Abstract
Purpose
The ambulatory patient experience is heavily influenced by wait times for provider care. Delayed patient visit start times may negatively affect overall satisfaction, and increased wait times affect the perception of the information, instructions, and treatment given by health care providers. Improving institutional practices overall requires the determination of the essential quality metrics that will make such an achievement possible. A protracted time leading up to the initiation of radiation therapy may promote poor satisfaction and perceived quality of care for both patients and referring providers alike, which may then create a barrier to patients being treated with radiation therapy. This institution piloted and sucessfully completed a study into improving the timeliness of initiation of patient radiation therapy for our patients.
Methods and Materials
This work sought to identify inefficiencies in radiation therapy treatment planning to shorten the time each patient waited for treatment. We examined the time between simulation to the start of the first fraction of treatment. This period includes simulation, contouring, treatment planning, and quality assurance of the plan.
Results
Before the study, the planning process would typically take 2 weeks. Target and organs-at-risk contouring were found to be the main inefficiency delaying treatment start dates. This delineating process includes drawing contours on radiologic images, typically computed tomography and magnetic resonance imaging. We focused on the time needed for the contouring process to be completed and took steps to increase efficiency. The length of time from simulation to contour approval was decreased by more than 60%, a reduction from an average of more than 4 days to less than 1.5 days. Overall planning time dropped from 2 weeks to less than 5 days.
Conclusions
Process improvements and implementation of task-specific tools improved the timeliness of patient treatments, reducing the overall planning time from simulation to treatments to less than 5 days. Continuous monitoring and modification of these processes revealed that the successes achieved toward better quality of care have been sustained.
Introduction
All health care institutions strive to provide quality care. The Institute of Medicine’s Committee on Quality of Health Care in America emphasizes that timeliness is a priority in providing quality health care.1 From a patient perspective, timeliness is a key facet of quality and a metric of the patient satisfaction experience.2, 3, 4 Timeliness of care broadly affects patient satisfaction throughout the health care enterprise. In radiation therapy, timeliness affects the efficacy of radiation therapy outcomes,1,5,6 which, in turn, affects patient survival in many treatment sites.2,7,8
The importance of timeliness in radiation therapy workflow must still be evaluated in the context of timeliness of the overall cancer care process, from diagnosis through every aspect of treatment.9,10 We describe a departmental effort, conducted in phases, to systematically reduce the time interval from patient simulation to treatment initiation at our institution. We report quantitative measures of timeliness immediately after interventions as well as the durability of the improvements over time.
Methods and Materials
The number of faculty and staff participating directly in this particular study included 14 physicians, 11 physicists, and 7 dosimetrists. Six external beam treatment units with 5 external beam planning systems were involved in the study.
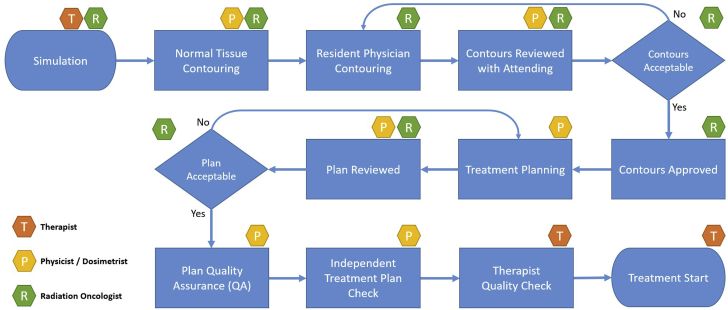
The steps taken to improve timeliness of patient treatments were comprised of 4 phases. Figure 1 shows the treatment planning flowchart starting from the patient simulation to the start of treatment. Briefly, phase I consisted of efforts to reduce the time from simulation to physician approval of contours. Phase II consisted of monitoring to ensure sustainability of any improvements. In phase III, interventions that were made were adjusted and modified to work well and to the greatest effect. Finally, phase IV was geared toward ensuring the robustness of the interventions for long-term sustainability.
Figure 1.
Treatment planning flowchart starting from the simulation to the start of treatment.
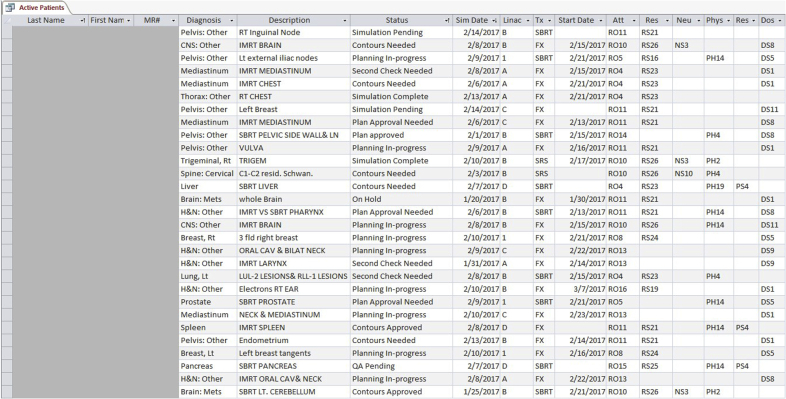
Tracking the time between each stage of the planning process was achieved through a database developed using Microsoft Access (Microsoft Corporation, Redmond, WA), shown in Figure 2. This in-house developed database allowed the flexibility to track and time stamp each step of the planning process. The database resided on a network server, which made it accessible to any computer on the network. A macro was created that would record any changes in the status of a patient’s plan as it progressed through the planning process. A time-stamp was saved with each change to record the time and date the changes were made.
Figure 2.
The patient database keeps track of patients within the planning process and notifies staff as important deadlines approach throughout the planning process.
Phase IA: Initial assessment of inefficiencies in the planning process
The initial phase of the project started in February 2011, when typical times from simulation to start of treatment averaged approximately 2 weeks. Our primary target was to limit this time interval from 2 weeks to 5 working days. Upon detailed analysis of the steps involved in the planning process between simulation and treatment, we concluded that 5 days was an achievable time frame in which to complete the contouring, planning, and quality assurance (QA) tasks needed to begin patient treatments. To exclude delays that were outside of our control, for example, authorizations for special imaging studies, conflicts in a patient’s schedule for personal reasons, or waiting for concurrent chemo to start, we excluded the top 10th percentile in terms of the greatest number of days. Upon review of the details of the outliers, we found this adequately excluded cases that were delayed owing to issues outside of our department and would skew our data owing to processes that were out of our control.
Emergency or “sim-and-treat” patients follow a different and expedited timeline for treatments that start the same or following day. These cases had much shorter time constraints compared with the cases we were focused on to increase efficiency. The main focus of improvement was the reduction in time from simulation to approved contours because an initial time study using the database showed that a disproportionate amount of time was spent on this step. For the plans that do not require measurements, the physics QA is completed within hours of the plan approval by the attending physician. For the plans requiring QA measurements, they are performed by the next business day after approval of the plan.
Phase IB: Focus on resolving inefficiencies
Guidelines were instituted that imposed time limits on each task. These changes in procedure were implemented for efficiency:
-
1.
Simulation-based radiation therapists entered patients into the database before simulation.
-
2.
The assigned planner was expected to attend the simulation and review patient setup and immobilization.
-
3.
Images needed for contouring were imported into the contouring system immediately after simulation.
-
4.
Improved communication during handoff of patients was improved.
Instead of entering patients into the database during simulation, patients were entered into the database at least a day before simulation. This allowed a physicist, who was designated as a planning supervisor, to assign a planner to each patient before the actual simulation.
Once assigned, the assigned planner would carry the plan through to the patient’s start of treatment. This included attending the simulation to observe what immobilization devices were used and how they were implemented. The planner would often times take an active part in choosing immobilization techniques to facilitate both planning and treatment delivery.
Imaging studies that were necessary for contouring were imported immediately after simulation to make them available for contouring. Simulation images were sent directly to the planning system from the simulator while images from picture archive and communication system, including magnetic resonance images, positron emission tomography images, and images from outside institutions, could be imported before simulation.
Communication among staff was essential for handoffs. Both email and other electronic modes of communication were explored to make communication more reliable and automatic.
Phase II: Monitor improvements for efficacy and sustainability
-
1.
Add more detail to the tracking ability of the database.
-
2.
Enable the patient database to track rate of progression through the planning process.
The second phase was to monitor the improvements made, both to observe the effects of the potential improvements as well as to confirm sustainability. The Microsoft Access database was initially used to prevent loss of communication between simulation therapist and planner during handoff. The database was modified from its initial use to accommodate time tracking of the patient’s plan through the planning process leading up to the start of treatment. A macro was added that would be triggered to create a timestamp after a change in the patient’s status. The status tracked where in the planning process a patient’s treatment plan was at any particular time. The status was changed manually by the planner. For example, a typical progression leading up to approved contours of a patient’s plan would be: “simulation complete,” “contours needed,” “contours ready for review,” to “contours approved.” From there, the planner would complete the treatment plan then change the status to “plan approval needed.” Each of these changes in status would trigger the macro to record the change. The information recorded would be the patient’s name and identification number, the status before the change, the status after the change, and the time and date of the change. The simulation date was also recorded as a point of reference.
Because the status had to be changed manually, the change in status suffered from a delay. If contours were approved by the attending physician who then sent an email to the planner, that email may not have been read by the planner for a time. The status would go unchanged until the planner became aware of the email and changed the status.
This observation phase not only allowed us to see the inefficiencies in our planning process, it also gave us ideas on how to fix them. Imposing the time limits to stress the process showed weaknesses in the process by causing an emphasized stress at choke points. Once identified, changes in process and implementation of tools allowed us to address the specific problems.
Phase III: Modify improvements and implement new tools
To maintain and further improve upon what was initially achieved, interventions were made to the planning process and notification systems:
-
1.
Contouring-specific software was implemented to standardize the contouring process.
-
2.
A physician dashboard was implemented to improve communication.
-
3.
A culture of improvement was created to promote feedback and encourage ideas for better quality of service.
-
4.
An incident-reporting website was implemented.
MIM software (MIM Software, Inc, Cleveland, OH) was used for contouring. Implementation of MIM as a contouring platform allowed contouring to be performed within a single software with a specific set of familiar tools, regardless of the treatment machine and planning software used. The contours developed within this platform were then transferred to the corresponding planning system for the assigned treatment machine for that patient.
A physician dashboard was implemented in the treatment planning room. The dashboard identified patients needing the attention of an attending physician, for example, needing contours drawn or plans reviewed. The goal was to allow the physician to know at a glance what was needed from them immediately upon entering the planning room.
Throughout this phase, the project was reviewed at the department’s weekly quality meeting and at various faculty and staff meetings to culturally reinforce its importance and to solicit all staff for possible adjustments or further improvements.
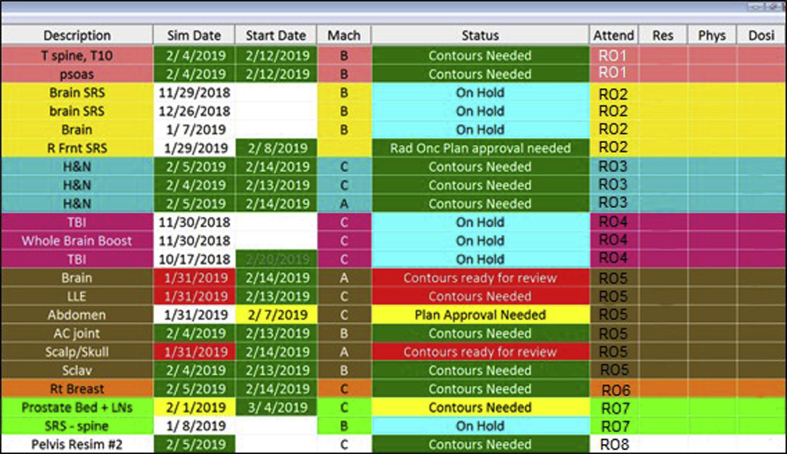
The dashboard developed into a constant visual reminder illustrating where every patient was at in the planning process. A new color-coded urgency scale was implemented (shown in Fig 3) to illustrate exigency. The color-coding was linked to the 48-hour time limit for physician approval of contours. For contours, the simulation date and the status would show in green less than 24 hours out from simulation. Once this was passed, they would show yellow until 48 hours was reached. After that, the 48-hour criterion was violated and these fields would be highlighted in red. Similarly, for the next step in the planning process, the same color-coding was implemented to express the urgency of plan approval as the 5-day rule for planning approached.
Figure 3.
Physician dashboard showing improved color coding. Colors were included to alert staff to overdue items, to group patients by physician, and to make it easier for physicians to spot their patients on the board. This version of the dashboard updates every 5 seconds from information drawn from the patient database.
Phase IV: Ensure robustness for long-term sustainability
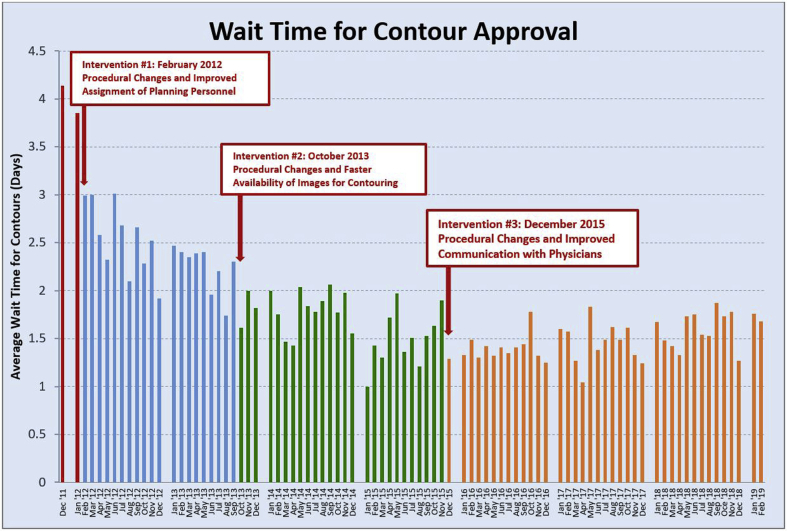
In the fourth phase of the project, the question of long-term sustainability was considered. Often, over time, programs such as this are forgotten about or staff may lose interest in sustained efforts to keep the program alive. Long-term observations from February 2014 through the end of 2015 showed sustained improvement (Fig 4). These improvements were then applied over a broader scope of the treatment planning process of care. To ensure long-term sustainability, steps were taken to make the changes an ongoing process to make it an organic part of the department’s day-to-day:
-
1.
Physician dashboard was improved to convey more information on timeliness to improve response times.
-
2.
Capabilities of the patient database were expanded to be used by staff beyond the planning process.
-
3.
Training and on-boarding of new faculty, residents, and staff were implemented to inform new employees of the utility and intricacy of clinical processes and the departmental standards for timeliness.
-
4.
Cultural commitment and communication among staff and faculty were stressed to ensure continuity even in the face of employee changes.
-
5.
Weekly quality team meetings were maintained and enhanced with monthly updates to the entire department.
Figure 4.
Data showing time from simulation to contour approval, revealing sustained improvement over the course of the 4 phases of the project.
The physician dashboard was improved to show more directed information from changes in the patient database. It went from being a query within the Access database to a standalone program, written in C++, which would poll the database for information to be displayed. The colors were also improved to group patients by physician and to better show the urgency of impending deadlines for contours and the 5-day rule for plan approval more accurately.
The patient database was also made accessible to all staff, from front desk personnel who schedule patients for simulation to therapists at the treatment machines anticipating the start of each new patient. Insurance authorization was included in the database. This information would dictate whether or not more complex treatment techniques like intensity modulated radiation therapy were authorized for treatment planning. Staff responsible for insurance authorization communicated changes in the authorization status to planners through the database in combination with other electronic communication.
Staff from the department of neurosurgery were also included to facilitate communication between the departments for the coplanning of radiosurgery patients. This raised awareness for all staff for each patient’s progress through the entire planning process.
The broadened scope of the project introduced increased complexity. Disparate types of staff doing completely different jobs had to be able to coordinate efforts, which became especially difficult during changes in staffing. The encouragement of constant discussion was achieved through open and frequent meetings specifically to address lapses in process efficiency associated with staff turnover. Effective and efficient on-boarding techniques became essential in easing new faculty and staff into the processes in place. This became especially useful as new residents entered the program and as senior residents graduated. These transitions were used as a test for the durability of our improvements.
Results
Before implementing the 5-day rule from simulation to start of treatment, it would typically take 2 weeks from the time of simulation for a patient to start treatment. Although we had enacted the 5-day rule, we realized that most of the time compression achieved was in the time for treatment planning. Once the 5-day rule was imposed, starting in December of 2011, the average time between simulation and approval of contours went from 4.14 days to 3.85 days. That left a little more than a single day for treatment planning. Constant changes and standardization in the notification of physicians were implemented that included emails and dashboards. In February 2012, there was a decrease in the average wait time to 2.99 days. Over the subsequent 2 years, which included the addition of the MIM software and the 48-hour limit for contouring, the consistent decrease in the wait time for contours continued. Ongoing monitoring through phase IV showed that by April of 2014 the wait time had dropped to 1.43 days (Fig 4). This allowed the planners more time for treatment planning within the 5 days allotted from the day of simulation. Further improvements in communication and implementation of newer technology such as the physician dashboard also combined to improve the timeliness of contouring.
Implementation of the MIM software also had a large effect on the sustainability of improvements. MIM standardized the contouring process because it was used to contour patients for all of the machines. Only some of the stereotactic radiosurgery patients were contoured either in iPlan (BrainLab AG, Munich, Germany) or in BrainLab’s Elements software (BrainLab AG, Munich, Germany) for patients with multiple brain metastases.
The patient database played in important role in bringing about improvements in efficiency. Starting from its use by the planners to track patients, it is now used throughout the department to track things from insurance authorization to completion of treatment. It now plays an integral role in the day-to-day operations of the department.
The physician dashboard played an important role in achieving our 5-day rule for bringing patients from simulation to treatment. It came in answer to our realization that contouring took too long. The dashboard was specifically targeted to get physicians to complete their contours and approve treatment plans in a timely manner. It also relieved pressure on the treatment planners to have an approved treatment plan within a day after getting approved contours. This went a long way in being able to sustain the 5-day rule.
Phase IV of the project was to ensure sustainability by making the changes robust enough to last (Fig 4), even when there were changes in personnel and staffing rotations throughout the department. By making the improvement process ongoing, it became culturally engrained in the department. By including timeliness improvements as part of the overall quality improvement culture of our department, they have now become something we continuously consider in our day-to-day operations.
Meetings that now address the planning process and the overall quality of patient care include:
-
1.
Clinical Physics and Dosimetry
-
2.
All clinical Faculty
-
3.
Quality working group
-
4.
Quality and Safety Oversight Committee
-
5.
Institutional Quality Council
Discussion
It is common to have improvements initially that do not necessarily persist. The aim of phase IV of the project was to ensure that the improvements achieved in prior phases were lasting and not a transient effect that went away with the faculty and staff’s attention to the problem. To accomplish this, improvements had to be process driven to become automatic. Technology became the greatest tool in keeping processes timely and efficient.
It was a challenge to bring our patient wait times for treatment down from 2 weeks to 5 business days. Currently, we schedule the patient’s start of treatment 5 days out from simulation.
The greatest tool in improvement was the introduction of the specialized technology—the specialized contouring software and the physician dashboard. This allows physicians and residents to contour from any computer, including personal computers on which they had exclusive access. This single contouring software also allows for targeted focus of the task of contouring. Of the 6 machines spread between the Westwood main campus and the Santa Monica satellite, we have 4 different types of machines implementing 5 different treatment planning systems. All 5 planning systems have their own distinct set of contouring tools. The contouring software both centralizes the task of contouring to 1 specific software while also greatly increasing the availability of contouring stations. The software is available on all nonvendor, hospital-based computers in the department.
The physician dashboard allows virtually automatic notification of tasks that need to be done. Time limitations placed on both contours and on plan approval continue to improve overall time as the urgency of these tasks is kept in the forefront of the workday. Constant reminders elevate the priority of these tasks through the dashboard and notification of physicians.
Other projects executed in parallel resulted in indirect improvement. Implementation of an incident reporting and learning system provides effective and immediate corrective action to address procedural oversights within processes unforeseen until actual clinical implementation. Staff are encouraged to report any unintended incidents or the possibility of an unintended event to this system. Reported incidents trigger an immediate assessment by quality officers who assess the severity of the reported incident and act on any incident requiring immediate attention. Weekly quality meetings then review the week’s incidents and assign focus groups to discuss solutions and implement corrective actions. Some of these actions, initiated by this system, have had the beneficial effect of mitigating any adverse effects brought about by changes made to improve timeliness of planning. However, the imposition of time limits and the introduction of the centralized contouring platform already mentioned have had the most effect.
Conclusions
In our pursuit of excellence in quality of patient care at our institution, we initiated a project specifically designed to address the timeliness of patient wait times before radiation therapy treatment initiation. The project saw the introduction of new processes and modifications of existing processes. It also saw the introduction of technologies used within radiation oncology and other medical subspecialties being applied to patient care. These changes brought about substantial improvements in the wait time. We later learned that to sustain decreased wait times, persistent attention was necessary to maintain a properly trained and well-coordinated faculty and staff. This was maintained by constant communication through an implemented notification system and regularly scheduled meetings. Implementing orientation modules to account for staffing changes helped mitigate this common challenge to sustaining a quality initiative within a department.
In conclusion, we found that the most effective way to implement improvement was to begin with a pilot project with a clear goal. Broadening the scope of the project after initial improvement was shown allowed for the effect of the project to increase. At each step, sustainability of the improvements was appropriately maintained through communication with all relevant members of the treatment team. Smaller, more frequent meetings of targeted staff where granular decisions were made were emphasized. Larger, less frequent meetings to keep staff apprised of changes were also incorporated. Having invested departmental staff was essential in this quality improvement. We hope that our experience serves to illustrate a process in which patient care and satisfaction may be improved in radiation oncology.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: Dr Agazaryan reports grants, personal fees, and nonfinancial support from Brainlab, personal fees, and nonfinancial support from Varian outside the submitted work. Mr Phillip Chow has nothing to disclose. Dr Lamb reports grants from the Agency for Health Care Research and Quality, grants and personal fees from ViewRay, Inc outside the submitted work. Dr Minsong Cao has nothing to disclose. Dr Raldow reports other from Intelligent Automation, Inc, other from Varian Medical Systems outside the submitted work. Dr Phillip Beron has nothing to disclose. Dr Hegde reports nonfinancial support and other from Soylent outside the submitted work. Dr Steinberg reports personal fees from ViewRay, personal fees from VisionRT, personal fees from Boston Scientific outside the submitted work.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Medicine Io, America CoQoHi . National Academy Press; Washington, DC: 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Institute of Medicine Committee on Quality of Healthcare. [Google Scholar]
- 2.Abdel-Rahman O. Impact of timeliness of adjuvant chemotherapy and radiotherapy on the outcomes of breast cancer: A pooled analysis of three clinical trials. Breast. 2018;38:175–180. doi: 10.1016/j.breast.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Albert J.M., Das P. Quality indicators in radiation oncology. Int J Radiat Oncol Biol Phys. 2013;85:904–911. doi: 10.1016/j.ijrobp.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Bullard J.T., Eberth J.M., Arrington A.K., Adams S.A., Cheng X., Salloum R.G. Timeliness of treatment initiation and associated survival following diagnosis of non-small-cell lung cancer in South Carolina. South Med J. 2017;110:107–113. doi: 10.14423/SMJ.0000000000000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Victoor A. Determinants of patient choice of healthcare providers: A scoping review. BMC Health Services Res. 2012;12:272. doi: 10.1186/1472-6963-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walling A.M., Beron P.J., Kaprealian T. Considerations for quality improvement in radiation oncology therapy for patients with uncomplicated painful bone metastases. J Palliat Med. 2017;20:478–486. doi: 10.1089/jpm.2016.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho A.S., Kim S., Tighiouart M. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124:3154–3162. doi: 10.1002/cncr.31533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiga A.W., Deppen S.A., Pinkerman R. Timeliness of care and lung cancer tumor-stage progression: How long can we wait? Ann Thorac Surg. 2017;104:1791–1797. doi: 10.1016/j.athoracsur.2017.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson J.K., Schultz E.M., Gould M.K. Timeliness of care in patients with lung cancer: A systematic review. Thorax. 2009;64:749–756. doi: 10.1136/thx.2008.109330. [DOI] [PubMed] [Google Scholar]
- 10.Gomez D.R., Liao K.P., Swisher S.G. Time to treatment as a quality metric in lung cancer: Staging studies, time to treatment, and patient survival. Radiother Oncol. 2015;115:257–263. doi: 10.1016/j.radonc.2015.04.010. [DOI] [PubMed] [Google Scholar]