Abstract
Purpose
As a means of limiting normal tissue toxicity, proton-beam therapy (PBT) is an emerging radiation modality for glioblastoma (GBM) reirradiation. However, data for recurrent GBM treated with PBT reirradiation is limited. Therefore, we analyzed treatment patterns, toxicities, and clinical outcomes of patients with recurrent GBM treated with PBT reirradiation using the multi-institutional Proton Collaborative Group registry.
Methods and Materials
Prospectively collected data for patients with recurrent GBM who underwent PBT while enrolled in Proton Collaborative Group study 01-009 (NCT01255748) were analyzed. We evaluated overall survival (OS), progression-free survival (PFS), and toxicity. Toxicities were scored per the Common Terminology Criteria for Adverse Events, version 4.0. Descriptive statistics were used to report patient, tumor, and treatment characteristics. Multivariable analyses (MVA) for toxicity were conducted using logistic regression. The Kaplan-Meier method was used to calculate OS and PFS. MVA for OS and PFS was conducted using Cox proportional-hazards models. The SAS statistical software was used for the analysis.
Results
We identified 45 recurrent patients with GBM who underwent PBT reirradiation between 2012 and 2018. The median time between initial GBM diagnosis and recurrence was 20.2 months. The median follow-up time from PBT reirradiation was 10.7 months. Median PFS was 13.9 months (95% confidence interval [CI], 8.23-20.0 months) and median OS was 14.2 months (95% CI, 9.6-16.9 months) after PBT reirradiation. One patient experienced an acute grade 3 toxicity, 4 patients experienced late grade 3 toxicity (no grade ≥4 toxicities). MVA revealed that prior surgery was associated with a 91.3% decreased hazard of death (hazard ratio: 0.087; 95% CI, 0.02-0.42; P < .01). No explanatory variables were associated with PFS or grade 3 toxicities.
Conclusions
This is the largest series to date reporting outcomes for PBT reirradiation of patients with recurrent GBM. Our analysis indicates that PBT is well tolerated and offers efficacy rates comparable with previously reported photon reirradiation.
Introduction
Glioblastoma (GBM) carries a poor prognosis despite modern advances in management, with a median survival of 14 months from the time of initial diagnosis.1, 2, 3 Standard treatment at the time of the initial diagnosis involves maximal safe resection with adjuvant chemoradiation, followed by chemotherapy alone. However, even with optimal management, patients will invariably experience a recurrence after treatment. At the time of recurrence, no standard treatment paradigm exists. Outside of a clinical trial, treatment options include reresection, chemotherapy, tumor-treating fields, reirradiation, or best supportive care.4,5
Although frequently used, reirradiation presents a dosimetric challenge given the tendency for in-/near-field recurrences, proximity to critical organs at risk (OARs), and previous radiation doses (typically 60 Gy) nearing OAR constraints.6,7 Consequently, proton-beam therapy (PBT) is an attractive radiation therapy (RT) modality in the setting of GBM reirradation owing to its inherent properties of improved dose distribution (over photon-based RT) and the resultant mitigation of normal tissue toxicity. Although the existing literature supports the use of photon-based RT for GBM reirradiation,8, 9, 10, 11, 12, 13 data are limited regarding the safety and efficacy of PBT reirradiation for recurrent GBM. Therefore, in the present study, we analyzed treatment patterns, toxicities, and clinical outcomes of patients with recurrent GBM treated with PBT reirradiation while enrolled in a prospective multi-institutional registry study.
Methods and materials
The Proton Collaborative Group (PCG) is a clinical research consortium of 12 proton therapy centers in the United States. Each member institution obtained institutional review board approval to conduct their respective registry studies. For our analysis, we analyzed prospectively collected data for patients with recurrent GBM who underwent PBT while enrolled in PCG study 01-009 (NCT01255748) between 2012 and 2018. We evaluated overall survival (OS), progression-free survival (PFS), and toxicity. Evidence of progression was assessed individually by each member institution. Toxicities were scored per the Common Terminology Criteria for Adverse Events, version 4.0. To allow for a comparison of the different radiation doses and fractionation schemes, we computed an equivalent dose in 2 Gy fractions (EQD2), using an α/β ratio of 10. Descriptive statistics were used to report patient, tumor, and treatment characteristics. A multivariable analysis (MVA) for toxicity was conducted using logistic regression. The Kaplan-Meier method was used to calculate rates of OS and PFS. MVA for OS and PFS were conducted using Cox proportional-hazards models. The statistical program used was SAS (version 9.4; SAS Institute, Cary, NC). All statistical analyses were performed using a P value significance level of 0.05.
Results
Patient and treatment characteristics
Within the PCG registry, 45 patients with an initial GBM diagnosis who developed recurrent GBM and were treated with PBT reirradiation between 2012 and 2018 were identified from 7 proton centers. The patient and treatment characteristics for this cohort are summarized in Table 1. Male and female sexes were equally represented (22 and 23 patients, respectively), and the median age at the time of recurrence was 54 years. The majority of patients had a favorable performance status with 30 patients (66%) having and Eastern Cooperation Oncology Group score of 0 or 1. Sixteen patients (35.5%) were O6-methylguanine-DNA methyltransferase (MGMT) methylated and 16 patients (35.5%) were MGMT unmethylated at the time of the initial diagnosis. MGMT status analysis was not available for 13 patients (29%). With regard to initial treatment, all patients underwent photon RT (n = 45) with a median initial dose of 60 Gy (range, 25-60 Gy). The vast majority of patients underwent prior surgical resection (n = 39) and chemotherapy (n = 41) for their initial diagnosis. The median time between initial diagnosis and time of recurrence was 20 months (range, 3-77 months).
Table 1.
Patient and initial treatment characteristics
| Parameter | All patients (N = 45) |
|
|---|---|---|
| N | % | |
| Sex, n | ||
| Male | 22 | 49% |
| Female | 23 | 51% |
| Age at the time of recurrence, y (range) | ||
| Median | 54 (27-81) | |
| Eastern Cooperative Oncology Group performance status score | ||
| 0-1 | 30 | 66% |
| >1 | 15 | 33% |
| O6-methylguanine-DNA methyltransferase status, n | ||
| Unknown | 13 | 29% |
| Methylated | 16 | 35.5% |
| Unmethylated | 16 | 35.5% |
| Prior resection, n | ||
| Yes | 39 | 87% |
| No | 6 | 13% |
| Prior chemotherapy, n | ||
| Yes | 41 | 91% |
| No | 4 | 9% |
| Prior radiation therapy dose, Gy (range) | ||
| Median | 60 (25-60) | |
| Time between initial diagnosis and recurrence, mo (range) | ||
| Median | 20 (3-77) | |
Table 2 summarizes the reirradiation treatment details. All patients were treated with PBT, 25 patients (56%) were treated with pencil-beam scanning, and 20 patients (44%) were treated with passive scattering. The median total reirradiation dose was 46.2 Gy (range, 25-60 Gy). The median fraction size was 2.2 Gy per fraction (range, 1.2-4 Gy/fraction). The majority of patients (n = 31) underwent a hypofractionated course (ie, >2 Gy/fraction), and 2 patients underwent twice-daily treatment. Five patients did not complete the intended treatment course. One of these patients received 25 Gy EQD2, and the remaining patients received ≥39 Gy EQD2. Two patients had an incomplete treatment course owing to disease progression and 3 patients due to complications (grade <3). Fourteen patients did not have any form of systemic therapy for their recurrent GBM management. The majority of patients (n = 31) also had some form of systemic therapy (Table 2).
Table 2.
Reirradiation treatment parameters
| Parameter | All patients (N = 45) |
|
|---|---|---|
| N | % | |
| Reirradiation dose, Gy (range) | ||
| Median | 46.2 (25-60) | |
| Fraction size, Gy/fraction (range) | ||
| Median | 2.2 (1.5-4) | |
| Equivalent dose in 2 Gy fractions, Gy (range) | ||
| Median | 47.1 (24.9-60.8) | |
| Proton modality, n | ||
| Pencil-beam scanning | 25 | 56% |
| Passive scattering | 20 | 44% |
| Systemic therapy, n | ||
| None | 14 | 31% |
| Temozolomide alone | 16 | 36% |
| Bevacizumab alone | 4 | 9% |
| Temozolomide + bevacizumab | 10 | 22% |
| Vorinostat + bevacizumab | 1 | 2% |
Outcomes
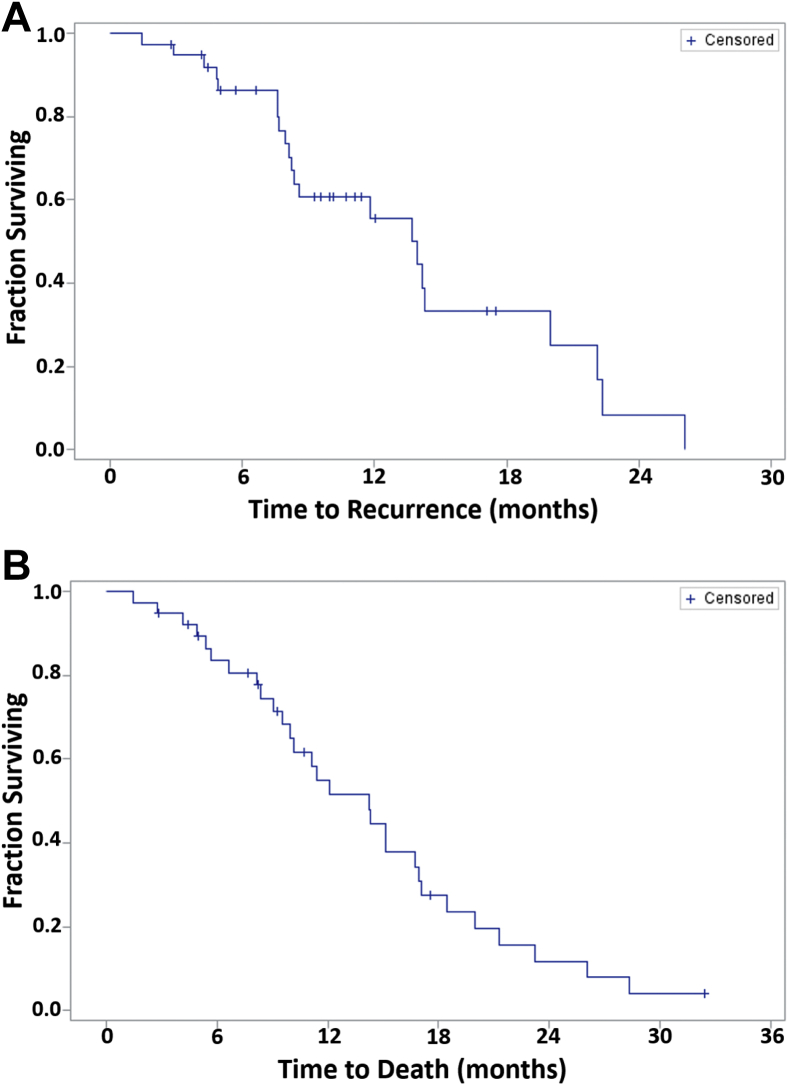
Figures 1A and B depict the PFS and OS for all patients from the time of PBT reirradiation. Table 3 summarizes the PFS, OS, and toxicity rates. The median follow-up time from PBT reirradiation was 10.7 months. The median PFS was 13.9 months (95% confidence interval [CI], 8.23-20.0 months) and median OS was 14.2 months (95% CI, 9.6-16.9 months) after PBT reirradiation. With regard to toxicity outcomes, a total of 5 patients experienced grade 3 toxicity. One patient experienced an acute grade 3 toxicity (ataxia) and 4 separate patients experienced a late grade 3 toxicity (neuropathy, cognitive disturbance, optic nerve disorder, and seizure). No acute or late grade 4 or 5 toxicities were observed. All observed late toxicities occurred in patients who had undergone a PBT reirradiation EQD2 in excess of 41 Gy.
Figure 1.
Kaplan-Meier curves for (A) overall survival and (B) progression-free survival for all patients treated with proton-beam therapy reirradiation (N = 45).
Table 3.
Clinical outcomes of proton beam therapy reirradiation patients
| Parameter | All patients (N = 45) |
|
|---|---|---|
| N | % | |
| Survival, mo (95% confidence interval) | ||
| Median progression-free survival | 13.9 (8.2-20) | |
| Median overall survival | 14.2 (9.6-16.9) | |
| Toxicity, n | ||
| Grade 3, acute | 1 | 2.2% |
| Grade 3, late | 4 | 8.8% |
| Grade 4+, any | 0 | 0% |
We performed univariate and multivariate analyses to determine factors associated with PFS or OS. Other than prior surgery, we did not identify any factors significantly associated with PFS or OS. For instance, age, performance status, MGMT status, time between initial and recurrent diagnosis, PBT modality (pencil beam scanning vs passive scatter), PBT reirradiation dose, or systemic therapy utilization were not associated with PFS or OS. However, prior surgery was associated with a 91.3% decreased hazard of death (hazard ratio: 0.087; 95% CI, 0.02-0.42; P < .01).
Discussion
In the present study, we report on the outcomes of patients with recurrent GBM treated with PBT reirradiation. To the best of our knowledge, this represents one of the largest GBM PBT reirradiation analyses to date. Outcomes after photon-based reirradiation have been well described, but the specific use of PBT reirradiation is limited.8, 9, 10, 11, 12, 13 In principle, PBT offers a distinct dosimetric advantage over photon RT given its inherent property of lacking an exit dose, which can help spare adjacent healthy tissue located beyond the target. This is useful in the reirradiation setting, especially for GBM reirradiation, given the typical initial radiation dose of 60 Gy and the likelihood of either previously having reached or at least contributed significantly toward maximum OAR tolerances. In a survey of central nervous system radiation oncology practitioners, the previously administered dose to OARs was one of the most important factors in terms of whether reirradiation was offered to patients with recurrent GBM.14 Thus, given the opportunity to spare the cumulative radiation dose to critical OARs, PBT may permit the safe delivery of RT in patients for whom reirradiation may not otherwise be safely feasible using photons.
Compared with previous studies evaluating the role of reirradiation in patients with recurrent GBM, our current analysis results compare favorably.8,9,12,13,15,16 The largest GBM reirradiation analysis is by Fogh et al, who analyzed 147 patients with recurrent high-grade gliomas undergoing hypofractionated photon RT.8 Of the 147 patients, 105 had GBM. These patients with recurrent GBM were treated with a reirradiation dose of 35 Gy in 10 fractions, and this cohort of patients with GBM had a median OS of 11 months from the time of reirradiation. Other GBM-reirradiation studies (all of which use photon RT) report a median OS in a similar range.
A literature review by Soccianti et al found a median OS range of 7.9 to 13 months for available studies.16 The study with the highest median OS was by Kong et al, with an OS of 13 months.17 Of note, Kong et al used a stereotactic radiosurgery approach with a median single-fraction dose of 16 Gy. Given the use of stereotactic radiosurgery, these GBM recurrences generally represented small-volume disease (on average 10.6 cm3 in size). With regard to the specific use of PBT for reirradiation of recurrent GBM, we identified one such analysis. Mizumoto et al analyzed 26 patients with recurrent gliomas who underwent reirradiation.18 Of the 26 patients, 14 patients had recurrent GBM and of these, 8 patients underwent PBT reirradiation. The median OS for these 8 patients was 11.9 months (range, 1.3-48 months).
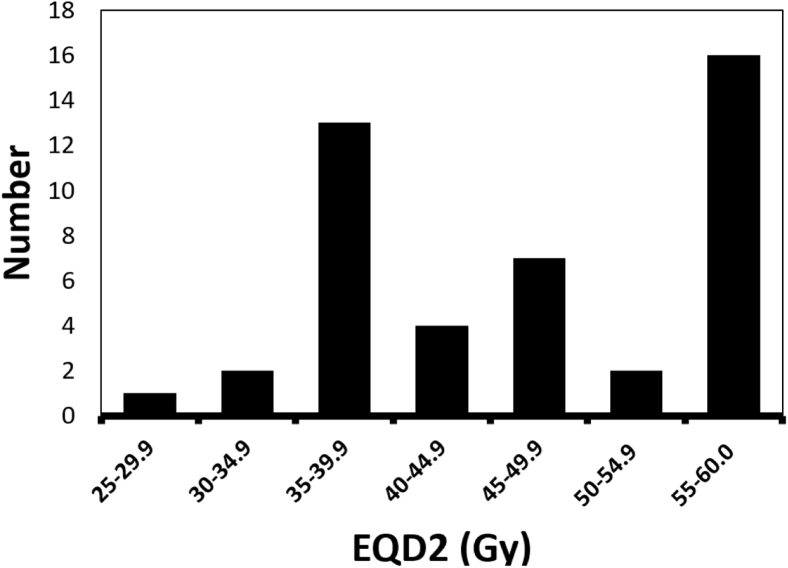
The observed higher median OS in our cohort may be related to a higher radiation dose compared with previous GBM reirradiation studies. Our median prescribed dose was 46.2 Gy with a median fraction size of 2.2 Gy per fraction (mean dose: 45.7 Gy; mean fraction size: 2.47 Gy/fraction), translating to an EQD2 of approximately 47.0 Gy. Fogh et al used a hypofractionation scheme of 35 Gy in 3.5 Gy per fraction, which represents an EQD2 of approximately 39.4 Gy. Of note, the majority of patients in our cohort received an EQD2 that was greater than that by Fogh et al., with 40 of our patients (88.9%) receiving an EQD2 of >39 Gy (Fig 2). This tendency for relatively higher doses in our cohort may reflect individual practitioner comfort given the use of PBT and its ability to likely preserve cumulative OAR constraints.
Figure 2.
Histogram depicting delivered equivalent dose in 2 Gy fractions for all patients (N = 45).
In determining prognostic factors associated with PFS or OS, only prior surgical resection was associated with an improved OS. We did not find the PBT delivery technique (passive scatter vs pencil-beam scanning), reirradiation dose, or time interval between diagnosis and recurrence to be significantly associated with our PFS, OS, or toxicity outcomes. Of note, we did not find that the addition of systemic therapy to PBT reirradiation affected OS. This is consistent with the results by Fogh et al, who similarly did not find a survival improvement with the addition of systemic therapy to hypofractionated photon RT.8 However, this is in contrast to the results by Niyazi et al who performed a single-institution retrospective study of high-grade gliomas (GBM and grade III gliomas).19 Niyazi et al reported that mean OS of reirradiation alone versus reirradiation + bevacizumab was 8 and 12 months, respectively.19 The role of bevacizumab with RT remains to be determined, and preliminary results from the prospective Radiation Therapy Oncology Group trial 1205 indicate that the addition of hypofractionated RT to bevacizumab improves PFS, but not OS.20
With regard to toxicities, we found that PBT reirradiation was generally well tolerated with a low overall incidence of toxicities. We found a total of 5 patients who experienced a grade 3 toxicity (1 acute grade 3, and 4 late-grade 3 toxicities), and no grade ≥4 toxicities. In reviewing the GBM reirradiation literature, reirradiation is generally well tolerated in photon-reirradiation series. Fogh et al8 reported 1 grade 3 toxicity (out of 147 patients), and other photon reirradiation series have also reported a low incidence. Fokas et al,11 Grosu et al,12 Arcicasa et al,10 and Combs et al9 all reported no grade ≥3 toxicities. The higher incidence grade 3 toxicities in our cohort may be a reflection of higher radiation doses (relative to existing photon-reirradiation series), as described in the EQD2 analysis, and may reflect the need for appropriate patient selection.
Although we used prospectively collected data from the PCG registry and had a relatively large sample size, some limitations should be noted. First, the PCG registry provides limited data regarding initial radiation course. Analyzing additional radiation-specific factors (eg, in-field vs out-of-field recurrence, degree of field overlap, previous treatment volume, previous OAR doses) would be important to fully assess the safety and efficacy of PBT reirradiation and help optimize patient selection. Additionally, patient-reported outcomes and quality-of-life data are not available, which would better characterize patients’ tolerance of PBT reirradiation.
With regard to PFS and OS measurements, OS is inherently a robust endpoint; however, PFS has a degree of uncertainty given the difficulty in distinguishing pseudo- versus disease progression.21,22 The PCG database does not provide primary radiographic data, and there is no standardized protocol for radiographic analysis between the 7 participating proton centers; thus, our PFS may not fully capture the true value. Also, given the single-cohort, nonrandomized nature of our study, confounding factors are possible and a direct comparison of our OS against that of previous studies is difficult. For instance, the limited availability of PBT and nonuniform health insurance approval for PBT may select for patient with better access to medical care and possibly better performance status (66% of our patients had Eastern Cooperation Oncology Group 0 or 1), which could lead to better survival outcomes23.
Conclusions
This is the largest series to date reporting outcomes for PBT-reirradiation of recurrent GBM patients. Our analysis indicates that PBT is well tolerated and offers efficacy rates similar to previously reported photon-reirradiation series. Future studies are warranted to prospectively determine the benefits and risks associated with PBT compared with photon RT, with a focus on quality-of-life.
Footnotes
Sources of support: Portions of this study were funded in part by the Keep Punching Foundation (MVM).
Disclosures: Dr Mark V. Mishra has received honoraria and travel funding from Varian outside the submitted work. Dr Lia M. Halasz reports grants from Abbvie outside the submitted work. The other authors have no conflicts of interest to report.
References
- 1.Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Johnson D.R., O’Neill B.P. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 4.Seystahl K., Wick W., Weller M. Therapeutic options in recurrent glioblastoma—An update. Crit Rev Oncol Hematol. 2016;99:389–408. doi: 10.1016/j.critrevonc.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Pace A., Dirven L., Koekkoek J.A.F. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18:e330–e340. doi: 10.1016/S1470-2045(17)30345-5. [DOI] [PubMed] [Google Scholar]
- 6.Gebhardt B.J., Dobelbower M.C., Ennis W.H., Bag A.K., Markert J.M., Fiveash J.B. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat Oncol. 2014;9:130. doi: 10.1186/1748-717X-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minniti G., Amelio D., Amichetti M. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010;97:377–381. doi: 10.1016/j.radonc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Fogh S.E., Andrews D.W., Glass J. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Combs S.E., Thilmann C., Edler L., Debus J., Schulz-Ertner D. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 10.Arcicasa M., Roncadin M., Bidoli E., Dedkov A., Gigante M., Trovo M.G. Reirradiation and lomustine in patients with relapsed high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999:43789–43793. doi: 10.1016/s0360-3016(98)00457-x. [DOI] [PubMed] [Google Scholar]
- 11.Fokas E., Wacker U., Gross M.W., Henzel M., Encheva E., Engenhart-Cabillic R. Hypofractionated stereotactic reirradiation of recurrent glioblastomas: A beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol. 2009;185:235–240. doi: 10.1007/s00066-009-1753-x. [DOI] [PubMed] [Google Scholar]
- 12.Grosu A.L., Weber W.A., Franz M. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–519. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.K., Thornton A.F., Greenberg H.S., Page M.A., Junck L., Sandler H.M. Results of re-irradiation of primary intracranial neoplasms with three-dimensional conformal therapy. Am J Clin Oncol. 1997;20:358–363. doi: 10.1097/00000421-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Krauze A.V., Attia A., Braunstein S. Expert consensus on re-irradiation for recurrent glioma. Radiat Oncol. 2017;12:193–194. doi: 10.1186/s13014-017-0928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed K.A., Kim S., Harrison L.B. Novel opportunities to use radiation therapy with immune checkpoint inhibitors for melanoma management. Surg Oncol Clin N Am. 2017;26:515–529. doi: 10.1016/j.soc.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Scoccianti S., Francolini G., Carta G.A. Re-irradiation as salvage treatment in recurrent glioblastoma: A comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol. 2018;126:80–91. doi: 10.1016/j.critrevonc.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Kong D.S., Lee J.I., Park K., Kim J.H., Lim D.H., Nam D.H. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer. 2008;112:2046–2051. doi: 10.1002/cncr.23402. [DOI] [PubMed] [Google Scholar]
- 18.Mizumoto M., Okumura T., Ishikawa E. Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Technical considerations based on experience at a single institution. Strahlenther Onkol. 2013;189:656–663. doi: 10.1007/s00066-013-0390-6. [DOI] [PubMed] [Google Scholar]
- 19.Niyazi M., Ganswindt U., Schwarz S.B. Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys. 2012;82:67–76. doi: 10.1016/j.ijrobp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Tsien C., Pugh S., Dicker A.P. Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): Initial outcomes and RT plan quality report. Int J Radiat Oncol Biol Phys. 2019;105:S78. [Google Scholar]
- 21.Ellingson B.M., Chung C., Pope W.B., Boxerman J.L., Kaufmann T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134:495–504. doi: 10.1007/s11060-017-2375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe L.S., Butman J.A., Mackey M. Differentiating pseudoprogression from true progression: analysis of radiographic, biologic, and clinical clues in GBM. J Neurooncol. 2018;139:145–152. doi: 10.1007/s11060-018-2855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryckman J.M., Ganesan V., Appiah A.K., Zhang C., Verma V. National practice patterns of proton versus photon therapy in the treatment of adult patients with primary brain tumors in the United States. Acta Oncol. 2019;58:66–73. doi: 10.1080/0284186X.2018.1512755. [DOI] [PubMed] [Google Scholar]