Abstract
Purpose
A bolus is usually required to ensure radiation dose coverage of extensive superficial tumors of the scalp or skull. Oftentimes, these boluses are challenging to make and are nonreproducible, so an easier method was sought.
Methods and Materials
Thermoplastic sheets are widely available in radiation oncology clinics and can serve as bolus. Two template cutouts were designed for anterior and posterior halves to encompass the cranium of children and adults.
Results
The created bolus was imaged using computed tomography, which demonstrated good conformity and minimal air gaps.
Conclusions
Although making a bolus for treating superficial tumors of the scalp or head and neck is challenging, the presented technique enables thermoplastic to be used as a bolus and is quick, easy, and reproducible.
Introduction
Superficial tumors involving the scalp or skull present technical challenges for radiation treatment planning owing to skin sparing of conventional beams. To provide adequate dose to the target and minimize brain dose, boluses are typically used.1 SuperFlab (Mick Radio-Nuclear Instruments, Mount Vernon, NY) is the most commonly used material to create bolus, but for the scalp this can be cumbersome, resulting in air gaps and irreproducibility. Although there are several methods to treat extensive scalp tumors, such as static electron fields,2 energy- and intensity modulated electron radiation therapy,3 helmet mold-based surface brachytherapy,4 and helical tomotherapy,5 intensity modulated radiation therapy (IMRT) is often the best technique to optimize dose homogeneity with clinically acceptable normal tissue dose.1,6 Motivation to create a template to rapidly produce a highly conformal custom bolus during computed tomography (CT) simulation came from the extended time it took to create a scalp bolus in these representative cases.
Case report 1
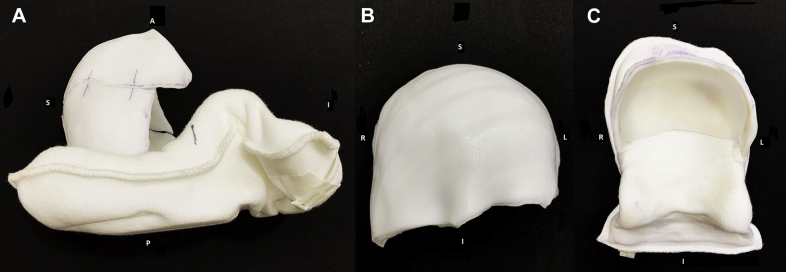
One potential scenario requiring a scalp bolus is a patient with osseous metastatic disease to the calvarium. In the index case, a 3-year-old girl presented with widespread bony disease from stage 4 neuroblastoma and symptomatic bony metastases involving her skull. After chemotherapy, the patient underwent right frontal craniotomy for partial resection of a skull mass and was treated with metaiodobenzylguanidine therapy with 18 mCi/kg with less than partial response. She was referred for radiation therapy owing to diffuse, persistently metaiodobenzylguanidine-positive sites in the calvarium. The patient was simulated in the supine position. Two adjoining boluses covering the whole scalp, 1 for the anterior half and another for the posterior half of the cranium, were made with thermoplastic (Fig 1). Total sedation time was approximately 1 hour and was largely due to the bolus creation time. She was treated with IMRT to a dose of 21.6 Gy in 12 fractions.
Figure 1.
Bolus for patient treated in case report 1. (A) Lateral view of complete bolus. (B) Frontal view of anterior half of bolus. (C) Frontal view of posterior half of bolus fused with headrest.
Case report 2
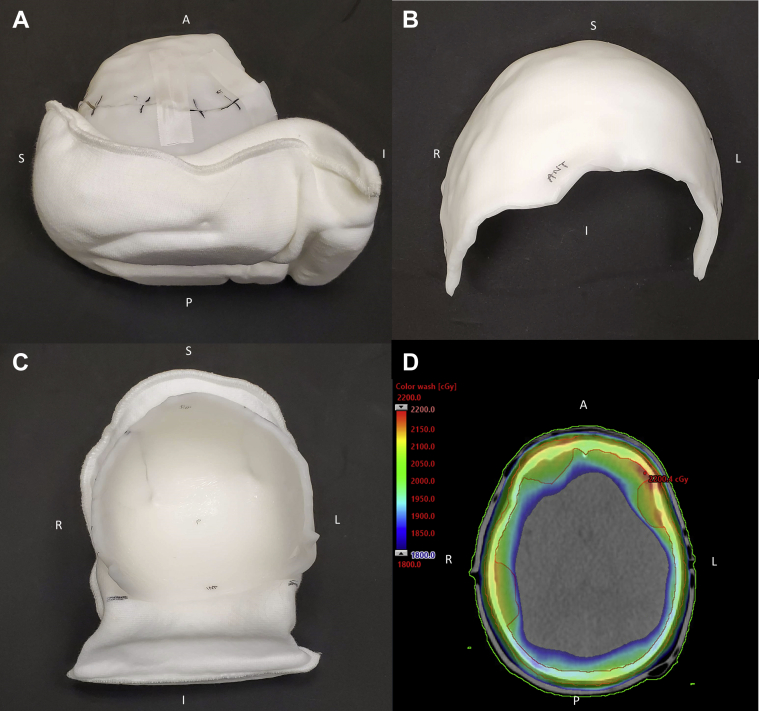
A 7-year-old child presented with progressive stage 4 neuroblastoma with multiple bone metastases, including the skull. The patient had undergone multiple chemotherapy treatments, each with varying success, and was referred to radiation oncology for palliative pain relief. The patient was simulated in the supine position. In this case, the templates were used and allowed the therapists to preheat appropriately sized sheets before the patient was brought into the simulation room. Two adjoining boluses were made so that the whole scalp was covered (Fig 2). Total sedation time was reduced to about 40 minutes. Treatment was to 20 Gy in 5 fractions.
Figure 2.
Bolus for patient treated in case report 2. (A) Lateral view of complete bolus. (B) Frontal view of anterior half of bolus. (C) Frontal view of posterior half of bolus. (D) Color wash showing 1800 to 2200 cGy dose to scalp.
Methods and Materials
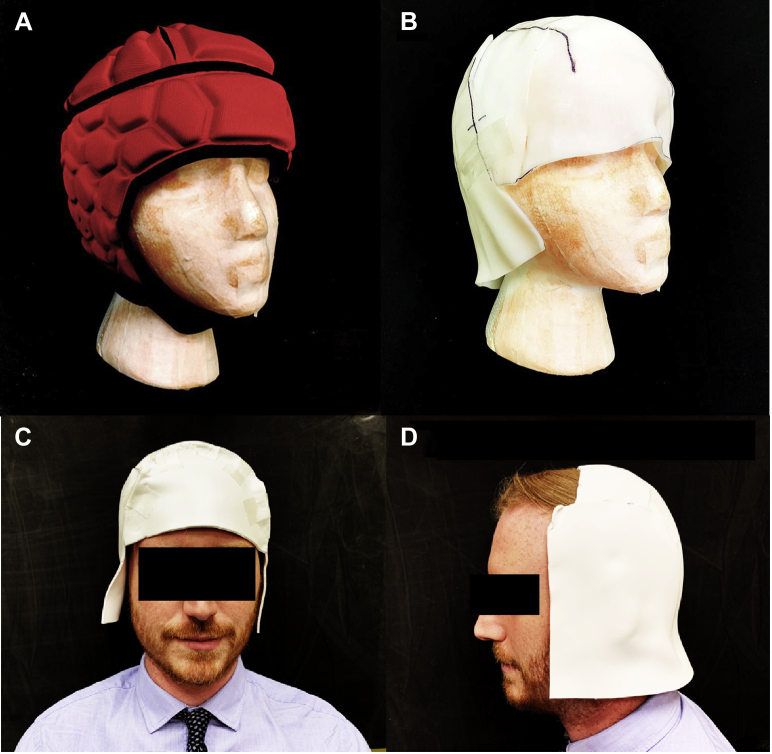
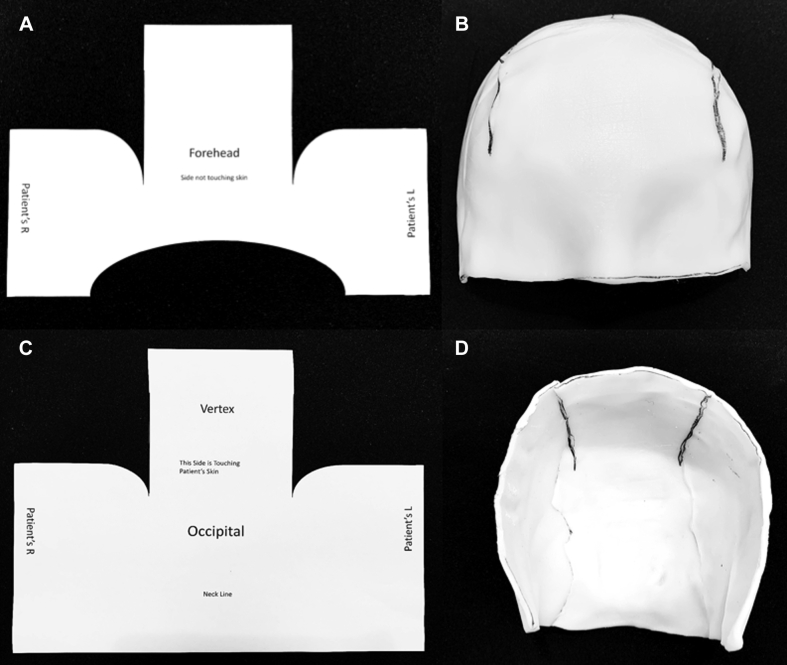
Inspiration came from the close-fitting design of a scrum helmet (Fig 3A) to make a similarly shaped custom bolus (Fig 3B). To reduce scalp bolus creation time for subsequent patients, templates were systematically developed starting with a foam head with a 51-cm head circumference (approximating a 2-5 year old [47.5-51.5 cm]).7 The templates were designed to make the posterior and anterior halves individually to facilitate application and removal (Fig 4).
Figure 3.
(A) Scalp and cranium radiation therapy using modulation (SCRUM) on foam head. (B) Bolus on foam head. (C) Volunteer facing forward. (D) Posterior bolus to demonstrate possible separate use of anterior and posterior halves.
Figure 4.
(A) Posterior template. (B) Formed thermoplastic using template. (C) Anterior template. (D) Formed thermoplastic using template.
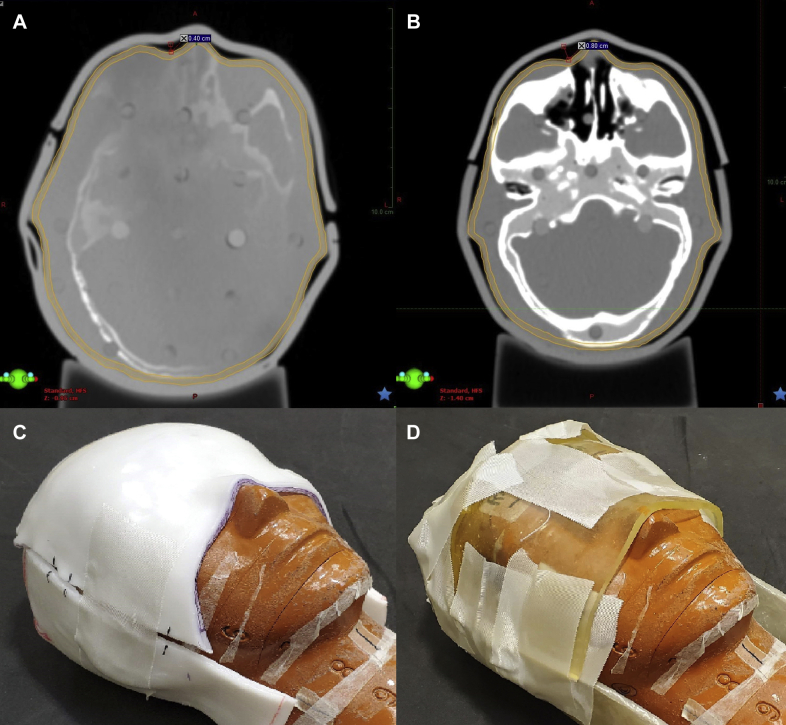
Once the templates were created, the posterior template was traced onto a solid, nonperforated 3-mm thick thermoplastic sheet (Qfix, Avondale, PA). The sheet was cut and placed in a dry convection oven (Par Scientific, Denmark) to soften while an anthropomorphic head phantom was placed on a Klarity headrest (Klarity Medical Products, OH), replicating simulation conditions. When the sheet was softened, it was placed posterior to the head and molded. The anterior portion was similarly molded (Fig 4D), and the 2 halves of the bolus were taped (Fig 5C) and imaged using a CT scanner (Fig 5A). Another set of templates was created for 57-cm head circumference, which corresponds to the mean value for the average adult male.8 The adult templates were tested on a volunteer with a head circumference of 56 cm (Fig 3C,D).
Figure 5.
(A) Axial view of phantom head with custom thermoplastic bolus with widest air gap depicted. (B) Axial view of SuperFlab bolus with widest air gap depicted. (C) Thermoplastic bolus setup on head phantom. (D) SuperFlab setup on head phantom.
To analyze conformality and dosimetry of both the thermoplastic and SuperFlab boluses, the head phantom was CT scanned with each bolus type. Contours were then created in Aria (Varian Medical Systems, Palo Alto, CA), which included a planning target volume (PTV), scalp, bones, body, and brain. A plan was then generated and the dosimetric results were analyzed (Fig 6).
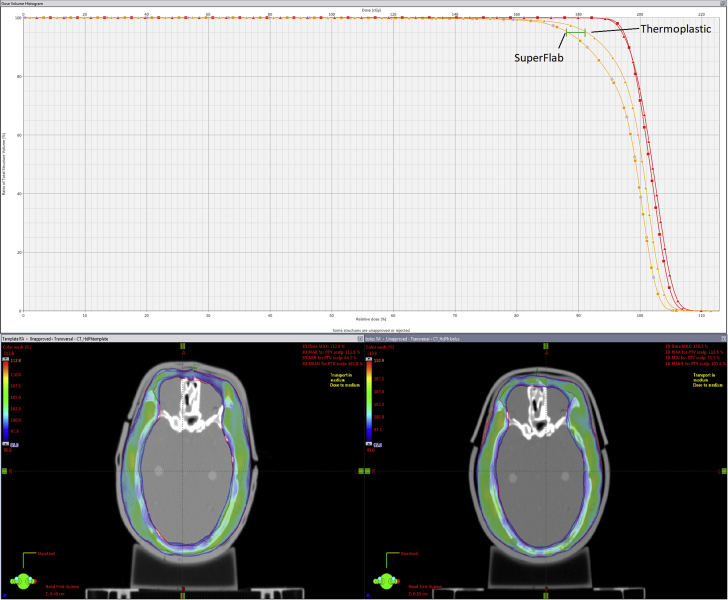
Figure 6.
(A) Dose histogram comparing thermoplastic versus SuperFlab with about 3% difference at D95%. (B) Color wash of thermoplastic showing prescribed dose of 95% to 113%. (C) Color wash of SuperFlab showing prescribed dose of 95% to 113%.
Bolus creation time was measured by noting the time between the first thermoplastic sheet being taken out of the convection oven and the time when the CT scan was completed. For the SuperFlab bolus, time was measured between when the sheets were being fitted to the completion of the CT scan.
Results
Conformality was analyzed using CT images, which showed minimal air gaps, with the largest (4 mm) at the nasal bridge (Fig 5A). A SuperFlab bolus was also created and showed gaps of up to 8 mm (Fig 5B). The adult bolus did not have significant gaps upon visual inspection (Fig 3C,D). The time to create thermoplastic bolus was reduced by approximately 20 minutes compared with SuperFlab creation.
As shown in Figure 6, there is about a 3% difference at D95% between thermoplastic and SuperFlab. There are also large air gaps and irregularities in SuperFlab and securing the bolus required a greater amount of tape (Fig 5D). In addition, the conformality of SuperFlab fluctuated each time it was placed on the head phantom, whereas thermoplastic conformality changed minimally owing to the rigid nature of the plastic after it had set.
Discussion
Involvement of the scalp and calvarium is seen in conditions such as squamous cell carcinoma, angiosarcoma, lymphoma, and melanoma.9 Infrequently, widespread involvement requires total scalp irradiation. This is more common in adults than in children.10,11 Thermoplastic bolus was described in a study by Mellenberg and Schoeppel,2 in which the authors used 2-mm thermoplastic masks as bolus and immobilization and added 5-mm bolus to achieve a 95% surface dose. However, care must be taken to eliminate airgaps that may lead to dosimetric implications.
The templates we created refine and standardize our earlier methods of creating scalp boluses. In the original method, a double-layered bolus was made with 4 parts (Figs E1 and E2). We gradually moved to an improved method using only 2 parts, as shown in the representative case (Figs 1 and 2). In this manuscript, we present templates to rapidly create custom thermoplastic halves for whole scalp boluses for adult or pediatric patients. Head circumferences of 51 and 57 cm were used as they correspond to the 50th percentile for children after the age of 5 years old and the mean for adults (mean age 35), respectively.7,8 However, the ductility of thermoplastic can accommodate head circumferences slightly larger than the 57-cm template.12 Additionally, the sheets can be trimmed for smaller head sizes, as was needed for our adult volunteer (Fig 3C,D). Although a single-layer bolus was used on our volunteer to demonstrate conformality, additional layers as in our patient example and for the adult head phantom may be added. Furthermore, the anterior and posterior templates can be used separately, depending on the extent of disease (Fig 3D).
Even with the ease of use of these templates as described, there are some limitations. While creating the 2 portions of the thermoplastic bolus, care must be taken to ensure that they do not fuse together so that the bolus can be easily removed from the patient’s head. This may be alleviated by using a barrier, such as a thin plastic wrap, to cover the edges where thermoplastic halves could fuse. Because the thermoplastic must be formed around the patient’s scalp using pressure, the cooperability of the patient can vary, especially if the lesions are painful. Inadequate pressure can lead to unwanted air gaps and a nonconforming bolus. Lastly, with thermoplastic bolus, 2 therapists may need to collaborate because the patient's head must be raised in order to place the thermoplastic underneath, and the head must be immobile while the thermoplastic cools so that the shape is retained. However, with SuperFlab, a single therapist can create bolus.
The necessity of a bolus has been studied in various radiation techniques. For example, Wolden et al11 studied outcomes of 31 pediatric patients with diffuse skull metastases from neuroblastoma treated with photon/electron whole-skull radiation therapy. They aimed to spare brain parenchyma by using lateral electron and megavoltage photon fields, which resulted in an 89% disease control 1 year after treatment began. In another study, Khuntia et al13 proposed tomotherapy for total scalp radiation, avoiding the need for a bolus. In response, Bedford et al14 studied an IMRT plan that simulated tomotherapy without a bolus, but found inadequate dose delivery to the PTV. In our case, we obtained PTV coverage within 5% to 7% of the prescribed dose with bolus and IMRT.
Other bolus techniques include the method presented by Mail et al,15 which used 4 thermoplastic shells with boluses between the inner and outer layers and required the patient to be in supine and prone positions. Several molds had to be made, which increased time and material needed. Our method does not require patients to change position because the anterior half can be made with the patient supine and the posterior half can be maneuvered into place by lifting the head. Similarly, the method proposed by Lin et al.16 used 2 Aquaplast sheets with bolus sandwiched in between, but this resulted in airgaps as big as 1.5 cm. Rather than using 2 materials, we used 1. Another method described using 2 neoprene wetsuit diving hoods as bolus.1 However, putting on 2 hoods a day, especially for children, can be cumbersome. Our department attempted this technique, but the electron density we obtained for neoprene hoods was insufficient to act as bolus. Finally, 3-dimensional printing of a bolus using types of thermoplastics such as acrylonitrile butadiene styrene and polyactic acid has been described in many studies.17, 18, 19, 20, 21, 22 This technique has not yet been widely adopted due to the need for additional equipment, lengthy print times, and specialized expertise. Thus, we present a streamlined and efficient process.
Conclusions
The existing literature on methods for creating scalp bolus for radiation therapy has compromises such as irreproducibility or impracticality. We developed a method that is easy, efficient, and reproducible on a daily basis. The materials required are readily available in radiation oncology clinics. The templates can be applied to both children and adult head circumferences and are included as supplemental materials for download.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.03.017.
Supplementary data
References
- 1.Hadziahmetovic M., Weldon M., Pearson M., Werner P., Siddiqui F. Scalp uniform bolus application (SCUBA) technique for homogenous scalp and regional nodal irradiation. Pract Radiat Oncol. 2014;4:e95–e99. doi: 10.1016/j.prro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Mellenberg D.E., Schoeppel S.L. Total scalp treatment of mycosis fungoides: The 4 × 4 technique. Int J Radiat Oncol Biol Phys. 1993;27:953–958. doi: 10.1016/0360-3016(93)90473-9. [DOI] [PubMed] [Google Scholar]
- 3.Yaparpalvi R., Fontenla D.P., Beitler J.J. Improved dose homogeneity in scalp irradiation using a single set-up point and different energy electron beams. BJR. 2002;75:670–677. doi: 10.1259/bjr.75.896.750670. [DOI] [PubMed] [Google Scholar]
- 4.Liebmann A., Pohlmann S., Heinicke F., Hildebrandt G. Helmet mold-based surface brachytherapy for homogeneous scalp treatment: A case report. Strahlenther Onkol. 2007;183:211–214. doi: 10.1007/s00066-007-1648-7. [DOI] [PubMed] [Google Scholar]
- 5.Locke J., Low D.A., Grigireit T., Chao K.S.C. Potential of tomotherapy for total scalp treatment. Int J Radiat Oncol Biol Phys. 2002;52:553–559. doi: 10.1016/s0360-3016(01)02593-7. [DOI] [PubMed] [Google Scholar]
- 6.Ostheimer C., Janich M., Hübsch P., Gerlach R., Vordermark D. The treatment of extensive scalp lesions using coplanar and noncoplanar photon IMRT: A single institution experience. Radiat Oncol. 2014;9:82. doi: 10.1186/1748-717X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Head circumference-for-age. https://www.who.int/childgrowth/standards/second_set/chts_hcfa_boys_p/en/ Available at:
- 8.Nguyen A.K.D., Simard-Meilleur A.-A., Berthiaume C., Godbout R., Mottron L. Head circumference in Canadian male adults: Development of a normalized chart. Int J Morphol. 2012;30:1474–1480. [Google Scholar]
- 9.Hata M. Radiation therapy for angiosarcoma of the scalp: Total scalp irradiation and local irradiation. Anticancer Res. 2018;38:1247–1253. doi: 10.21873/anticanres.12346. [DOI] [PubMed] [Google Scholar]
- 10.Skórska M. Total skin electron beam (TSEB) therapy in pediatric patients: A review of the literature. Rep Pract Oncol Radiother. 2013;19:109–113. doi: 10.1016/j.rpor.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolden S.L., Barker C.A., Kushner B.H. Brain-sparing radiotherapy for neuroblastoma skull metastases. Pediatric Blood & Cancer. 2008;50:1163–1168. doi: 10.1002/pbc.21384. [DOI] [PubMed] [Google Scholar]
- 12.Mălăescu I.M., Marin C.N., Spunei M. Comparative study on the surface dose of some bolus materials. Int J Med Phys, Clin Eng Radiat Oncol. 2015;4:348–352. [Google Scholar]
- 13.Khuntia D., Jaradat H., Orton N., Tomé W., Mehta M.P., Welsh J.S. Helical tomotherapy as a means of administering total or partial scalp irradiation: In regards to Bedford et al. (Int J Radiat Oncol Biol Phys. 2005;62:1549-1558) Int J Radiat Oncol Biol Phys. 2006;64:1288–1289. doi: 10.1016/j.ijrobp.2005.11.020. author reply 1289-1290. [DOI] [PubMed] [Google Scholar]
- 14.Bedford J.L., Childs P.J., Hansen V.N., Warrington A.P., Mendes R.L., Glees J.P. In response to Dr. Khuntia et al. Int J Radiat Oncol Biol Phys. 2006;64:1289–1290. [Google Scholar]
- 15.Mail N., Al-Ghamdi S.M., Chantel C., Sedhu F., Rana A., Saoudi A. Customized double-shell immobilization device combined with VMAT radiation treatment of basosquamous cell carcinoma of the scalp. J Appl Clin Med Phys. 2019;20:84–93. doi: 10.1002/acm2.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S.H., Latronico D., Teslow T., Bajaj G.K. A highly reproducible bolus immobilization technique for the treatment of scalp malignancies. Med Dosim. 2008;33:30–35. doi: 10.1016/j.meddos.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Baltz G.C., Chi P.-C.M., Wong P.-F. Development and validation of a 3D-printed bolus cap for total scalp irradiation. J Appl Clin Med Phys. 2019;20:89–96. doi: 10.1002/acm2.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dipasquale G., Poirier A., Sprunger Y., Uiterwijk J.W.E., Miralbell R. Improving 3D-printing of megavoltage X-rays radiotherapy bolus with surface-scanner. Radiat Oncol. 2018;13:203. doi: 10.1186/s13014-018-1148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehler E., Sterling D., Dusenbery K., Lawrence J. Workload implications for clinic workflow with implementation of three-dimensional printed customized bolus for radiation therapy: A pilot study. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0204944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craft D.F., Kry S.F., Balter P., Salehpour M., Woodward W., Howell R.M. Material matters: Analysis of density uncertainty in 3D printing and its consequences for radiation oncology. Med Phys. 2018;45:1614–1621. doi: 10.1002/mp.12839. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto K., Shiinoki T., Yuasa Y., Hanazawa H., Shibuya K. Efficacy of patient-specific bolus created using three-dimensional printing technique in photon radiotherapy. Phys Med. 2017;38:1–9. doi: 10.1016/j.ejmp.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Burleson S., Baker J., Hsia A.T., Xu Z. Use of 3D printers to create a patient-specific 3D bolus for external beam therapy. J Appl Clin Med Phys. 2015;16:5247. doi: 10.1120/jacmp.v16i3.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.