Abstract
The backbone of treatment for patients with advanced-stage Hodgkin lymphoma is systemic therapy. The use of radiation therapy as a component of combined-modality treatment in this setting is controversial. In this review, we describe the data in support of and against the use of radiation therapy for stage III and IV Hodgkin lymphoma. Specifically, we review the data for the use of radiation therapy in the consolidation and partial-response settings, including for patients with initial bulky disease. We also discuss the use of radiation therapy in the era of more modern systemic therapies, including checkpoint inhibitors and brentuximab vedotin.
Introduction
Hodgkin lymphoma (HL) is a rare disease, with an estimated 8110 new diagnoses and 1000 deaths from HL in 2019.1 HL is broadly divided into classical subtypes versus nodular lymphocyte-predominant HL. Treatment paradigms for classical HL are typically separated by the distinction of early favorable disease, early unfavorable disease, and advanced-stage disease. Approximately one-third of patients with HL present with advanced-stage disease at diagnosis. Although the estimated 5-year survival rate for stage I to II disease is excellent at >90%, survival outcomes are worse in stage III to IV disease, with survival rates ranging from 56% to 90%.2 As the development of effective systemic therapies has significantly improved survival for patients with advanced-stage HL (AS-HL) during recent decades, the challenge is to minimize the toxicity of treatment while maintaining a high cure rate. The mainstay of therapy for AS-HL is chemotherapy, typically with consolidation radiation therapy (RT) reserved for patients with initial bulky disease or poor response to chemotherapy. Herein, we review the literature and the role of RT for AS-HL.
Defining Advanced-Stage Hodgkin Lymphoma
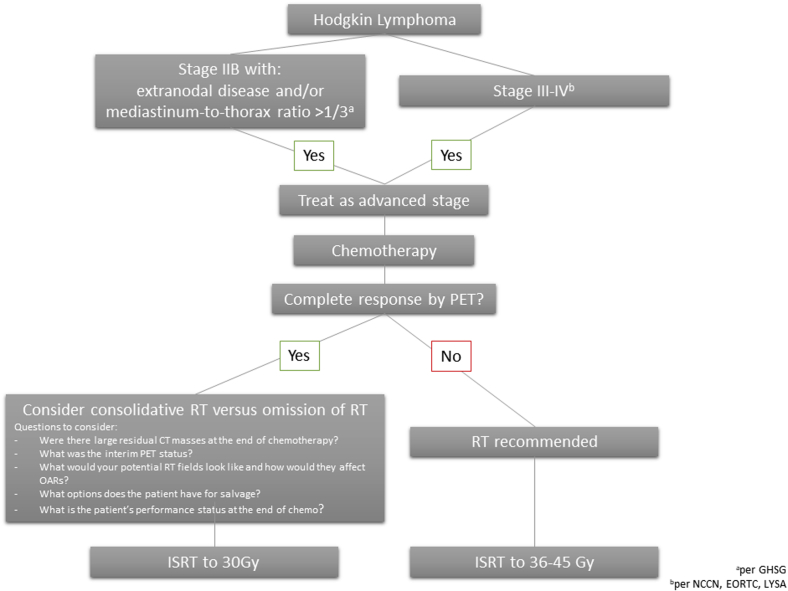
As with favorable versus unfavorable early-stage disease, various cooperative groups and research trials define AS-HL slightly differently (Fig 1).3 When reviewing clinical trials, noting inclusion criteria is thus critical. The National Comprehensive Cancer Network (NCCN), European Organization for Research and Treatment (EORTC), and Lymphoma Study Association define advanced stage as Ann Arbor stage III to IV disease. The German Hodgkin Study Group (GHSG) also includes Ann Arbor stage IIB disease with the following risk factors as advanced-stage disease: mediastinum-to-thorax ratio ≥0.33 or extranodal disease.
Figure 1.
Schematic of a treatment algorithm for management of advanced-stage Hodgkin lymphoma in the positron emission tomography era.
Radiation Therapy in the Consolidative Setting
Consolidation RT after effective systemic therapy remains controversial, with data supporting both its addition and its omission (Table 1). Of note, most of these data arose in the prepositron emission tomography (PET) era.
Table 1.
Studies incorporating radiation therapy for the treatment of advanced stage Hodgkin lymphoma
| Trial | Chemotherapy | Radiation | PET era | Outcome |
|---|---|---|---|---|
| UK Lymphoma Group LY094 | ABVD vs ChIVPP/PABIOE vs ChIVIPP/EVA | Recommended for initial bulky disease or residual CT mass | No | PFS and OS superior for RT despite more bulky disease and less CR on retrospective analysis |
| EORTC11 | MOPP-ABV | Pts with CR randomized to RT vs no RT; RT for all pts w/ PR | No | No difference in EFS or OS for RT for pts with CR; rates of EFS and OS for PR → RT similar to pts with CR |
| GHSG HD1212 | escBEACOPP ×8 vs escBEACOPP ×4 —> BEACOPP | Pts w/bulky disease ≥5 cm or residual disease ≥1.5 cm randomized to RT vs no RT | No | On subgroup analysis, RT improved FFTF in pts w/ residual disease but not pts w/ initial bulky disease with CR |
| GHSG HD157 | escBEACOPP ×6-8 vs BEACOPP14 | RT for persistent PET avid mass ≥2.5 cm | Yes | Low relapse rates in PET negative pts who did not receive RT (4-y PFS 92.6%); PFS for PET positive pts who received RT 86.2% |
| RATHL9 | ABVD → ABVD or AVD vs BEACOPP | Pts with negative interim PET not recommended for RT (consolidative RT in only 6.5% of pts) | Yes | 3-y PFS 67.5% and OS 87.8% |
| ECOG E249614 | ABVD vs Stanford V | RT for all pts with bulky disease (75% in Stanford V arm and 41% in ABVD arm) | No | 5-y OS 88% in both arms |
| GITIL/FIL trial HD 060715 | ABVD → escBEACOPP vs BEACOPP ± rituximab | Pts w/ bulky disease but negative final PET randomized to RT or no RT | Yes | No SS difference in 3-y PFS w/RT (97%) vs no RT (93%); also no SS when limited analysis to bulky disease >10 cm: 3-y PFS w/RT (94%) vs no RT (86%) |
| FIL trial HD 0801 (abstract only)16 | ABVD | Pts with bulky disease and CR on PET randomized to RT or no RT | Yes | 10.3% 3-y PFS benefit in RT arm but not SS |
| ECHELON-118 | Bv + AVD vs ABVD | In both chemotherapy arms pts received RT for residual disease (n = 54-55 in each arm) | Yes | 2-y PFS 82% for Bv + AVD and 77% for ABVD |
Abbreviations: ABV = doxorubicin, bleomycin, vinblastine; ABVD = adriamycin, bleomycin, vinblastine, and dacarbazine; BEACOPP = bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone; CR = complete response; ChIVIPP = chlorambucil, vinblastine, procarbazine, and prednisone; CT = computed tomography; ECOG = Eastern Cooperative Oncology Group; EFS = event-free survival; EORCT = European Organization for Research and Treatment; Esc = escalated; EVA = etoposide, vincristine, and doxorubicin; FFTF = freedom from treatment failure; FIL = Fondazione Italiana Linfomi; GHSG = German Hodgkin Study Group; GITIL/FIL = Gruppo Italiano Terapie Innovative nei Linfomi/Fondazione Italiana Linfomi; MOPP = mechlorethamine, vincristine, procarbazine, prednisone; OS = overall survival; PABIOE = doxorubicin, bleomycin, vincristine, etoposide, and prednisolone; PET = positron emission tomography; PFS = progression free survival; PR = partial response; pts = patients; RATHL = Response-adjusted therapy for advanced Hodgkin lymphoma; RT = radiation therapy; SS = statistically significant.
In the historic EORTC study, when the 421 patients with AS-HL with a complete response (CR) after 6 to 8 cycles of MOPP-ABV (mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine) hybrid chemotherapy were randomized to no further treatment versus 24 Gy of involved-field RT (IFRT), the 5-year event-free survival rate was 84% in the no-further-treatment group and 79% in the IFRT group (P = .35).4 There was also no difference in 5-year overall survival (91% for the no further treatment group vs 85% for the IFRT group). Notably, this study occurred in the pre-PET era and patients who had a partial response (PR) by computed tomography (CT) criteria received 30 Gy (with a boost of 4-10 Gy, if necessary) to the involved nodal regions as described in subsequent sections.
The more modern UK Lymphoma Group LY09 trial was a prospective chemotherapy trial for patients with AS-HL comparing ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) with one of 2 other multidrug regimens: ChIVPP (chlorambucil, vinblastine, procarbazine, and prednisone)/PABIOE (doxorubicin, bleomycin, vincristine, etoposide, and prednisolone) or hybrid ChIVPP/EVA (etoposide, vincristine, and doxorubicin).5 The use of RT was not mandated on the protocol but was recommended for patients with initial bulky disease or those in whom a residual mass was present on CT imaging at the end of chemotherapy; ultimately, 43% of the cohort (n = 300) received consolidative RT. Subsequent retrospective analysis of this prospectively collected data found that RT was preferentially given to patients with bulky disease (63% vs 28%) and a PR to chemotherapy (50% vs 9%). Progression-free survival (PFS; hazard ratio 0.43; 95% confidence interval, 0.30-0.60) and overall survival (hazard ratio 0.47; 95% confidence interval, 0.29-0.77) were both superior for patients receiving RT, despite more bulky disease at baseline and a higher rate of PR by CT criteria. Again, these data were collected in the pre-PET era, and it is possible that these patients would have been designated PET-negative after systemic therapy today. Furthermore, RT maximum doses were variable and ranged from 30 to >39 Gy. Most patients (55%) received 39 Gy, which is more than recommended by the International Lymphoma Radiation Oncology Group (ILROG) in the consolidative setting today, as ILROG recommends a range of 36 to 40 Gy for chemotherapy-refractory disease.6
With the widespread availability of PET imaging over the past decade, PET results at the end of chemotherapy are being used increasingly to guide decision-making regarding consolidative RT. Most commonly, patients who are PET-negative, defined as Deauville 1, 2, or 3, at the end of chemotherapy will have consolidative RT omitted.7 The GHSG HD15 study was one of the first noninferiority trials to use PET and CT criteria to guide the use of RT at the end of chemotherapy.8 In this study, patients with a persistent PET-positive mass that was 2.5 cm or larger received 30 Gy RT after 8 cycles of escalated-BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone), 6 cycles of dose-escalated BEACOPP, or 8 cycles of BEACOPP14. PET-positive versus PET-negative disease was defined according to a central review panel according to published criteria.7,9 Patients who were PET-negative did not receive RT and, of 529 PET-negative patients, only 4.5% (n = 24) had disease progression or relapse. The 4-year PFS rate was 92.6% for these patients, supporting the idea that AS-HL patients who are PET-negative at the end of chemotherapy do not need consolidation RT.
Similarly, in the Response-adjusted therapy for advanced Hodgkin lymphoma trial, patients received 2 cycles of ABVD followed by interim PET-CT; patients with a negative PET-CT were randomized to ABVD or AVD in cycles 3 to 6, whereas those with positive PET findings received BEACOPP.10 Positive interim PET was defined as a Deauville score of 4 or 5 (ie, uptake moderately above the liver at the initial site or markedly above the liver at any site including new sites of disease).11 Patients who achieved a negative interim PET scan were generally not recommended consolidation RT. Overall in the trial, only 6.5% of patients received consolidation RT and the 3-year PFS rate was 67.5%, and the overall survival rate was 87.8%. Of note, this was a noninferiority trial that at the time of publishing had failed to meet its prespecified noninferiority margin and further details regarding the failures and their characteristics need to be described.
Consolidation radiation therapy for patients with initial bulky disease
A specific consideration within the consolidation setting is whether patients who present initially with bulky disease would benefit from radiation therapy despite PET-negativity at the end of chemotherapy. In the aforementioned GHSG HD12 trial,12 on subgroup analysis, RT did not improve freedom from treatment failure in patients with initial bulky disease with a CR. Accordingly, the GHSG trials HD15 and HD18 did not recommend consolidation RT for patients with bulky disease. However, in the Eastern Cooperative Oncology Group trial E2496, which randomized patients with either stage III to IV HL or stage I to II HL with bulky disease to ABVD (6-8 cycles) versus Stanford V (doxorubicin, vinblastine, chlormethine, vincristine, bleomycin, etoposide, prednisone; 12 weeks) chemotherapy, all patients with bulky disease received RT after chemotherapy.13 For patients in the ABVD arm, bulky disease was defined as a mass greater than one-third of the maximum intrathoracic diameter on standing posteroanterior chest x-ray. The RT dose delivered was 36 Gy. For patients in the Stanford V arm, RT (36 Gy) was delivered to any pretreatment sites of disease >5 cm. Based on the results of this study published in 2013, ABVD plus consolidation radiation to initial bulky sites of disease is a viable option for AS-HL in North America.
Two more recent studies have examined the role of consolidation radiation to bulky lesions in AS-HL despite the achievement of rapid early response to chemotherapy: the Italian GITIL/FIL (Gruppo Italiano Terapie Innovative nei Linfomi/Fondazione Italiana Linfomi) trial HD 060714 and FIL (Fondazione Italiana Linfomi) trial HD 0801.15 The GITIL/FIL trial HD 0607 enrolled patients with stage IIB to IVB HL and, after 2 cycles of ABVD, randomly assigned patients with positive PET to 4 cycles of escalated BEACOPP versus standard BEACOPP with or without rituximab. Interim PET was defined as positive if the Deauville score was 4 to 5. Patients with a negative PET after the first 2 cycles of ABVD remained on ABVD for a total of 6 cycles, and those with initial bulky disease who also remained negative on final PET restaging were randomly assigned to consolidation RT (n = 148) or no RT (n = 148). Bulky disease was defined as ≥5 cm, and the radiation dose was 30 Gy. The 3-year PFS rate for RT was 97% versus 93% without RT, which was not statistically significant. When limiting the analysis to patients with initial bulky disease >10 cm, the 3-year PFS rate was 94% for RT versus 86% for no RT, a large difference although still not statistically significant. The authors thus concluded that consolidation RT can be safely omitted in patients who achieve both a rapid early response on interim PET scan and a durable response to chemotherapy on end-of-treatment PET scan despite initial bulky disease. However, it is important to note the sample size was not calculated on the RT substudy and these results should therefore be interpreted with caution, particularly given the difference between the RT and no RT arms.
In FIL HD0801, patients were assessed with interim PET after an initial 2 cycles of ABVD. Patients with a positive interim PET, defined according to Juweid criteria,9 proceeded with a phase II portion of the trial to salvage treatment.16 Patients with initial bulky disease who had a negative interim PET and remained PET-negative after a full 6 cycles of ABVD (n = 116) were then randomized on a phase III portion of the trial to either RT to initial sites of bulky disease or no RT. The phase III portion of the trial has only been published thus far in abstract form.15 An intention-to-treat analysis showed similar PFS in both arms, and the per-protocol analysis showed that consolidation RT was associated with a 10.3% PFS benefit at 3 years and a 7.5% benefit at 5 years compared with no RT, although the findings were not statistically significant. The authors note there was a limited sample size and low number of events.
Radiation Therapy for Patients With a Partial Response to Chemotherapy
In contrast to the consolidation setting, the use of RT is more widely accepted for patients with AS-HL who have a PR on PET to chemotherapy. Most trials in both the CT and PET eras have shown that RT is an important component of treatment. In the EORTC trial,17 patients who only achieved a partial response (PR) after the first 4 cycles of MOPP (mustargen, oncovin, procarbazine, prednisone)-ABV then went on to receive an additional 2 to 4 cycles of chemotherapy. Patients who then experienced a CR after a total of 6 cycles were randomized to IFRT or observation as described in a previous section. However, all patients who remained in PR after 6 cycles were to receive IFRT to 30 Gy plus an additional dose to extranodal sites with or without a boost. The rates of event-free survival and overall survival in patients with a PR who received RT were similar to patients with a CR, suggesting a potential valuable role for RT in patients who achieve only a PR to chemotherapy. Of note, this study occurred in the pre-PET era, although the GHSG HD15 study also appears to support this observation.
The GHSG HD12 trial12 compared 8 cycles of escalated BEACOPP with 4 cycles of escalated BEACOPP followed by 4 cycles of nonescalated BEACOPP. For each chemotherapy arm, patients with bulky disease ≥5 cm or residual disease ≥1.5 cm were subsequently further randomized to consolidation RT (30 Gy) versus no RT. On subgroup analysis, RT improved freedom from treatment failure in patients with residual disease after chemotherapy. After HD12, the GHSG HD15 and HD18 trials examined whether interim PET could be used to de-escalate chemotherapy for patients with favorable PET responses.8,18 Owing to the results of the HD12 trial, RT was recommended for all patients with residual PET-avid lesions ≥2.5 cm after chemotherapy in all arms in both trials. Among 739 patients with a persistent CT mass measuring ≥2.5 cm, PET was positive in 26% (n = 191), and these patients went on to receive RT with 92% protocol-adherence. The 4-year PFS rate for these patients was 86.2% (lower than the PFS for PET-negative patients as described earlier), although these patients received additional RT.
Radiation Therapy in the Setting of Modern Systemic Therapies
In recent years, there have been numerous advances in systemic therapies, including checkpoint inhibitors and brentuximab vedotin (Bv), an antibody-drug conjugate targeting CD30. Although exciting, these new drugs come with high price tags and their potential synergies with RT remain relatively unknown.
Currently, Bv appears to be well tolerated with RT. Bv with AVD chemotherapy has been shown in patients with AS-HL to improve modified PFS by approximately 5% compared with ABVD chemotherapy.19 Of note, in both the Bv + AVD and ABVD groups, 54 and 55 patients received subsequent RT, respectively, for residual disease. Similarly, investigators at the Memorial Sloan Kettering Cancer Center found in a multicenter pilot study that Bv with AVD followed by both 30 Gy and 20 Gy of consolidation involved-site radiation therapy (ISRT) was well tolerated in patients. Although the study is intended for early-stage unfavorable patients, it is important to note that, in each cohort, approximately one-third of patients would have AS-HL by GHSG criteria.20 Currently, Bv with AVD is approved by the US Food and Drug Administration as well as the European Medicines Agency for previously untreated AS-HL.
At this time, there are no checkpoint inhibitors that are approved in the frontline setting for AS-HL. However, they have shown tremendous promise in the relapsed or refractory setting after autologous stem cell transplant and in heavily pretreated patients.21, 22, 23, 24 Often, these checkpoint inhibitors are used in conjunction with RT in other solid malignancies and appear well tolerated. Whether RT can be used synergistically with checkpoint inhibitors in the frontline setting for AS-HL remains unknown. We await results of the Southwest Oncology Group Cancer Research Network trial S1826, which is a randomized trial comparing Bv or the immune checkpoint inhibitor nivolumab with combination AVD chemotherapy in patients with newly diagnosed AS-HL.25 In this trial, RT is allowed at the completion of systemic therapy in both arms at the discretion of the treating physician.
Radiation Therapy Fields and Doses
In the treatment of AS-HL, the primary reason physicians may avoid RT involves the potentially large radiation fields and toxicity risks. However, RT fields have decreased in size, and technology has improved over time with the advent of improved treatment planning techniques and PET imaging. The use of CT-based simulation, deep-inspiration breath hold technique, and daily image guidance during RT have allowed for more conformal and precise treatments with improved protection of normal tissues.6
The conundrum thus arises as to the recommended modern field sizes for AS-HL in the era of PET-adapted approaches to systemic therapy: all sites of initial disease, only sites of initial bulk, sites positive on interim PET, or only sites positive on final postchemotherapy PET assessment? The radiation fields and doses reported in the trials included in this review are summarized in Table 2. Radiation to all sites of initial disease was largely limited to the EORTC study from the pre-PET era.17 Therefore, best suited for more modern reference are trials from the PET era. According to both International Radiation Oncology Group (ILROG) and NCCN guidelines, when radiation is used for AS-HL, the appropriate treatment is ISRT, which allows for smaller fields limited to the area of involved nodal sites without treating uninvolved adjacent nodal regions or uninvolved normal tissues; ILROG states that larger IFRT or extended-field RT should be limited for salvage treatments on a case-by-case basis.6,26 Per ILROG, many centers reserve RT for AS-HL patients who have not achieved CR to chemotherapy, and the target is thus “the residual mass (gross tumor volume) after chemotherapy” with a margin added “to account for uncertainties and motion.” Per ILROG, “usually a margin of 1 cm in sufficient, but in the chest and upper abdomen a larger margin in the superior-inferior direction is needed to account for respiratory motion.”6 NCCN evidence-based guidelines currently state to consider ISRT after systemic therapy for initially bulky or selected PET-positive sites.26 When opting to consolidate with postchemotherapy radiation to sites of initial bulky disease, the definition of initial bulky disease has most commonly been defined as diameter >5 cm.13, 14, 15 The recommended dose for bulky disease ranges from 30 to 36 Gy at 1.5 to 2.0 Gy per fraction, and for residual PET-positive disease postchemotherapy is 36 to 40 Gy, per ILROG, or 36 to 45 Gy, per NCCN.6,26,27
Table 2.
Radiation therapy details for studies incorporating radiation therapy for the treatment of advanced stage Hodgkin lymphoma
| Trial | Radiation fields | PET era | Radiation dose |
|---|---|---|---|
| UK Lymphoma Group LY094 | Initial bulky disease (>1/3 transthoracic ratio or >10 cm outside of chest) or residual CT mass | No | Variable (minimum 30 Gy ranging to >39 Gy) |
| EORTC11 | IFRT to all sites of originally involved nodal areas + extranodal sites with or without a boost | No | 24 Gy to nodal sites if CR; 30 Gy (± 4-10 Gy boost) to nodal sites if PR; 18-24 Gy to extranodal sites |
| GHSG HD1212 | Sites of initial bulky disease (defined as ≥5 cm) or residual disease ≥1.5 cm on CT; also bone lesions with instability risk; whole anatomic region encompassed in volume | No | 30 Gy |
| GHSG HD157 | RT for persistent postchemotherapy PET-avid mass ≥2.5 cm and bone lesions regardless of residual PET activity if instability/fracture risk; 1.5 cm margin used | Yes | 30 Gy |
| ECOG E249614 | Bulky disease (defined as mass greater than one-third of the maximum intrathoracic diameter on standing posteroanterior chest x-ray in ABVD arm; any pretreatment sites of disease >5 cm in Stanford V arm) | No | 36 Gy |
| GITIL/FIL trial HD 060715 | Bulky disease (defined as ≥5 cm) | Yes | 30 Gy |
| FIL trial HD 0801 (abstract only)16 | Bulky disease (any initial mass with diameter >5 cm) | Yes | Not yet reported |
Abbreviations: CR = complete response, CT = computed tomography; ECOG = Eastern Cooperative Oncology Group; EORTC = European Organization for Research and Treatment; FIL = Fondazione Italiana Linfomi; IFRT = involved-field radiation therapy; GHSG = German Hodgkin Study Group; GITIL/FIL = Gruppo Italiano Terapie Innovative nei Linfomi/Fondazione Italiana Linfomi; PET = positron emission tomography, PR = partial response.
Patterns of relapse studies prove to be informative for designing the optimal RT field for AS-HL patients. For GHSG H15, which gave RT postchemotherapy to residual PET-positive disease ≥2.5 cm and bone lesions, Kriz et al reported the patterns of relapse for 152 irradiated patients for whom RT documents were available (68% of the irradiated patients).28 RT was given locally with a 1.5-cm margin around the gross tumor volume, which was defined as the residual PET-positive tumor as well as any CT correlates in the same area. Of the 28 (11%) patients who experienced relapse after RT, 39% of the relapses occurred within the RT field, 25% out of field, and 36% mixed in field and out of field. When the authors examined the out-of-field relapses, they did not occur at sites that would have been encompassed even by larger IFRT fields, suggesting that local RT used by the GHSG was adequate. In SWOG S0816, interim PET after 2 cycles of ABVD was used to assign patients to additional cycles of ABDV (if PET-negative) versus escBEACOPP (if PET-positive).29 Although no radiation therapy was allowed, 74 patients (22%) experienced a relapse at a median follow-up of 3.3 years with 32% of evaluable relapses occurring only at sites of initial disease, 53% at only new sites, and 6% at both initial and new sites. The median baseline size of initial sites of disease that recurred was 3.5 cm with only 5 (24%) of these initial lesions measuring >5 cm and only 2 (9.5%) measuring >10 cm. The authors conclude that the low number of relapses at sites of initial disease, with only 2 relapses occurring at sites of previous bulk >10 cm, suggests that consolidation RT in these patients may have provided only a modest benefit.
Population Databases and Meta-analyses
Although the data provided by clinical trials are inconclusive, both a Surveillance, Epidemiology, and End Results analysis and a meta-analysis have assessed the role of RT in AS-HL and add to our understanding. In the Surveillance, Epidemiology, and End Results analysis, the addition of RT appears to consistently improve overall survival for all stages, including stages III and IV.30 However, a Cochrane meta-analysis showed that combined-modality chemotherapy and RT compared with chemotherapy alone was only associated with improved overall survival for early-stage HL.31
Summary
Ultimately, the data regarding the ideal treatment paradigm and the specific role of RT for AS-HL remain inconclusive. Based on the data at hand, it increasingly appears that in the PET-era the role of consolidation RT in PET-negative AS-HL patients is less important, particularly if the patient did not present with bulky disease upfront. In patients with initial bulky disease, the addition of RT after effective systemic therapy may still provide a meaningful PFS benefit, especially for patients who have limited salvage options.
In patients with a partial response on PET after systemic therapy, it is reasonable to treat PET-positive areas with RT, even after the use of novel agents such as Bv. With modern technology including CT-based simulation, deep-inspiration breath hold, and daily image guidance, radiation can be delivered safely with accurate disease targeting and maximal sparing of the organs at risk. Thus, RT in this situation should not be avoided out of fear of toxicity, especially if it offers the patient a chance to be cured.
Footnotes
Sources of support: none.
Disclosures: Drs Lockney and Yang have no conflicts of interest to disclose.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Hasenclever D., Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 3.Eichenauer D.A., Aleman B.M.P., Andre M. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv19–iv29. doi: 10.1093/annonc/mdy080. [DOI] [PubMed] [Google Scholar]
- 4.Aleman B.M., Raemaekers J.M., Tirelli U. Involved-field radiotherapy for advanced Hodgkin's lymphoma. N Engl J Med. 2003;348:2396–2406. doi: 10.1056/NEJMoa022628. [DOI] [PubMed] [Google Scholar]
- 5.Johnson P.W., Sydes M.R., Hancock B.W. Consolidation radiotherapy in patients with advanced Hodgkin's lymphoma: Survival data from the UKLG LY09 randomized controlled trial (ISRCTN97144519) J Clin Oncol. 2010;28:3352–3359. doi: 10.1200/JCO.2009.26.0323. [DOI] [PubMed] [Google Scholar]
- 6.Specht L., Yahalom J., Illidge T. Modern radiation therapy for Hodgkin lymphoma: Field and dose guidelines from the international lymphoma radiation oncology group (ILROG) Int J Radiat Oncol Biol Phys. 2014;89:854–862. doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Cheson B.D., Pfistner B., Juweid M.E. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 8.Engert A., Haverkamp H., Kobe C. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): A randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 9.Juweid M.E., Stroobants S., Hoekstra O.S. Use of positron emission tomography for response assessment of lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 10.Johnson P., Trotman J., Federico M. Interim PET-CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;375:999–1000. doi: 10.1056/NEJMc1609333. [DOI] [PubMed] [Google Scholar]
- 11.Barrington S.F., Mikhaeel N.G., Kostakoglu L. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borchmann P., Haverkamp H., Diehl V. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin's lymphoma: Final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29:4234–4242. doi: 10.1200/JCO.2010.33.9549. [DOI] [PubMed] [Google Scholar]
- 13.Gordon L.I., Hong F., Fisher R.I. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–691. doi: 10.1200/JCO.2012.43.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallamini A., Tarella C., Viviani S. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: Long-term results of the GITIL/FIL HD 0607 Trial. J Clin Oncol. 2018;36:454–462. doi: 10.1200/JCO.2017.75.2543. [DOI] [PubMed] [Google Scholar]
- 15.Ricardi U., Levis M., Evangelista A. OC-0502 Role of consolidation RT to bulky lesions of advanced Hodgkin lymphoma: Results of FIL HD0801 trial. Radiother Oncol. 2019;133:S258–S259. [Google Scholar]
- 16.Zinzani P.L., Broccoli A., Gioia D.M. Interim positron emission tomography response-adapted therapy in advanced-stage Hodgkin lymphoma: Final results of the phase II part of the HD0801 Study. J Clin Oncol. 2016;34:1376–1385. doi: 10.1200/JCO.2015.63.0699. [DOI] [PubMed] [Google Scholar]
- 17.Aleman B.M., Raemaekers J.M., Tomisic R. Involved-field radiotherapy for patients in partial remission after chemotherapy for advanced Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 2007;67:19–30. doi: 10.1016/j.ijrobp.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Borchmann P., Goergen H., Kobe C. PET-guided treatment in patients with advanced-stage Hodgkin's lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2018;390:2790–2802. doi: 10.1016/S0140-6736(17)32134-7. [DOI] [PubMed] [Google Scholar]
- 19.Connors J.M., Jurczak W., Straus D.J. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018;378:331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A., Casulo C., Yahalom J. Brentuximab vedotin and AVD followed by involved-site radiotherapy in early stage, unfavorable risk Hodgkin lymphoma. Blood. 2016;128:1458–1464. doi: 10.1182/blood-2016-03-703470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansell S.M., Lesokhin A.M., Borrello I. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R., Zinzani P.L., Fanale M.A. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younes A., Santoro A., Shipp M. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armand P., Rodig S., Melnichenko V. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37:3291–3299. doi: 10.1200/JCO.19.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Immunotherapy (nivolumab or brentuximab vedotin) plus combination chemotherapy in treating patients with newly diagnosed stage III-IV classic Hodgkin lymphoma: NCT03907488. 2019. https://clinicaltrials.gov/ct2/show/NCT03907488 Available at:
- 26.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Hodgkin Lymphoma. Version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf Available at:
- 27.Constine L.S., Yahalom J., Ng A.K. The role of radiation therapy in patients with relapsed or refractory Hodgkin lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;100:1100–1118. doi: 10.1016/j.ijrobp.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Kriz J., Reinartz G., Dietlein M. Relapse analysis of irradiated patients within the HD15 trial of the German Hodgkin Study Group. Int J Radiat Oncol Biol Phys. 2015;92:46–53. doi: 10.1016/j.ijrobp.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 29.Press O.W., Li H., Schoder H. US Intergroup Trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34:2020–2027. doi: 10.1200/JCO.2015.63.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Master S., Koshy N., Wilkinson B. Effect of radiation therapy on survival in Hodgkin's lymphoma: A SEER data analysis. Anticancer Res. 2017;37:3035–3043. doi: 10.21873/anticanres.11658. [DOI] [PubMed] [Google Scholar]
- 31.Franklin J.G., Paus M.D., Pluetschow A. Chemotherapy, radiotherapy and combined modality for Hodgkin's disease, with emphasis on second cancer risk. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003187.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]