Abstract
Purpose
To evaluate the safety and efficacy of definitive chemoradiotherapy (CRT) for patients with clinical T1N0M0 esophageal adenocarcinoma.
Methods and Materials
This was a retrospective study of patients with clinical T1N0 adenocarcinoma of the esophagus treated with curative-intent CRT between 2004 and 2017 at 2 tertiary care centers. Patients received CRT instead of esophagectomy owing to medical comorbidities or patient preference. Toxicities were evaluated according to Common Terminology Criteria for Adverse Events version 4.03. The Kaplan-Meier method was used to estimate overall, progression-free, and disease-specific survivals.
Results
Twenty-eight patients were included for analysis. Median age was 76 years (range 55-90). The majority of patients were male (93%) and had a history of Barrett’s esophagus (71%). Tumor characteristics included distal esophagus location (93%), clinical stage T1b (86%), and median length of 2 cm (range, 1-9). Prior endoscopic resection was performed in 57%.
The median follow-up was 44 months (range, 4-146). The acute grade 3 adverse events were observed in 7 patients (25%). One patient died of complications potentially related to chemoradiation. Eight patients (29%) had disease progression at a median of 7.6 months after CRT. First site of progression was local only (14%), local and regional (11%), or distant (4%). Salvage locally directed treatment was performed in 3 of 4 patients with local-only recurrence. The 3-year overall survival, progression-free, and disease-specific rates were 78%, 62%, and 81%, respectively.
Conclusion
CRT is a safe and effective curative treatment strategy for select patients with clinical T1N0M0 esophageal adenocarcinoma.
Introduction
Esophageal cancer is one of the most fatal malignancies causing 572,000 new cases and 509,000 deaths worldwide as estimated in 2018.1 With improvements of early cancer detection and diagnostic accuracy, clinical T1N0M0 esophageal cancer is increasingly diagnosed and may be amenable to less radical curative treatment.
The Japanese Esophageal Society practice guidelines support the use of endoscopic resection (ER), such as endoscopic mucosal resection and endoscopic submucosal dissection (ESD), for patients with esophagus cancer limited to the epithelium or lamina propria mucosa. Patients with disease invading into the submucosa (T1b) have higher risks of occult lymph node involvement and are at an increased risk of developing distant metastasis.2 Therefore, these patients are typically recommended more radical therapy in the form of esophagectomy or chemoradiotherapy (CRT).3
Although esophagectomy is an effective approach for patients with stage I disease,4, 5, 6, 7, 8, 9, 10, 11, 12 it is associated with substantial morbidity and mortality.13, 14, 15 CRT may allow an opportunity for organ preservation and potentially better quality of life. The Japan Clinical Oncology Group Study (JCOG) 9708 trial evaluated patients with clinical stage 1 squamous cell carcinoma (SCC) treated with definitive CRT and reported a 4-year overall survival of 81%.16 The more recently presented JCOG 0502 trial, which compared esophagectomy and CRT for patients with T1 esophageal SCC, reported 5-year overall survival (OS) of 87% in the esophagectomy arm and 86% in CRT arm (adjusted hazard ratio [HR] 1.05; 95% confidence interval [CI], 0.67-1.64).17 The conclusion of the JCOG trials was that organ-preserving CRT is an acceptable treatment option for patients with stage IA esophageal SCC.
SCC has been shown to be more responsive to CRT, thereby limiting our ability to extrapolate these data to patients with adenocarcinoma. To our knowledge, there are no published series evaluating the safety and efficacy of definitive CRT for stage I esophageal adenocarcinoma. Current National Comprehensive Cancer Network (NCCN) guidelines endorse the use of ER as an alternative to esophagectomy for patients with T1a disease and select patients with superficial T1b disease; however, CRT is not included as a standard treatment option for this cohort.18
The purpose of this study was to analyze the adverse events (AE), survival outcomes, and recurrence patterns for patients with clinical stage I esophageal adenocarcinoma treated with definitive CRT.
Methods and Material
Patients
Eligible patients included those with histologically confirmed, clinical stage T1N0M0 esophageal or gastroesophageal junction adenocarcinoma treated within 2 tertiary cancer centers between January 2004 and May 2017. Patients were prescribed definitive CRT to a dose of at least 40 Gy, did not undergo esophagectomy as planned initial therapy and had complete follow-up information. Patients with lymph node involvement, distant metastasis, or those who received planned preoperative CRT were excluded.
Diagnosis and treatment
Pretreatment workup included esophagogastroduodenoscopy with biopsies, endoscopic ultrasound to determine the depth of tumor invasion and regional lymph node staging, computed tomography (CT) of the chest, abdomen, and pelvis, and positron emission tomography (PET)/CT. Patients could have undergone endoscopic mucosal or submucosal resection for diagnostic or therapeutic intent, although most had known residual disease. Patient comorbid illnesses were assessed with the Charlson Comorbidity Index.19
RT was delivered with 3-dimensional conformal radiation therapy, intensity modulated radiation therapy, or proton beam radiation therapy (RT). Concurrent chemotherapy was administered, most commonly with regimens containing platinum, taxane, or 5-fluorouracil (5-FU). Acute (less than 90 days after treatment) and late (90 days or longer after treatment) AEs were defined according to Common Terminology Criteria for Adverse Events version 4.03.
Follow-up
Patients were typically followed up every 3 months for the first 2 years, every 6 months for the third and fourth year, and then annually. Follow-up examinations included physical examination, hematologic and biochemic testing, esophagogastroduodenoscopy with biopsies, and diagnostic imaging. Local recurrence was defined as recurrence or persistence at the primary tumor site; regional recurrence sites were defined as regional lymph nodes; distant recurrence sites were defined as nonregional lymph nodes or distant organs. In-field locoregional recurrence was defined as the epicenter of the recurrent tumor being located within the 95% isodose of prescription dose and out of field recurrence was defined as the epicenter of the recurrent tumor being located outside of the 95% isodose. Recurrences and progressions were diagnosed by histologic confirmation or, when biopsy was not available, clinically as a mass with significant increase of standardized uptake value on PET/CT.
Statistical analysis
Survival time was defined from the date of CRT completion to the date of events or censor. Survival outcomes including overall survival (OS), progression-free survival (PFS), and disease-specific survival (DSS) were estimated using the Kaplan-Meier method. The log-rank test was used to compare the distribution of survival time and the median survival time between groups. The cumulative incidence of local recurrence was estimated using the competing risk model, with death as a competing risk. Univariable analyses were performed to assess for potential associations between baseline patient or treatment factors with outcomes using the Cox proportional hazard model. All analyzed covariates are included in Table E1. Statistical analyses were performed using SPSS 25.0 (SPSS Inc, Chicago, IL). Two-sided P values less than .05 were considered significant.
Results
Baseline characteristics
From 2004 to 2017, 28 consecutive patients underwent definitive CRT and were eligible for analysis. The patient characteristics were summarized in Table 1. The majority of patients were male (93%), had T1b tumors (86%), and had tumors within the distal esophagus (93%). Sixteen (57%) patients underwent ER before definitive CRT; however, only one patient underwent ER without invasive cancer at the margin.
Table 1.
Baseline characteristics
| Characteristics | All patients (n = 28, %) |
|---|---|
| Sex | |
| Male | 26 (93) |
| Female | 2 (7) |
| Age (y), median (range) | 76 (55-90) |
| ECOG PS | |
| 0 | 16 (57) |
| 1-2 | 10 (36) |
| 2 | 2 (7) |
| Charlson Comorbidity Index, median (range) | 1 (0-6) |
| Barrett esophagus | |
| No | 8 (29) |
| Yes | 20 (71) |
| Tumor length (cm), median (range) | 2.0 (1.0-9.0) |
| Grade | |
| G2 | 15 (54) |
| G3 | 13 (46) |
| Tumor location | |
| Middle thoracic | 2 (7) |
| Lower thoracic + GEJ | 26 (93) |
| Lymphovascular invasion | |
| No | 7 (25) |
| Yes | 9 (32) |
| Unknown | 12 (43) |
| T category | |
| T1a | 4 (14) |
| T1b | 24 (86) |
| Treatment before CRT | |
| No | 11 (39) |
| ER | 16 (57) |
| Chemotherapy | 1 (4) |
| ER margin status | |
| Positive invasive cancer | 14 (88) |
| Positive high-grade dysplasia | 1 (6) |
| Unknown | 1 (6) |
| Radiation modality | |
| 3DCRT | 9 (32) |
| IMRT | 12 (43) |
| Protons | 7 (25) |
| Median radiation dose, range (Gy) | 50.4 (40.0-50.4) |
| Concurrent chemotherapy regimen | |
| Carboplatin/taxane | 8 (29) |
| Platinum/5FU | 7 (25) |
| Taxane/5FU | 12 (43) |
| Irinotecan | 1 (4) |
| Reason not receiving surgery | |
| Medical comorbidity | 15 (54) |
| Patient preference | 13 (46) |
Abbreviations: 3DCRT = 3-dimensional conformal radiation therapy; CRT = chemoradiation; ECOG PS = Eastern Cooperative Oncology Group performance status; ER = endoscopic resection; GEJ = gastroesophageal junction; IMRT = intensity modulated radiation therapy.
Treatment-related adverse events
Acute grade 3 (G3) AEs were observed in 7 patients (25%), including esophagitis (2 patients), nausea and vomiting (2 patients), dysphagia (1 patient), thrombocytopenia (1 patient), or pulmonary embolus (1 patient). No patients experienced an acute G4 event, although 1 patient (4%) who was 84 years old, had a 30-pack year smoking history, and moderate-severe chronic kidney disease experienced an acute G5 event of myocardial infarction, respiratory failure, and death 20 days after treatment. Two patients (7%) had late G3 esophageal stricture. Two (7%) patients underwent placement of a feeding tube, one during CRT because of acute esophagitis and late G3 esophageal stricture, and the other after completion of CRT and required it for approximately 2 months due to G3 dysphagia. Three patients (11%) experienced late G1-2 pulmonary AE in the form of pleural effusion. No patients experienced late cardiac AEs.
Recurrence patterns
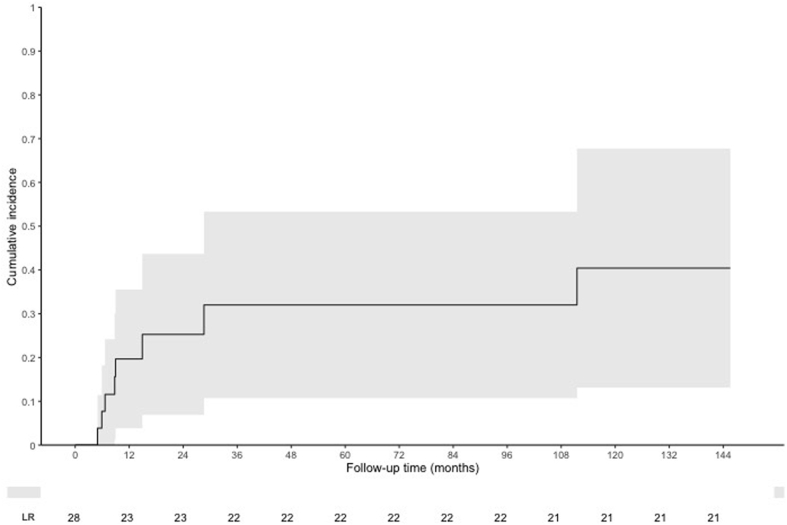
Cancer recurrence or progression occurred in 8 (29%) patients at a median of 7.6 months (range, 1.8-111.5) after completion of CRT. One of 4 (25%) patients with T1a disease and 7 of 24 (29%) patients with T1b disease experienced recurrence (Table 2, Fig 1). First sites of recurrence included 4 (14%) local only, 3 (11%) local and regional, and 1 (4%) distant metastasis. The 1-, 2-, and 3-year cumulative local recurrence rate were 17.9%, 17.9%, and 21.4%. All local recurrences occurred within RT treatment volumes. One of 3 regional LN recurrences occurred outside of the RT treatment volume within a left gastric lymph node.
Table 2.
Characteristics for 8 patients with recurrence
| No. | Sex | Age | T | Tumor length | Grade | LVI | Treatment before RT | RT modality | Recurrence pattern | Relation to RT field∗ | Salvage approach |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 90 | T1b | 9 | G2 | Unknown | None | 3DCRT | Local and regional | In field | Chemotherapy |
| 2 | Male | 78 | T1b | 2 | G3 | None | None | 3DCRT | Local and regional | In field | Chemotherapy |
| 3 | Female | 67 | T1a | 6 | G2 | Unknown | None | IMRT | Local | In field | ER + cryotherapy |
| 4 | Male | 80 | T1b | 5 | G3 | Present | ER | 3DCRT | Local | In field | None |
| 5 | Male | 80 | T1b | 1 | G2 | Unknown | ER | IMRT | Local | In field | Cryotherapy |
| 6 | Male | 59 | T1b | 6 | G3 | Unknown | Chemotherapy | IMRT | Local and regional | In field and out of field | Chemotherapy |
| 7 | Male | 66 | T1b | 2 | G3 | None | ER | IMRT | Local | In field | Esophagectomy (ypT1bN0) |
| 8 | Male | 81 | T1b | 2 | G3 | None | ER | IMRT | Distant (bone) | Out of field | None |
Abbreviations: 3DCRT = 3-dimensional conformal radiation therapy; ER = endoscopic resection; IMRT = intensity modulated radiation therapy; LVI = lymphovascular invasion; RT = radiation therapy.
In field locoregional recurrence was defined as the epicenter of the recurrent tumor being located within the 95% isodose of prescription dose, and out of field recurrence was defined as those occurring outside of the 95% isodose.
Figure 1.
Cumulative incidence of local recurrence using competing risk method.
Salvage therapy included local treatment (ER, cryotherapy, or esophagectomy) for 3 patients with local recurrence, or salvage chemotherapy for 3 patients who experienced local and regional recurrence. One patient who experienced local recurrence and 1 patient who experienced distant recurrence did not receive salvage therapy owing to poor performance status. Patients with local-regional recurrence survived for a median time of 77 months and had a 3-year OS rate of 71%. These survival endpoints were similar to patients who did not experience disease recurrence (median OS 69 months, 3-year OS rate 89%).
Survival outcomes
Median follow-up time was 44.2 months (range, 4-146 months). At last follow-up, 12 (43%) patients were alive without recurrence and 3 (11%) patients were alive with recurrent disease. Thirteen (46%) patients died, with death attributable to esophageal cancer (n = 6), infectious diseases (pneumonia, urinary tract infection, and extremity cellulitis; n = 3), unrelated malignancies (lung cancer and prostate cancer; n = 2), treatment complications (myocardial infarction, atrial fibrillation, and respiratory failure; n = 1), or from an unknown reason unrelated to cancer (n = 1).
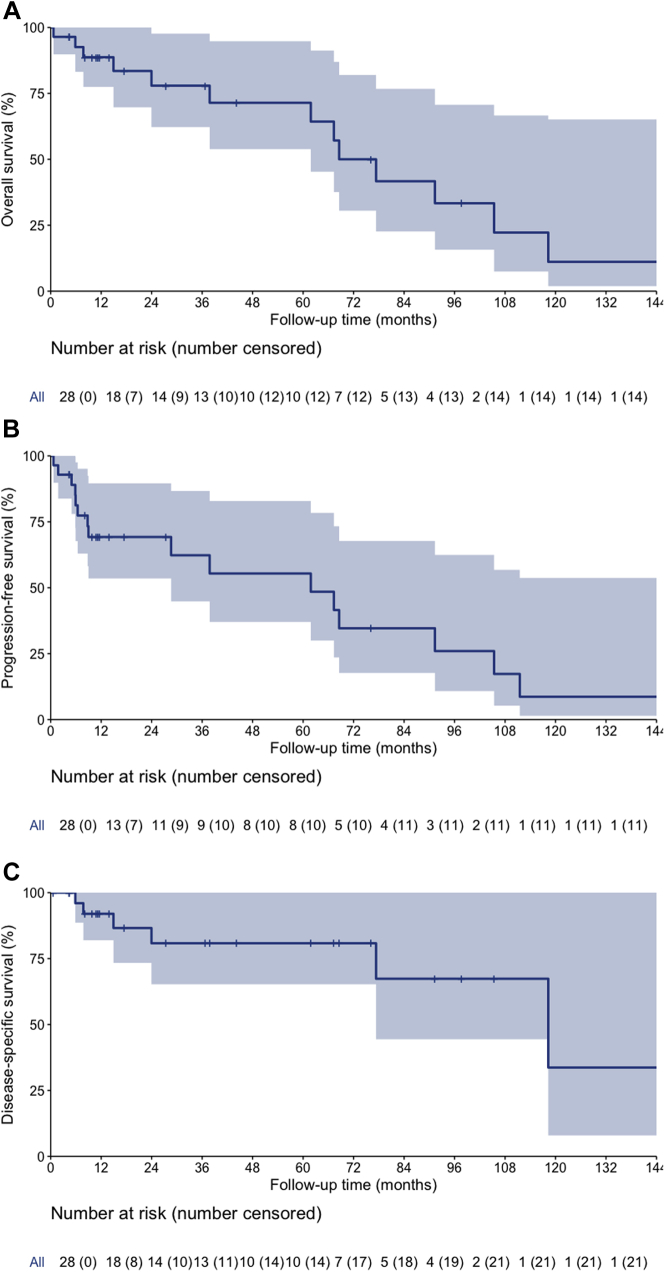
The median and 3- and 5-year OS, PFS, and DSS were 69 months (95% CI, 53-85), 78% (95% CI, 62-98%), and 71% (95% CI, 54-95%); 62 months (95% CI, 12-112), 62% (95% CI, 45-87%), and 55% (95% CI, 37-83%); and 118 months (95% CI, 59-178), 81% (95% CI, 65-100%), and 81% (95% CI, 65-100%), respectively (Fig 2A-C).
Figure 2.
(A) Overall survival curves for patients with stage T1N0M0 esophageal adenocarcinoma who received definitive chemoradiotherapy (CRT; shaded area represented 95% confidence interval [CI]). (B) Progression-free survival curves for patients with stage T1N0M0 esophageal adenocarcinoma who received definitive CRT (shaded area represented 95% CI). (C) Disease-specific survival curves for patients with stage T1N0M0 esophageal adenocarcinoma who received definitive CRT (shaded area represented 95% CI).
Patients with tumor length greater than 2 cm had significantly worse 3-year PFS (48% vs 70%; HR 3.3; 95% CI, 1.1-10.3; P = .028) and DSS (67% vs 91%; HR 5.5; 95% CI, 1.0-29.4; P = .026). Grade 3 histology trended toward worse 3-year DSS (70% vs 91%; HR 5.8; 95% CI, 0.7-48.1; P = .066). There were no significant differences in survival between patients with T1a or T1b disease.
Discussion
We report on a cohort of 28 patients with T1N0M0 esophageal adenocarcinoma treated with definitive CRT and demonstrate a median and 3- and 5-year OS of 69 months, 78%, and 71%, respectively. Acute and late treatment-related AEs were acceptable. These data support the use of definitive CRT as a safe and effective curative treatment strategy for select patients with clinical T1N0M0 esophageal adenocarcinoma.
To our knowledge, there are no other published data assessing the safety and efficacy of definitive CRT for patients with stage I esophageal adenocarcinoma. Current National Comprehensive Cancer Network guidelines endorse the use of ER or esophagectomy, but do not include CRT as a standard curative treatment strategy.18 Despite this, practice patterns per a National Cancer Database study suggest that 21% of patients age 80 or older with stage I esophageal adenocarcinoma receive CRT as curative intent therapy, and CRT was associated with similar survival outcomes to esophagectomy.20 However, National Cancer Database analyses have a multitude of limitations in regards to accurate reporting of comparative effectiveness.21 Therefore, our series helps fill a knowledge gap in the existing literature by providing important safety and efficacy data regarding the role of definitive CRT for patients with stage I adenocarcinoma of the esophagus.
Esophagectomy has been considered the gold standard treatment for clinical stage T1N0M0 esophageal adenocarcinoma, with most contemporary series demonstrating 5-year OS of 77% to 85%, and 5-year DSS of 79% to 88% (Table 3).4, 5, 6, 7, 8, 9, 10, 11, 12 In our study, the 5-year OS and DSS were 71% and 81%, respectively, which seem comparable to outcomes for patients who undergo esophagectomy for T1N0 adenocarcinoma. This is notable considering that in our series the median patient age was 76 years and half of the patients had significant medical comorbidities, with 25% of patients having a Charlson Comorbidity Index >3. A limitation in comparing outcomes of surgical versus nonsurgical treatments is that many of the esophagectomy series report outcomes based on pathologic stage, although our cohort treated with CRT is reliant upon clinical staging. Pathologic tumor or lymph node upstaging occurs in 20% to 30% of patients with stage I disease.6,10 Therefore, one would expect a pathologically staged T1bN0 cohort to have better outcomes than that of a clinically staged T1bN0 cohort. Acknowledging these issues, we feel that definitive CRT may be a reasonable alternative to esophagectomy, especially for patients who are suboptimal candidates for esophagectomy due to advanced age and/or comorbidities.
Table 3.
Summary of outcomes from select esophagectomy series for T1 esophageal adenocarcinoma
| Series | N | Staging method | Median age (years) | T1a (%)/T1b (%) | LR (%) | 5-y OS (%) | 5-y DSS (%) |
|---|---|---|---|---|---|---|---|
| Barbour et al5 | 85 | P | 65 | 41/59 | - | 77 | 82 |
| Altorki et al4 | 75 | P | 68 | 40/60 | - | 78 | 87 |
| Pennathur et al11 | 100 | P | 68 | 29/71 | 20 | 62 | - |
| Leers et al7 | 126 | P | 64 | 60/40 | - | 78 | 98/79 (T1a/T1b) |
| Liu et al8 | 90 | P | 64 | 59/41 | 10 | 91/58 (T1a/T1b) | - |
| Dickinson et al6 | 51 | C (T1) | 66 | 45/55 | - | 77 | - |
| Westerterp et al12 | 120 | P | 65 | 45/55 | 8 | 68 (disease free survival) | - |
| Mohiuddin et al9 | 38 | P | 66 | 0/100 | - | 79 | - |
| Molena et al10 | 23 | C (T1b post ESD) | 67 | 35/30 | - | - | 88 |
Abbreviations: C = clinical; DSS = disease-specific survival; LR = local recurrence; P = pathologic; OS = overall survival.
CRT is a standard definitive treatment option for locally advanced esophageal cancer and for stage I esophageal SCC.16,17,22 RTOG 0436 demonstrated a 3-year OS of 28% for patients with locally advanced esophageal cancer treated with definitive CRT, with similar outcomes observed for patients with SCC or ACA.23 As would be expected, outcomes with definitive CRT for stage I adenocarcinoma in our series of patients appear significantly better than those in patients who received CRT for locally advanced adenocarcinoma. The JCOG 9708 trial evaluating definitive CRT for patients with stage I esophageal SCC demonstrated a clinical response rate of 88% and a 3-year OS of 85%.16 The more recently presented JCOG 0502 trial, which compared esophagectomy and CRT in a nonrandomized manner for patients with T1 esophageal SCC, reported 3-year OS of 95% in the esophagectomy arm, and 93% in the CRT arm (adjusted HR 1.05; 95% CI, 0.67-1.64).17 Outcomes in our series of patients with stage I adenocarcinoma appear similar or slightly inferior to those of patients with stage I SCC, although this could be explained by the advanced patient age and medical comorbidities in our series.
In our series, 25% of patients experienced local recurrence, which was the most common site of recurrence. Importantly, 3 of 4 patients who experienced local-only disease progression underwent potentially curative salvage therapy. No patients experienced isolated regional lymph node recurrence, supporting the notion that CRT is effective at clearing clinically occult regional lymph node metastasis.24
One hypothesis is that further escalation of treatment to the primary mucosal tumor may reduce the risk of local tumor persistence or recurrence after CRT. For example, some series suggest that there may be a benefit to ER for maximal gross tumor cytoreduction before CRT in patients with SCC. Hamada et al reported a 3-year OS of 87% and local recurrence of 3% with ESD-CRT for patients with stage I esophageal SCC.25 Preliminary data from JCOG0508 trial also support the safety and efficacy of this approach, with 3 year OS of 91%.26 Kawaguchi et al retrospectively compared outcomes of ESD-CRT versus CRT alone for patients with SCC, clinical stage T1bN0 or T1aN0 with LVI. ESD-CRT was associated with better 3-year OS (90% vs 63%, P = .12), and fewer local recurrences (0 vs 19%, P = .03) compared with CRT alone.27 Additionally, modest RT dose escalation could be considered. In our series, all patients received RT doses of ≤50.4 Gy; however, prospective studies have reported that an RT regimen of 60 Gy in 30 fractions with concurrent chemotherapy is well tolerated for patients with stage I esophageal SCC.16,17 Additional studies may consider exploring the role of ESD-CRT and RT dose escalation for patients with stage I ACA treated with definitive CRT.
This study has limitations, including the retrospective design, small sample size, potential selection biases, and lack of patient-reported outcomes. Additionally, longer term follow-up is needed to assess for durability of disease control and for late RT effects. However, these data are provocative and provide a valuable addition to current literature supporting the efficacy and safety of definitive CRT for patients with T1N0 esophageal adenocarcinoma.
Conclusion
This study indicated that CRT is a safe and effective curative treatment strategy for select patients with clinical T1N0M0 esophageal adenocarcinoma. Prospective trials evaluating this strategy are warranted.
Footnotes
Sources of support: Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Disclosures: Dr Lin reports grants from Hitachi Chemical Diagnostics, Beyond Spring Pharmaceuticals, STCube Pharmaceuticals, is on the advisory board for Beyond Spring Pharmaceuticals, STCube Pharmaceuticals, AstraZeneca Inc, and is on the speaker bureau for AstraZeneca Inc, Varian Medical Systems.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.03.020.
Supplementary data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yamashina T., Ishihara R., Nagai K. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol. 2013;108:544–551. doi: 10.1038/ajg.2013.8. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa Y., Uno T., Oyama T. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: Part 1. Esophagus. 2019;16:1–24. doi: 10.1007/s10388-018-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altorki N.K., Lee P.C., Liss Y. Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: Implications for endoscopic treatment. Ann Surg. 2008;247:434–439. doi: 10.1097/SLA.0b013e318163a2ff. [DOI] [PubMed] [Google Scholar]
- 5.Barbour A.P., Jones M., Brown I. Risk stratification for early esophageal adenocarcinoma: Analysis of lymphatic spread and prognostic factors. Ann Surg Oncol. 2010;17:2494–2502. doi: 10.1245/s10434-010-1025-0. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson K.J., Wang K., Zhang L. Esophagectomy outcomes in the endoscopic mucosal resection era. Ann Thorac Surg. 2017;103:890–897. doi: 10.1016/j.athoracsur.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 7.Leers J.M., DeMeester S.R., Oezcelik A. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271–278. doi: 10.1097/SLA.0b013e3181fbad42. [DOI] [PubMed] [Google Scholar]
- 8.Liu L., Hofstetter W.L., Rashid A. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol. 2005;29:1079–1085. [PubMed] [Google Scholar]
- 9.Mohiuddin K., Dorer R., El Lakis M.A. Outcomes of surgical resection of T1bN0 esophageal cancer and assessment of endoscopic mucosal resection for identifying low-risk cancers appropriate for endoscopic therapy. Ann Surg Oncol. 2016;23:2673–2678. doi: 10.1245/s10434-016-5138-y. [DOI] [PubMed] [Google Scholar]
- 10.Molena D., Schlottmann F., Boys J.A. Esophagectomy following endoscopic resection of submucosal esophageal cancer: A highly curative procedure even with nodal metastases. J Gastrointest Surg. 2017;21:62–67. doi: 10.1007/s11605-016-3210-3. [DOI] [PubMed] [Google Scholar]
- 11.Pennathur A., Farkas A., Krasinskas A.M. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048–1054. doi: 10.1016/j.athoracsur.2008.12.060. discussion 54-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westerterp M., Koppert L.B., Buskens C.J. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497–504. doi: 10.1007/s00428-005-1243-1. [DOI] [PubMed] [Google Scholar]
- 13.In H., Palis B.E., Merkow R.P. Doubling of 30-day mortality by 90 days after esophagectomy: A critical measure of outcomes for quality improvement. Ann Surg. 2016;263:286–291. doi: 10.1097/SLA.0000000000001215. [DOI] [PubMed] [Google Scholar]
- 14.Low D.E., Kuppusamy M., Hashimoto Y., Traverso L.W. Comparing complications of esophagectomy and pancreaticoduodenectomy and potential impact on hospital systems utilizing the accordion severity grading system. J Gastrointest Surg. 2010;14:1646–1652. doi: 10.1007/s11605-010-1325-5. [DOI] [PubMed] [Google Scholar]
- 15.Sturm E.C., Zahnd W.E., Mellinger J.D., Ganai S. Survival implications of increased utilization of local excision for cT1N0 esophageal cancer. Ann Surg. 2019;270:295–301. doi: 10.1097/SLA.0000000000002782. [DOI] [PubMed] [Google Scholar]
- 16.Kato H., Sato A., Fukuda H. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708) Jpn J Clin Oncol. 2009;39:638–643. doi: 10.1093/jjco/hyp069. [DOI] [PubMed] [Google Scholar]
- 17.Kato K., Igaki H., Ito Y. Parallel-group controlled trial of esophagectomy versus chemoradiotherapy in patients with clinical stage I esophageal carcinoma (JCOG0502) American Society of Clinical Oncology. 2019;37:7. [Google Scholar]
- 18.NCCN NCCN clinical practice guidelines in oncology: Esophageal and esophagogastric junction cancer. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf Version 1. Available from:
- 19.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 20.Moreno A.C., Verma V., Hofstetter W.L., Lin S.H. Patterns of care and treatment outcomes of elderly patients with stage I esophageal cancer: Analysis of the National Cancer Data Base. J Thorac Oncol. 2017;12:1152–1160. doi: 10.1016/j.jtho.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Soni P.D., Hartman H.E., Dess R.T. Comparison of population-based observational studies with randomized trials in oncology. J Clin Oncol. 2019;37:1209–1216. doi: 10.1200/JCO.18.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper J.S., Guo M.D., Herskovic A. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 23.Suntharalingam M., Winter K., Ilson D. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: The NRG Oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol. 2017;3:1520–1528. doi: 10.1001/jamaoncol.2017.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sgourakis G., Gockel I., Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: A systematic review. World J Gastroenterol. 2013;19:1424–1437. doi: 10.3748/wjg.v19.i9.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamada K., Ishihara R., Yamasaki Y. Efficacy and safety of endoscopic resection followed by chemoradiotherapy for superficial esophageal squamous cell carcinoma: A retrospective study. Clin Transl Gastroenterol. 2017;8:e110. doi: 10.1038/ctg.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muto M., Minashi K., Nihei K. Efficacy of combined endoscopic resection and chemoradiotherapy for clinical stage I esophageal squamous cell carcinoma (ESCC): A single-arm confirmatory study (JCOG0508) J Clin Oncol. 2016;34:4013. [Google Scholar]
- 27.Kawaguchi G., Sasamoto R., Abe E. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol. 2015;10:31. doi: 10.1186/s13014-015-0337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.