Abstract
Purpose
We report on the clinical performance of a fully automated approach to treatment planning based on a Pareto optimal, constrained hierarchical optimization algorithm, named Expedited Constrained Hierarchical Optimization (ECHO).
Methods and materials
From April 2017 to October 2018, ECHO produced 640 treated plans for 523 patients who underwent stereotactic body radiation therapy (RT) for paraspinal and other metastatic tumors. A total of 182 plans were for 24 Gy in a single fraction, 387 plans were for 27 Gy in 3 fractions, and the remainder were for other prescriptions or fractionations. Of the plans, 84.5% were for paraspinal tumors, with 69, 302, and 170 in the cervical, thoracic, and lumbosacral spine, respectively. For each case, after contouring, a template plan using 9 intensity modulated RT fields based on disease site and tumor location was sent to ECHO through an application program interface plug-in from the treatment planning system. ECHO returned a plan that satisfied all critical structure hard constraints with optimal target volume coverage and the lowest achievable normal tissue doses. Upon ECHO completion, the planner received an e-mail indicating the plan was ready for review. The plan was accepted if all clinical criteria were met. Otherwise, a limited number of parameters could be adjusted for another ECHO run.
Results
The median planning target volume size was 84.3 cm3 (range, 6.9-633.2). The median time to produce 1 ECHO plan was 63.5 minutes (range, 11-340 minutes) and was largely dependent on the field sizes. Of the cases, 79.7% required 1 run to produce a clinically accepted plan, 13.3% required 1 additional run with minimal parameter adjustments, and 7.0% required ≥2 additional runs with significant parameter modifications. All plans met or bettered the institutional clinical criteria.
Conclusions
We successfully implemented automated stereotactic body RT paraspinal and other metastatic tumors planning. ECHO produced high-quality plans, improved planning efficiency and robustness, and enabled expedited treatment planning at our clinic.
Introduction
Automated treatment planning and its clinical application has been an active research subject in radiation therapy (RT) for many years.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 The goals of automated treatment planning are to improve the efficiency, consistency, and quality of treatment plans for individual patients. Various automated treatment planning strategies have been investigated, including (1) knowledge-based planning (KBP), which extracts knowledge from the prior clinically approved treatment plans and generates clinically acceptable plans8, 9, 10, 11,15,17; (2) multicriteria optimization (MCO), which allows users to navigate the prior generated Pareto surface to create clinical plans,1,6,13; and (3) constrained hierarchical optimization (also known as prioritized optimization or lexicographic optimization) to create an optimized plan based on clinical priorities.2,4,5,16,18
Our institution has recently developed an automated approach to intensity modulated RT treatment planning using the constrained hierarchical optimization approach,4,5,16 which is internally referred to as the Expedited Constrained Hierarchical Optimization (ECHO) system.18 The clinical criteria the institution requires to be always met are formulated as hard constraints and therefore strictly enforced by the optimization. Other clinical criteria defined as “desired” (eg, better planning target volume [PTV] coverage and lower normal-organ doses) are optimized by solving sequential constrained optimization problems. Using application program interface capabilities, ECHO has been implemented as a plug-in that can be launched directly from the commercial treatment planning system (TPS) Eclipse (Varian Medical Systems, Palo Alto, CA).
ECHO creates automated plans using external optimization solvers and is completely independent from the TPS optimization engine. The system only requires the users to prepare the contours and beam arrangement before launching the plug-in. In a preclinical retrospective study of 75 paraspinal patients (25 plans for 24 Gy in a single fraction and 50 plans for 27 Gy in 3 fractions), ECHO paraspinal plans were found to be dosimetrically superior compared with clinically treated manual plans with respect to tumor coverage, plan conformity as measured by the Paddick conformity index (PCI),19 and organ-at-risk (OAR) sparing.18 Our preclinical study also demonstrated the deliverability of ECHO plans with duty cycles (ie, total monitor units of all beams divided by the prescription dose per fraction in cGy) with acceptable range and that ECHO passed rigorous quality assurance (QA) procedures.18
Since April 2017, ECHO has been implemented at our clinic for the automated planning of stereotactic body RT (SBRT) paraspinal and other metastatic cases. In this manuscript, we report our initial clinical experience using ECHO. This study was approved by the institutional review board of our institution.
Methods and materials
Patient populations
From April 2017 to October 2018, 523 patients underwent SBRT for paraspinal or other metastatic lesions with 640 different ECHO-produced plans at our institution. Table 1 shows the characteristics of the patients and plans. A total of 182 plans were for 24 Gy in a single fraction, 387 plans were for 27 Gy in 3 fractions, and the rest were for various prescription doses with varied fractionations. Most plans were for paraspinal tumors, with 69, 302, and 170 in cervical, thoracic, and lumbosacral spine, respectively. Ninety-nine plans were for other metastatic tumor sites (hereafter referred to as metastases for simplicity), including 89 plans for bone metastases in the pelvis, shoulder, and ribs, and 10 plans were for nodal tumors.
Table 1.
Characteristics of SBRT paraspinal and other metastatic tumor patients planned with ECHO
| 24 Gy × 1 noPreRT |
9 Gy × 3 noPreRT |
9 Gy × 3 preRT |
8 Gy × 5 preRT |
Other | Total | |
|---|---|---|---|---|---|---|
| Paraspinal (541 ECHO plans) | ||||||
| C spine | 13 | 40 | 8 | 5 | 3 | 69 |
| T spine | 99 | 111 | 62 | 17 | 13 | 302 |
| L-S spine | 66 | 58 | 33 | 8 | 5 | 170 |
| Other metastatic tumors (99 ECHO plans) | ||||||
| Pelvic bones | - | 41 | - | - | 15 | 56 |
| Shoulder area bones | - | 15 | - | - | 2 | 17 |
| Ribs | - | 12 | - | - | 4 | 16 |
| Nodes | - | 7 | - | - | 3 | 10 |
Abbreviations: ECHO = Expedited Constrained Hierarchical Optimization; noPreRT = no relevant prior radiation therapy; Other = plans with miscellaneous fractionations with or without preRT; PreRT = pertinent prior radiation therapy treatment affecting OAR dose constraints; SBRT = stereotactic body radiation therapy.
Simulation and contours
All patients had computed tomography (CT) simulation based on our departmental SBRT simulation procedures. For paraspinal patients, a CT simulation scan was usually acquired immediately after a separate CT myelogram to highlight the spinal cord/cauda. CT slice thickness was 0.2 cm. The gross target volume, clinical target volume (CTV), PTV, spinal cord, or cauda as defined by myelogram, and other OARs, such as the esophagus, brachial plexus, and large and small bowel, were contoured. The PTV was created using a 0.3 cm expansion of the CTV if the thecal sac or esophagus was not included. Ootherwise, the PTV was pulled back to the CTV boundary.20
ECHO planning clinical workflow
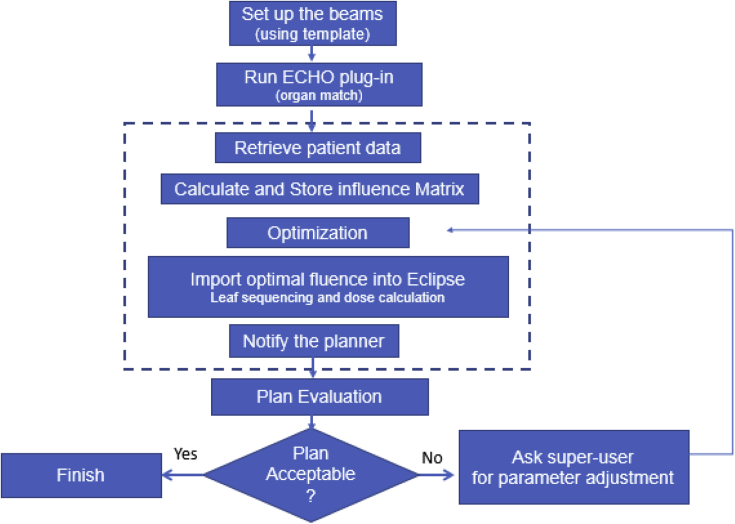
Figure 1 illustrates the ECHO planning clinical workflow. The first step is to insert a plan using one of the predefined Eclipse plan templates, with 9 fixed fields created for ECHO SBRT. For paraspinal plans, the fields were mostly posterior with gantry angles 20° apart for a 160° total span, except for C-spine cases, which had 7 posterior beams and 2 oblique anterior beams to avoid going through the shoulder area. For metastases plans, different beam arrangement templates were provided based on the location of the tumor, with beams 20° apart for a total span of 160°. These templates account for pathlength considerations and clearance issues.
Figure 1.
Expedited Constrained Hierarchical Optimization system clinical workflow.
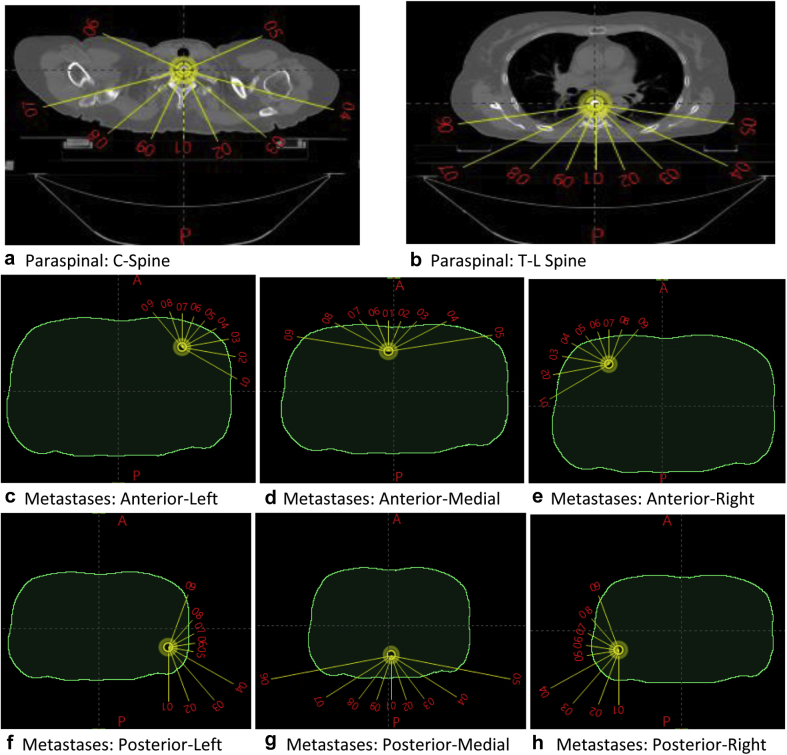
Figure 2 illustrates the default Eclipse plan templates we created for the ECHO SBRT plans. The collimator angles for the template beams were alternated between 0° and 90° by default. Planners can adjust the beam parameters based on the patient’s special anatomic features, tumor location, and clearance considerations after loading a template. Planners can also reduce the number of beams if the fractional dose is <8 Gy. For paraspinal cases, each field had a default 0.3 cm multileaf collimator (MLC) margin and 0.5 cm jaw margin around the PTV. For metastases cases, a default 0.1 cm MLC margin around the PTV was set (a tighter margin than paraspinal cases to further improve conformality). Truebeam (Varian Medical Systems, Palo Alto, CA) machines with 6X-FFF were used for plan delivery, and most plans were delivered on a machine with a high-definition MLC.
Figure 2.
Default plan templates for paraspinal and metastases Expedited Constrained Hierarchical Optimization system stereotactic body radiation therapy plans. The collimator angles for the template beams alternated between 0° and 90° by default.
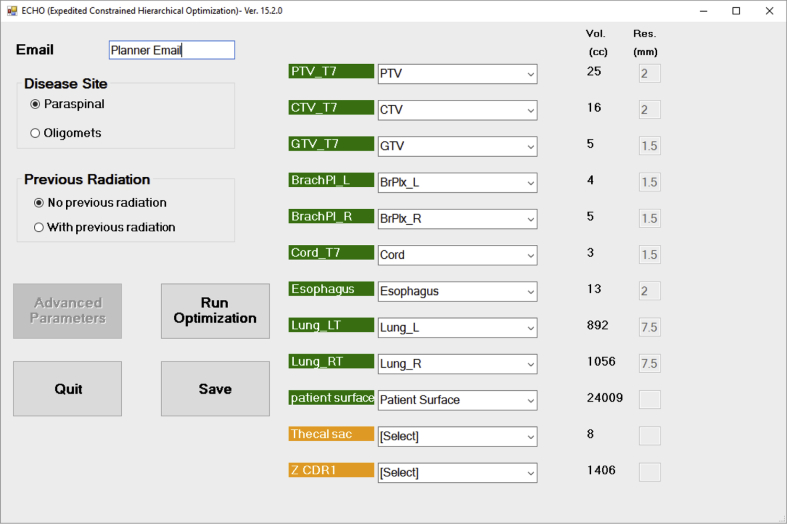
Once the beams are set, the planner opens the ECHO custom graphic user interface (Fig 3) by launching the plug-in from the Eclipse tool menu. The planner first selects the disease site and whether that site had pertinent previous RT, and ECHO loads the appropriate optimization template accordingly. For plans with pertinent previous RT, more stringent requirements on previously treated OARs were incorporated. For some organs (cord, cauda, esophagus), a lower predefined dose constraint was defined in the parameter file inside ECHO. For example, maximum cord dose for re-treatment was 18 Gy in 5 fractions instead of the 30 Gy used without previous treatment, and maximum dose to re-treatment esophagus was 25 Gy in 5 fractions instead of 50 Gy without previous treatment.
Figure 3.
Expedited Constrained Hierarchical Optimization system user interface, launched as a plug-in from Eclipse. The planner matches each contour to the appropriate optimization structure (green) and acknowledges any contours that will not be used in optimization (orange). The “Advanced Parameters” button will be only available for designated superusers. Only structures in green are used in optimization.
For other organs (eg, stomach or bowel), the user is prompted in the interface to input the required dose constraint, which is based on the previously delivered dose and departmental equivalent total dose calculations guidelines. To resolve nomenclature differences, the planner then matches each contour to the appropriate optimization structure and acknowledges any contours that will not be used in optimization. No additional pseudo or dummy structures for optimization purposes are contoured by the planner. A planner’s e-mail will be automatically logged with the case based on the TPS login. The planner runs ECHO by clicking the “Run Optimization” button. The “Advanced Parameters” button, which provides some optimization parameter–tuning capabilities (eg, changing OAR maximum/mean dose criteria), is only accessible to a few designated superusers so that the performance of the ECHO can be monitored and recorded.
After launching ECHO, a plan is created in the background, completely automated and invisible to the planner. ECHO first pulls the patient data needed for optimization (eg, images, contours, and beam data) from Eclipse and then calculates and stores the influence matrix, which contains the dose contribution of each beamlet at each voxel of the patient’s body. Next, the optimization algorithm is activated to generate the plan based on hierarchical constrained optimization. Details of the optimization algorithm can be found in Zarepisheh et al.18
In summary, the ECHO approach consists of 3 constrained optimization steps to maximize target coverage, minimize OAR doses, and smooth the fluence map for delivery efficiency, plus a correction step to incorporate leaf sequencing and scattering contributions into optimization. After the optimization has been completed, the fluence map for each beam is imported back into Eclipse for final forward dose calculations using an Eclipse AAA dose algorithm with a 0.125 cm grid size.
Upon ECHO completion, the planner receives an e-mail indicating the plan is ready for review. The planner evaluates the plan against the clinical planning criteria. The plan is accepted and approved by the physician if all clinical criteria are met; otherwise, the planner requests that a superuser use the “Advanced Parameters” capability to modify the optimization parameters and initiate another run with ECHO.
Quality assurance and delivery
QA based on departmental clinical policies was performed and passed before the treatment delivery of all ECHO plans. At our institution, every single-fraction SBRT plan has patient-specific dosimetry QA measurements before treatment, and all single-fraction ECHO SBRT plans passed this QA test. Leaf motion trajectory logfiles for each beam for all plans were also analyzed after each plan’s delivery in our department. No out-of-tolerance delivery discrepancy was recorded for any beam of delivered ECHO plans.
Results
Planning time
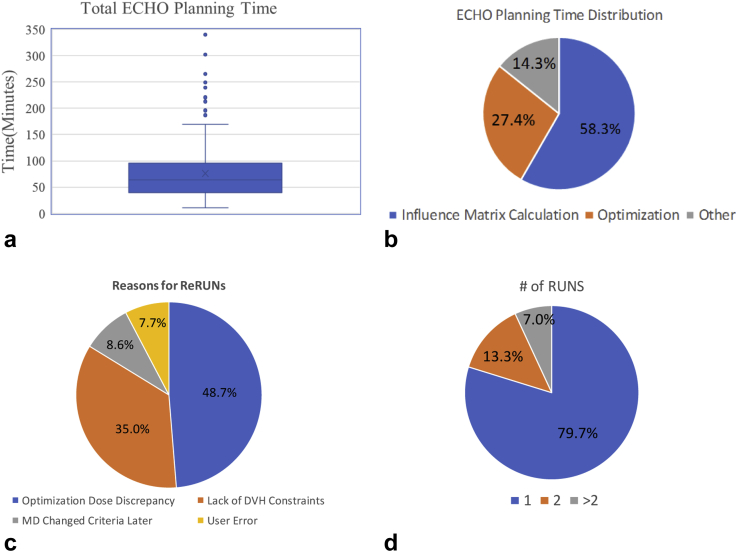
On our computational platform (6 Windows 7 servers with Intel Xeon E5-2680 2.5 GHz CPU and 64 GB RAM), the median time to produce 1 ECHO plan was 63.5 minutes (range, 11-340 minutes; Fig 4a). Of the ECHO plans, 46.3% were generated within 1 hour, 40.7% required between 1 and 2 hours, and only 13.0% took >2 hours. The timing was mainly dependent on the PTV, which determines the field sizes. The PTV median size was 84.3 cm3 (range, 6.9-633.2 cm3). As shown in Figure 4b, on average, 58.3% of the time was spent on influence matrix calculation, 27.4% on optimization, and 14.3% on the rest of operations (eg, importing the optimal fluence into Eclipse and performing the correction step and final dose calculations).
Figure 4.
(a) Total planning time, (b) time dedicated to different parts of calculation, (c) number of runs to create a satisfactory plan, and (d) reasons for multiple runs.
Number of runs
As shown in Figure 4c, 79.7% cases only required 1 run to produce clinically acceptable plans, 13.3% required 1 additional run with optimization parameter adjustment, and 7.0% of all cases required ≥2 additional runs with more parameter modifications. These additional runs were performed and recorded by a few designated superusers after discussion with the planners. As shown in Figure 4d, 48.7% of reruns were due to the discrepancy between the optimization dose and final dose calculation. This typically occurred for re-treatment cases or unusual anatomy and manifested as OARs not meeting the clinical constraints.
Of the reruns, 35.0% were a result of ECHO’s current lack of dose-volume constraints, and adjusted maximum dose constraints for some organs were needed to achieve dose volume constraints. In addition, 8.6% of the reruns were due to changes in treating physicians’ patient-specific criteria preferences. The remaining 7.7% of reruns were recorded as user error. Reruns do not require new influence matrix calculations because the same beam arrangements and contours were used, resulting in a much shorter plan generation time.
Plan quality metrics
All ECHO plans met or improved upon institutional clinical criteria, passed QA processes, and were delivered successfully. We analyzed the dose-volume histogram data for plans with common prescription dose schemes (ie, 24 Gy × 1, 9 Gy × 3, and 8 Gy × 5 for paraspinal cases and 9 Gy × 3 for metastases cases). The institutional ideal and acceptable clinical criteria for target volume coverage and major dose-limiting OARs are listed in Table 2 and illustrated in Figure 5 in green (ideal) and red (acceptable) lines.
Table 2.
Institutional clinical criteria for stereotactic body radiation therapy paraspinal and other metastatic tumor plans
| Plan type | Clinical criteria |
|
|---|---|---|
| Ideal | Acceptable | |
| Paraspinal | ||
| PTV | ||
| V100% (%) | ≥90% | ≥80% |
| V95% (%) | ≥95% | ≥90% |
| Metastases | ||
| PTV | ||
| V100% (%) | ≥98% | ≥95% |
| Single fraction | ||
| CTV | ||
| D(smallest 0.035cc )(Gy) | 15 | |
| GTV | ||
| D(smallest 0.035cc )(Gy) | 15 | |
| Cord | ||
| Dmax (Gy) | ≤14 | |
| D0.35cc (Gy) | ≤10∗ | |
| Cauda | ||
| Dmax (Gy) | ≤18 | |
| D5cc (Gy) | ≤14 | |
| Esophagus | ||
| D2.5cc (Gy) | ≤14 | ≤18 |
| Bowel | ||
| D5cc (Gy) | ≤12 | |
| Three fractions | ||
| Cord | ||
| Dmax (Gy) | ≤24 (13.9†) | ≤26 (14.5†) |
| D0.35cc (Gy) | ≤18 (10†) | |
| Cauda | ||
| Dmax (Gy) | ≤26 (15†) | |
| D5cc (Gy) | ≤22 (13†) | |
| Esophagus | ||
| Dmax (Gy) | ≤27.6 | ≤30 (20.4†) |
| D5cc (Gy) | ≤16.8 | ≤22 |
| Five fractions | ||
| Cord | ||
| Dmax (Gy) | ≤30 (17†) | ≤30 (18†) |
| D0.35cc(Gy) | ≤23 (14†) | |
| Cauda | ||
| Dmax (Gy) | ≤35 (20†) | |
| D5cc (Gy) | ≤30 (17†) | |
| Esophagus | ||
| Dmax (Gy) | ≤32.2 | ≤50 (25†) |
| D5cc (Gy) | ≤19 | ≤27.5 |
Abbreviations: CTV = clinical target volume; GTV = gross target volume; PTV = planning target volume.
10 Gy must not transect the entire cord contour on any axial slice.
Criteria with pertinent previous radiation therapy.
Figure 5.
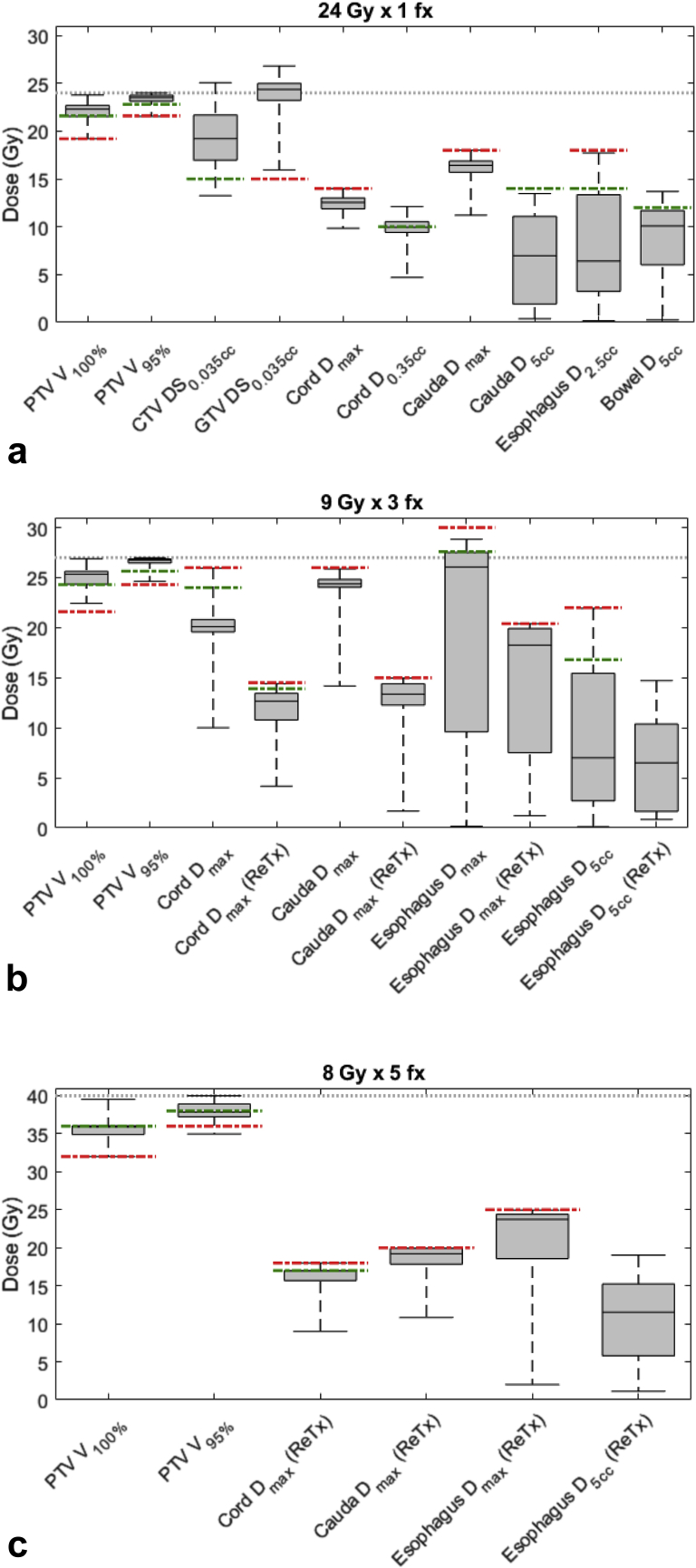
Planning target volume (PTV) coverage and critical structures doses for paraspinal plans. The dotted line indicates prescription dose level, green lines ideal criteria, and red lines acceptable criteria. Of note, if no green or red lines are drawn, there were no established criteria. (a) A total of 178 paraspinal plans at 24 Gy × 1 (PTV median size: 62.7 cm3 [range, 8.8-324.5 cm3]), (b) 312 paraspinal plans at 9 Gy × 3 (PTV median size: 100.5 cm3 [range, 11.3-633.2 cm3]), and (c) 30 paraspinal re-treatment plans at 8 Gy × 5 (PTV median size: 162.8 cm3 [range, 33.4-621 cm3]).
For 24 Gy × 1 paraspinal prescription (Fig 5a), 178 plans were treated. The median PTV size was 62.4 cm3 (range, 8.8-324.5 cm3). In general, treatments with this fractionation do not typically involve previous RT to the same treatment site. All ECHO plans achieved the acceptable target coverage, and 70.2% achieved the ideal target coverage criteria of PTV V100% ≥90% and PTV V95% ≥95%. Minimum dose (measured as the dose to the smallest 0.035 cm3) to CTV and GTV was on average 19.3 ± 2.9 Gy and 23.3 ± 2.6 Gy, respectively. Hotspots >105% outside the PTV were <3 cm3 (desired guideline) for 95% of cases, and no plan had hotspots >115% located outside the PTV (desired guideline). In addition to maximum dose, the dose transecting the spinal cord in any slice was limited to ≤10 Gy for all plans. PCI was 0.85 ± 0.06 for this group. The duty cycles (total monitor units of all beams divided by the prescription dose per fraction in cGy) was 5.5 ± 1.1 for 24 Gy × 1 paraspinal plans.
For 9 Gy × 3 paraspinal cases (Figure 5b), 312 plans were treated. The median PTV size was 100.5 cm3 (range, 11.3-633.2) cm3. A total of 76.0% of plans achieved the ideal target coverage criteria of PTV V100% ≥90% and PTV V95% ≥95%. For 209 plans, the cases were without pertinent treatment to the same site. In addition to maximum dose, dose transecting the spinal cord in any slice was limited to 18 Gy or less for all plans. For 103 plans with pertinent previous RT, dose constraints to the spinal cord and esophagus were very restrictive, as indicated in Table 2 and Figure 5b. PCI score for this group was 0.86 ± 0.05. The duty cycles for this group were 6.8 ± 2.0.
For 8 Gy × 5 paraspinal re-treatment cases (Fig 5c), 30 plans were treated. The median PTV size was 162.8 cm3 (range, 33.4-621.9 cm3). The maximum point doses to the re-treatment cord and cauda were limited to 18 Gy and 20 Gy, respectively. PTV V100% was 89.0 ± 5.1, and PTV V95% was 94.6 ± 3.4. Figure 5c shows plan metrics for these most difficult paraspinal plans with previous RT. PCI for this group was 0.76 ± 0.07. The duty cycles for 8 Gy × 5 paraspinal re-treatment group were 8.5 ± 2.9. The increased duty cycles for this group reflect the high dose gradient required to meet the stringent re-treatment OAR constraints.
For 9 Gy × 3 metastases cases, 75 plans were treated. The median PTV size was 88.0 cm3 (range, 9.0-598.2 cm3), and all ECHO plans except 1 achieved acceptable target coverage, with PTV V100% ≥95%. One case resulted in PTV V100% = 92% because the physician requested further restriction of the dose to the lumbar-sacral plexus abutting the PTV. In addition, 87.8% of plans achieved PTV V100% ≥98% (ideal coverage criteria). The PCI score was 0.90 ± 0.05 for this group. The duty cycles for this group were 4.0 ± 1.5.
Discussion
The goal of ECHO development was to provide an automated planning platform that would optimize normal tissue sparing while still achieving target volume treatment goals, something that requires significant trial and error without constrained optimization methods. A second goal of ECHO is to improve plan consistency, which is also difficult to achieve in a large institution with planners and physicians with different levels of experience and expertise. Finally, ECHO achieves an optimal result that is not dependent on the quality of a learning library of previously treated patient examples. ECHO produced clinically acceptable SBRT plans for patients with paraspinal and other metastatic tumors with a wide range of PTV sizes and OAR constraint requirements. A great deal of effort was spent in making ECHO optimization results close to final deliverable results by smoothing the fluence map within the optimization and incorporating MLC leaf sequencing dosimetric and scattering effects into the optimization with a correction loop.18 This was a critical step to avoid rerunning plans because of significant differences between the ECHO-optimized and Eclipse planning system recomputed (final) deliverable dose distributions.
Of the ECHO plans, 87% were generated within 2 hours using our existing computational platform, and 79.7% of plans were clinically accepted with only 1 time run. ECHO demonstrated the ability to produce high-quality SBRT plans in terms of target coverage and dose conformity under a high prescription dose and very restrictive OAR dose criteria. As a result of the consistent performance of ECHO in producing high-quality paraspinal and metastases plans efficiently, we have currently reduced the time between simulation and treatment at our clinic by 1 day for paraspinal and other metastatic tumor SBRT plans.
Influence matrix calculations took 58.3% of the total optimization time on average, which is highly dependent on field sizes and dose calculation speed. A faster dose calculation process by vendors (eg, GPU-based dose calculation) or upgrading of computer hardware would accelerate this process and improve the efficiency of ECHO to shorten the planning time.
As part of ECHO’s clinical implementation, we designated a few superusers to monitor ECHO’s performance and gather data on the circumstances under which additional runs of ECHO were required. Approximately half of the reruns were due to discrepancies between the optimization dose and final dose calculation for OARs in situations including previous RT to the same site or unusual anatomy. We are in the process of updating our user interface to allow for a limited range of OAR optimization parameter adjustments by the planners to accommodate patient-specific criteria changes due to physician preference or patient treatment history. Approximately 35% of rerun cases were due to lack of dose-volume constraints and adjusting the maximum dose constraints for organs in the current implementation of ECHO. A computationally efficient technique to incorporate dose-volume constraints into fluence map optimization has been developed and will be implemented into ECHO in the near future.21
Automated treatment planning algorithms are currently available in multiple commercial treatment planning systems. One commercially available algorithm is based on KBP.8, 9, 10, 11 KBP requires significant resources for model building based on preexisting clinical plans and is not easily adaptable to the new clinical practices. Once the model is built, however, KBP can quickly produce new plans by leveraging the predicted achievable plan for the new patient. Lin et al.22 demonstrated that building KBP models with constrained hierarchical optimization plan data could result in a high-quality KBP model to be used to quickly adapt KBP to changes in clinical practice.
MCO is another commercially available automated planning system6,13 that generates a pool of Pareto optimal plans upfront based on a few key clinical objectives and then allows the user to navigate among them and select the plan with the preferred clinical criteria trade-offs. Although MCO enjoys adaptability to the clinical changes, it is a semiautomated treatment planning system that still relies on user experience and skills.
ECHO can produce Pareto optimal plans efficiently and independent of user experience.18 The system is easily adaptable to dynamic and changing clinical practice in terms of prescription doses, fractionation schema, and OAR criteria. A limitation of ECHO is that the system requires significant effort to establish a template of goals, objective functions, and parameters for a given disease site because each site has different clinical criteria and priorities. We expect that this will become easier as we gain more experience with other treatment sites.
Since the implementation of ECHO plans in April 2017, we have used ECHO to generate approximately 30 paraspinal or other metastatic tumor SBRT plans per week throughout our clinical network. We are also working on expanding ECHO to other disease sites and developing a delivery-efficient volumetric modulated arc technique.
Conclusions
We successfully implemented a constrained hierarchical optimization method at our clinic for automated SBRT paraspinal and other metastatic tumors planning. ECHO has achieved the expected goals of producing consistently high-quality clinical plans in a reasonable time that pushes normal tissue sparing as much as possible while respecting disease treatment goals. This has further resulted in an improved clinical workflow and shorter times between simulation and treatment at our clinic.
Footnotes
Sources of support: This work was partially supported by the MSK Cancer Center Support Grant/Core Grant (NIH P30 CA008748) and the Enid A. Haupt Endowed Chair Fund.
Disclosures: Drs Hong, Deasy, Mageras, Mechalakos, and Zarepisheh and Ms Hunt have a patent pending, entitled “Methods and systems for automatic radiation therapy treatment planning.” Dr Yamada reports fees from BrainLab, as well as personal fees from Vision RT, Institute for Medical Education, and University of Wollongong, and other fees from the Chordoma Foundation outside the submitted work.
References
- 1.Halabi T., Craft D., Bortfeld T. Dose-volume objectives in multicriteria optimization. Phys Med Biol. 2006;51:3809–3818. doi: 10.1088/0031-9155/51/15/014. [DOI] [PubMed] [Google Scholar]
- 2.Breedveld S., Storchi P.R., Keijzer M. A novel approach to multicriteria inverse planning for IMRT. Phys Med Biol. 2007;52:6339–6353. doi: 10.1088/0031-9155/52/20/016. [DOI] [PubMed] [Google Scholar]
- 3.Jee K.W., McShan D.L., Fraass B.A. Lexicographic ordering: Intuitive multicriteria optimization for IMRT. Phys Med Biol. 2007;52:1845–1861. doi: 10.1088/0031-9155/52/7/006. [DOI] [PubMed] [Google Scholar]
- 4.Wilkens J.J., Alaly J.R., Zakarian K. IMRT treatment planning based on prioritizing prescription goals. Phys Med Biol. 2007;52:1675–1692. doi: 10.1088/0031-9155/52/6/009. [DOI] [PubMed] [Google Scholar]
- 5.Clark V.H., Chen Y., Wilkens J. IMRT treatment planning for prostate cancer using prioritized prescription optimization and mean-tail-dose functions. Linear Algebra Appl. 2008;428:1345–1364. doi: 10.1016/j.laa.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monz M., Kufer K.H., Bortfeld T.R. Pareto navigation: Algorithmic foundation of interactive multicriteria IMRT planning. Phys Med Biol. 2008;53:985–998. doi: 10.1088/0031-9155/53/4/011. [DOI] [PubMed] [Google Scholar]
- 7.Breedveld S., Storchi P.R., Heijmen B.J. The equivalence of multicriteria methods for radiotherapy plan optimization. Phys Med Biol. 2009;54:7199–7209. doi: 10.1088/0031-9155/54/23/011. [DOI] [PubMed] [Google Scholar]
- 8.Chanyavanich V., Das S.K., Lee W.R. Knowledge-based IMRT treatment planning for prostate cancer. Med Phys. 2011;38:2515–2522. doi: 10.1118/1.3574874. [DOI] [PubMed] [Google Scholar]
- 9.Moore K.L., Brame R.S., Low D.A. Experience-based quality control of clinical intensity modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81:545–551. doi: 10.1016/j.ijrobp.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Wu B., Ricchetti F., Sanguineti G. Data-driven approach to generating achievable dose-volume histogram objectives in intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;79:1241–1247. doi: 10.1016/j.ijrobp.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Appenzoller L.M., Michalski J.M., Thorstad W.L. Predicting dose-volume histograms for organs-at-risk in IMRT planning. Med Phys. 2012;39:7446–7461. doi: 10.1118/1.4761864. [DOI] [PubMed] [Google Scholar]
- 12.Breedveld S., Storchi P.R., Voet P.W. Icycle: Integrated, multicriterial beam angle, and profile optimization for generation of coplanar and noncoplanar IMRT plans. Med Phys. 2012;39:951–963. doi: 10.1118/1.3676689. [DOI] [PubMed] [Google Scholar]
- 13.Craft D.L., Hong T.S., Shih H.A. Improved planning time and plan quality through multicriteria optimization for intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:e83–e90. doi: 10.1016/j.ijrobp.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voet P.W., Dirkx M.L., Breedveld S. Toward fully automated multicriterial plan generation: A prospective clinical study. Int J Radiat Oncol Biol Phys. 2013;85:866–872. doi: 10.1016/j.ijrobp.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Zarepisheh M., Long T., Li N. A DVH-guided IMRT optimization algorithm for automatic treatment planning and adaptive radiotherapy replanning. Med Phys. 2014;41:061711. doi: 10.1118/1.4875700. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari P.B. Automating intensity modulated radiation therapy treatment planning by using hierarchical optimization. Engineering and Applied Science Theses & Dissertations. 2015;12:140. [Google Scholar]
- 17.Wang H., Dong P., Liu H. Development of an autonomous treatment planning strategy for radiation therapy with effective use of population-based prior data. Med Phys. 2017;44:389–396. doi: 10.1002/mp.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarepisheh M., Hong L., Zhou Y. Automated intensity modulated treatment planning: The expedited constrained hierarchical optimization (echo) system. Med Phys. 2019;46:2944–2954. doi: 10.1002/mp.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):219–222. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 20.Cox B.W., Spratt D.E., Lovelock M. International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597–e605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee S, Hong L, Deasy JO, Zarepisheh M. Integrating soft and hard dose-volume constraints into hierarchical constrained IMRT optimization. Med Phys. 2019; in press. [DOI] [PMC free article] [PubMed]
- 22.Lin Y.H., Hong L.X., Hunt M.A. Use of a constrained hierarchical optimization dataset enhances knowledge-based planning as a quality assurance tool for prostate bed irradiation. Med Phys. 2018;45:4364–4369. doi: 10.1002/mp.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]