND rates reported using the NCDB database, the comparable rates reported using the SEER-Medicare13 warn of a possible practice phenomenon which we must continue to study if we hope to improve the care of penile cancer patients. Second, the use of the NCDB database may lead to sampling bias as the NCDB only queries records from CoC-approved facilities, which may affect the generalizability of our results. Review of other disease sites by Mettlin et al 30, has shown that patient and disease treatment characteristics between NCDB and SEER (a national representative sample) are comparable which may mitigate this bias. Third, the overall improvement on overall survival seen in patients receiving lymphadenectomy may be a function of younger and healthier patients undergoing the procedure. To minimize this potential bias in our survival analysis we used propensity adjustment to reduce impact of known confounding variables 10, however, it is possible that residual bias from unmeasured factors may remain. Finally, the NCDB database does not allow for assessment of patient choice on whether to undergo or forgo lymphadenectomy as part of their treatment plan. We attempted to reduce this bias by including only patients in whom a lymphadenectomy is an absolute indication (T1b-4 N0/x-1) in all existing best practice guidelines7,16.
Conclusion:
In hospitals reporting to NCDB, the overall rate of I-LND utilization for invasive penile cancer was only 27.2%. The use of I-LND saw a statistically significant increase over the study period from 23.3% in 2004 to 33.6% in 2014, which is encouraging. There was a significant increase in median overall survival in patients who received I-LND, justifying use of I-LND utilization an important quality metric for performance reporting in patients with invasive penile cancer.
Footnotes
Financial Sources: None
Conflicts of Interest: None
Andres F. Correa, MD: No financial disclosures, No Disclosures
Elizabeth Handorf, PhD: No financial disclosures
Shreyas S. Joshi, MD: No financial disclosures
Daniel M. Geynisman, MD: No financial disclosures
Alexander Kutikov, MD: No financial disclosures
David Y. Chen, MD: No financial disclosures
Robert G. Uzzo, MD: No financial disclosures
Rosalia Viterbo, MD: No financial disclosures
Richard E. Greenberg, MD: No financial disclosures
Marc C. Smaldone, MD, MSHP: No financial disclosures
Contributor Information
Andres F. Correa, Department of Surgical Oncology, Division of Urologic Oncology, Fox Chase Cancer Center, Philadelphia PA; Department of Urology Creighton University, Omaha NE.
Elizabeth Handorf, Department of Biostatistics and Bioinformatics, Fox Chase Cancer Center, Philadelphia PA.
Shreyas S. Joshi, Department of Surgical Oncology, Division of Urologic Oncology, Fox Chase Cancer Center, Philadelphia PA.
Daniel M. Geynisman, Department of Hematology and Oncology, Division Genitourinary Oncology, Fox Chase Cancer Center, Philadelphia PA.
References:
- 1.ACS. What Are the Key Statistics About Penile Cancer? 2017; https://www.cancer.org/cancer/penile-cancer/about/key-statistics.html. Accessed March 21, 2017, 2017.
- 2.Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. The Journal of urology. March 2005;173(3):816–819. [DOI] [PubMed] [Google Scholar]
- 3.Leijte JA, Kirrander P, Antonini N, Windahl T, Horenblas S. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. European urology. July 2008;54(1):161–168. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RA, Slopnick EA, Ferry EK, Zhu H, Kim SP, Abouassaly R. Disparity between pre-existing management of penile cancer and NCCN guidelines. Urologic oncology. March 28 2017. [DOI] [PubMed]
- 5.Chipollini J, Tang DH, Sharma P, Baumgarten AS, Spiess PE. Patterns of Regional Lymphadenectomy for Clinically Node-negative Patients With Penile Carcinoma: Analysis From the National Cancer Database From 1998 to 2012. Clin Genitourin Cancer. April 26 2017. [DOI] [PubMed]
- 6.Protzel C, Alcaraz A, Horenblas S, Pizzocaro G, Zlotta A, Hakenberg OW. Lymphadenectomy in the surgical management of penile cancer. European urology. May 2009;55(5):1075–1088. [DOI] [PubMed] [Google Scholar]
- 7.Clark PE, Spiess PE, Agarwal N, et al. Penile Cancer: Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11(5):594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. Journal of surgical oncology. January 2004;85(1):1–3. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. June 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 10.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Statistics in medicine. July 20 2013;32(16):2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDougal WS, Kirchner FK Jr., Edwards RH, Killion LT. Treatment of carcinoma of the penis: the case for primary lymphadenectomy. The Journal of urology. July 1986;136(1):38–41. [DOI] [PubMed] [Google Scholar]
- 12.Lont AP, Horenblas S, Tanis PJ, Gallee MP, van Tinteren H, Nieweg OE. Management of clinically node negative penile carcinoma: improved survival after the introduction of dynamic sentinel node biopsy. The Journal of urology. September 2003;170(3):783–786. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TV, Hsiao W, Delman KA, Jani AB, Brawley OW, Master VA. Extensive inguinal lymphadenectomy improves overall 5-year survival in penile cancer patients: results from the Surveillance, Epidemiology, and End Results program. Cancer. June 15 2010;116(12):2960–2966. [DOI] [PubMed] [Google Scholar]
- 14.Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson Cancer Center Experience. The Journal of urology. April 2002;167(4):1638–1642. [PubMed] [Google Scholar]
- 15.Bouchot O, Rigaud J, Maillet F, Hetet JF, Karam G. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. European urology. June 2004;45(6):761–765; discussion 765–766. [DOI] [PubMed] [Google Scholar]
- 16.Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N. EAU Guidelines on Penile Cancer: 2014 Update. European urology.67(1):142–150. [DOI] [PubMed] [Google Scholar]
- 17.Senthil Kumar MP, Ananthakrishnan N, Prema V. Predicting regional lymph node metastasis in carcinoma of the penis: a comparison between fine-needle aspiration cytology, sentinel lymph node biopsy and medial inguinal lymph node biopsy. British journal of urology. March 1998;81(3):453–457. [DOI] [PubMed] [Google Scholar]
- 18.Saisorn I, Lawrentschuk N, Leewansangtong S, Bolton DM. Fine-needle aspiration cytology predicts inguinal lymph node metastasis without antibiotic pretreatment in penile carcinoma. BJU Int. June 2006;97(6):1225–1228. [DOI] [PubMed] [Google Scholar]
- 19.Am S, Lc H, El T. Demographic, Clinical, and Treatment Trends Among Women Diagnosed with Vulvar Cancer in the U.S. Gynecologic oncology. December/21 2008;108(3):577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Balch CM, Bentrem DJ, et al. Complete lymph node dissection for sentinel node-positive melanoma: assessment of practice patterns in the United States. Annals of surgical oncology. June 2008;15(6):1566–1576. [DOI] [PubMed] [Google Scholar]
- 21.Matulewicz RS, Flum AS, Helenowski I, et al. Centralization of Penile Cancer Management in the United States: A Combined Analysis of the American Board of Urology and National Cancer Data Base. Urology. April 2016;90:82–88. [DOI] [PubMed] [Google Scholar]
- 22.Tobias-Machado M, Tavares A, Ornellas AA, Molina WR Jr., Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. The Journal of urology. March 2007;177(3):953–957; discussion 958. [DOI] [PubMed] [Google Scholar]
- 23.Slaton JW, Morgenstern N, Levy DA, et al. TUMOR STAGE, VASCULAR INVASION AND THE PERCENTAGE OF POORLY DIFFERENTIATED CANCER: INDEPENDENT PROGNOSTICATORS FOR INGUINAL LYMPH NODE METASTASIS IN PENILE SQUAMOUS CANCER. The Journal of urology. 2001/04/01/ 2001;165(4):1138–1142. [PubMed] [Google Scholar]
- 24.Society AC. How Is Penile Cancer Staged? 2016; https://www.cancer.org/cancer/penile-cancer/detection-diagnosis-staging/staging.html, 2017.
- 25.Aita G, da Costa WH, de Cassio Zequi S, et al. Pattern of invasion is the most important prognostic factor in patients with penile cancer submitted to lymph node dissection and pathological absence of lymph node metastasis. BJU Int. October 2015;116(4):584–589. [DOI] [PubMed] [Google Scholar]
- 26.Derbel O, Heudel PE, Cropet C, et al. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort). PLoS ONE. 02/03 07/14/received 06/15/accepted 2017;12(2):e0158406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cindolo L, Bada M, Nyirady P, et al. THE ADHERENCE TO THE EAU GUIDELINES ON PENILE CANCER TREATMENT COULD INFLUENCE THE SURVIVAL: MULTICENTER, RETROSPECTIVE, EUROPEAN STUDY. AUA National Meeting 2017; Boston, MA. [Google Scholar]
- 28.Stephenson AJ, Sheinfeld J. Management of patients with low-stage nonseminomatous germ cell testicular cancer. Current treatment options in oncology. September 2005;6(5):367–377. [DOI] [PubMed] [Google Scholar]
- 29.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA oncology. February 23 2017. [DOI] [PubMed]
- 30.Mettlin CJ, Menck HR, Winchester DP, Murphy GP. A comparison of breast, colorectal, lung, and prostate cancers reported to the National Cancer Data Base and the Surveillance, Epidemiology, and End Results Program. Cancer. May 15 1997;79(10):2052–2061. [DOI] [PubMed] [Google Scholar]


