Abstract
Since the outbreak of the new coronavirus pneumonia (COVID-19) in December 2019, more than 23 million people worldwide have been diagnosed with SARS-CoV-2. In response to this pandemic, a global mobilization of scientific, industrial and political support has ensued. However, more than 8 months later, as studies multiply and several governments are embarking on a resumption of their activities, the threat still remains. Our efforts to understand the evolution of the virus and the means to defeat it, at the dawn of a possible new wave, have raised more questions than provided clear and unequivocal answers. Compared to diseases caused by previously known human coronavirus, COVID-19 shows higher transmissibility, as a matter of fact “deeply concerning” cases continue to increase. Under these circumstances, and based on the information we have collected so far, this paper provides an overview of the epidemiological status of COVID-19 by considering, first through comparisons with other coronaviruses, similarities that may guide prevention measures and potentially effective therapies. From this starting point, we aimed to discuss the evidence around the efficacy of masks and respirators for different group of the population. Finally, we address therapeutic aspects including perspectives of vaccines and some antimicrobial agents such as remdesivir, favipiravir, chloroquine, hydroxychloroquine in combination with azithromycin and immunomodulators.
Keywords: Vaccines, outbreaks, coronavirus, SARS-CoV-2 remdesivir, favipiravir, dexamethasone, tocilizumab, hydroxychloroquine, interferon.
SUMMARY
1. Introduction
2. Virology and comparison of SARS-CoV-2 with SARS/MERS-CoV
3. Incubation, airborne transmissibility and mutation
4. Respiratory protection
5. Clinical features of COVID-19 and treatment strategies
5.1. Current vaccine studies
5.2. Remdesivir
5.3. Favipiravir
5.4. Immunomodulators
5.5. Dexamethasone
5.6. Chloroquine and its derivatives
5.7. Azithromycin/Hydroxychloroquine
6. Conclusion
1. Introduction
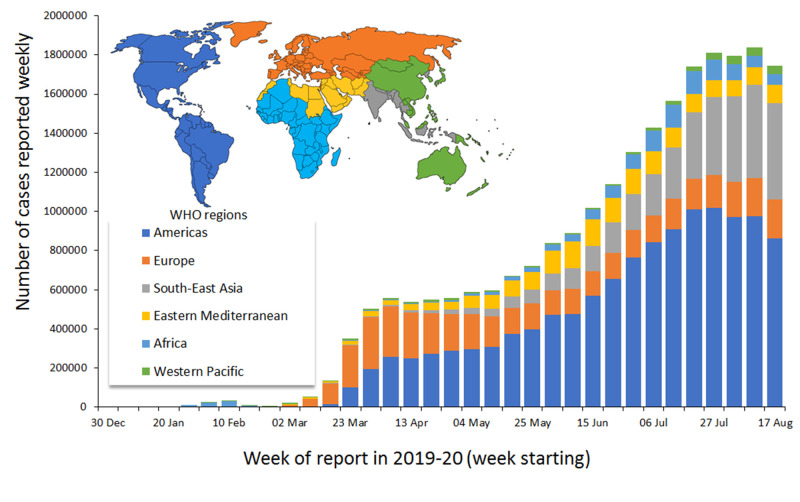
The new HCoV was discovered in December 2019 in the city of Wuhan, located in Hubei Province, China1,2. Initially informally called "Wuhan Coronavirus", the strain was officially named SARS-CoV-2 by the ICTV on 11 February 2020, based on its taxonomic and phylogenetic similarities with SARS-CoV, which caused the SARS epidemic from 2002 to 20031. The World Health Organization named the disease caused by SARS-CoV-2 coronavirus disease (COVID-19)1. The first cases of SARS-CoV-2 were detected in Wuhan, China, while the pattern of human-to-human transmission of the virus was confirmed after it was observed that several individuals who had not visited the city of Wuhan were also affected3. The situation rapidly spread beyond the borders of Hubei and China, causing an epidemic of more than 118,000 cases in 114 countries and the subsequent declaration of a pandemic status by the World Health Organization on March 11, 20204. As of August 23, 2020, the number of confirmed cases was 23 077 756, including 800 909 deaths, suggesting a general mortality rate lower than 4%, among confirmed cases, since many infections are asymptomatic or produce mild symptoms, thus, not being diagnosed (Figure 1)5. Individuals working in the healthcare sector remain, to this day, among the most affected subjects, as already reported in an ICN report in early May, to the effect that COVID-19 would have infected at least 90 000 healthcare workers. These figures include statistics from only 30 countries6.
Figure 1. Number of confirmed COVID-19 cases, by date of report and WHO region, December 30, 2019 through August 23, 2020.
Adapted from WHO5, under the Creative Commons Attribution 3.0 IGO license (CC BY 3.0 IGO) http://creativecommons.org/licenses/by/3.0/igo/legalcode
These mortality rates and the groups of subjects at risk are strongly influenced by many factors, including health prevention measures, individual protection and the extent of early detection of infection, in order to isolate problem cases. The novelty of the disease has caused these issues to be the subject of much debate and even contradiction, such as the capability of the different tests to detect the entire virus rather than debris; arising the concern that such tests are being misinterpreted to suggest COVID-19-positive patients when, in reality, they are not7,8. By taking on a dimension where the health and epidemiological stakes are mixed with the political and economic challenges, the situation is sometimes confusing for the layman. This article, therefore, seeks to address the problem of the pandemic by firstly looking at the structural aspect of the virus, secondly at its mode of transmission and life cycle, and finally at some therapeutic and protective approaches developed to combat previous viruses of the human coronavirus family, such as SARS/MERS-CoV.
2. Virology and comparison of SARS-CoV-2 with SARS/MERS-CoV
Each SARS-CoV-2 virion has an estimated size of 50-200 nm in diameter, and its shape varies from round to oval9,10. The SARS-CoV viral particles, on the other hand, have a smaller diameter of 50-80 nm as measured by electron microscopy11-13. The genome of SARS-CoV-2, initially considered stable, consists of a single-stranded RNA of 29,903 nucleotides, containing 14 open reading frames (ORFs), from which four ORFs encode known structural proteins such as S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins14,15. In terms of homology, the genome of SARS-CoV-2 is 50% and 79.5% identical to that of MERS-CoV and SARS-CoV, respectively16,17. The similarity between SARS-CoV-2 and SARS-CoV increases to 96%, from a structural point of view, at the level of protein E. This conservation in the E sequence could be explained by its key role as a transmembrane protein18. Nevertheless, glycoprotein S, which is responsible for attachment and fusion with the host cell, shows the weakest similarity (76%) between the two specimens SARS-CoV-2 and SARS-CoV19. In addition, protein S is believed to have an affinity for the ACE2 receptor that can be exploited in several ways, but the level of comprehension on this matter needs to be further investigated20-22. That said, recent studies have failed to demonstrate, beyond doubt, the existence of other virus-like spicule-free structures, despite similar diameter bodies, in autopsies in COVID-19 patients. A cross-species comparison has shown that SARS-CoV-2 is 91% identical to a coronavirus present in Javanese pangolins, with 99% similarity at the site of binding to the ACE2 receptor, ensuring host specificity, and 96% identical to the coronavirus Beta-CoV/bat/Yunnan/RaTG13/2013 found in the Chinese bat, Rhinolophus affinis23-28. These similarities leave no doubt as to the origin and the possible crossing of the species barrier.
3. Incubation, airborne transmissibility and mutation
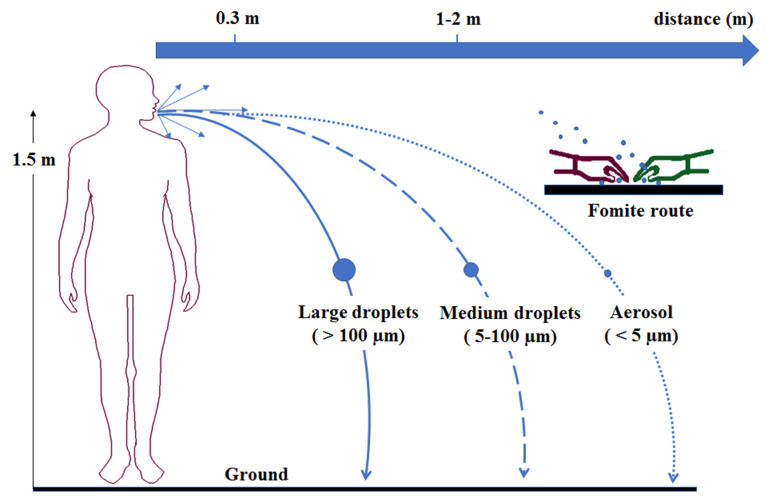
Once infected, the incubation period of the virus seems, according to the different tests, to be widely variable. The generally advanced interval is 2-14 days29. These conclusions are mainly based on 1) WHO observations of 2-10 days; 2) reports from the Chinese National Health Commission initially indicating a period of 10-14 days; and 3) the Centers for Disease Control and Prevention in the USA, which has estimated the incubation period to be between 2 and 14 days29. However, this does not exclude longer periods, as reported by Bai et al. of 0-24 days30. Human-to-human transmission of these types of viruses can occur through direct, indirect (fomite) or respiratory droplet contact (Figure 2). In this respect, a distinction is made between three categories: large droplets (or postilion) which fall to the ground rapidly near the source of emission, medium droplets (or coarse aerosols) with an aerodynamic diameter greater than 5 μm and aerosols with an aerodynamic diameter less than 5 μm31,32. Accurate data on how far a droplet or smaller particle and aerosol, emitted by an infected patient, can travel before settling on surfaces are scarce, but it is well known that the distance may vary depending on ventilation, turbulence of the surrounding air, the nasopharyngeal load, and the size of the particle.
Figure 2. Illustration of different transmission routes of SARS-CoV-2.
Aerosols (<5 μm) are responsible for the short-range airborne route, long-range airborne route, and indirect contact route. Large droplets are responsible for the direct spray route and indirect contact route. Fomite route refers to contaminated surfaces. (adapted with permission from references33,34)
While the spread of the virus in the population depends on its transmissibility, R is used as a "diagnostic" tool for the latter. In epidemiology, the basic reproductive number (R) is defined as the expected number of cases of infection directly generated by a case in a population where all individuals are susceptible to the pathogen35. For example, measles, which is transmitted by air, has an R between 12 and 1836. However, SARS-CoV-2 has an R of about 3, which is far lower than measles, but higher than MERS-CoV and SARS-CoV37. On the other hand, its fatality rate would be lower than 4%, as mentioned above, compared to a rate of approximately 10% for measles in some regions, equal to SARS, and 34% for MERS38. In sum, as is the trend generally observed for many viruses, low pathogenicity is often associated with high transmissibility, which according to our best observations, appears to be the case for coronaviruses family. This observation seems to become clearer as time progresses. As a matter of fact, in a recent genomic study carried out on the geographical distribution of SARS-CoV-2 mutations, from a library of 48 635 complete genomes, a global emergence of the D614G mutation was observed39. This mutation of the SARS-CoV-2 (D614G) protein S is associated with an increase in the number of binding and recognition elements on the surface of the virus for the ACE2 receptor and, consequently, in the capacity of SARS-CoV-2 to infect and transmit to a host. While such a mutation does not seem to be associated with an increase in the pathogenicity of the virus40, the fact remains that the D614G strain may not be the only explanation for the very recent change in the profile of the epidemic in the population. Several other hypothetical factors can be considered, such as the average age of newly infected subjects, the widespread awareness of basic sanitary and hygienic measures and the advances, still in progress, of screening methods and care to avoid patients being overlooked. The analysis of this statistical data requires nonetheless great caution in terms of interpretation, since when calculating the fatal risk of infection (IFR), a much lower mortality risk of barely 0.3-0.6% is observed for SARS-CoV-241. Without neglecting the fact that, as we are still in the midst of a pandemic, lack of hindsight, the results are still fragmentary for the moment. Let us also add that it is difficult to clearly detect and prevent transmission in a population, except through extensive screening, due in particular, to a transmission rate by asymptomatic (or mildly symptomatic) subjects of about 55%42. For these reasons, concerning asymptomatic cases that are difficult to assess, the effects and transmissibility of SARS-CoV-2 between mother and foetus are all the more still poorly known. The same is true for transmission via breast milk43.
4. Respiratory protection
Before discussing treatments, let us first address the issue of respiratory protection. As mentioned above, SARS-CoV-2 is mainly spread through the airways via fine aerosol droplets, although the definition of aerosol itself is still debated. Significant loads of SARS-CoV-2 have been reported in the saliva of patients with COVID-19, not to mention growing voices calling for recognition of the potential for airborne spread of the virus44-46. As a result, several studies have investigated the effectiveness of various nasobuccal means of filtering these transmission vectors. Currently, three types of respiratory protection are mainly suggested and put forward in the literature and by certain health authorities. These are respirators, medical masks and homemade masks. In a meta-analysis of 19 randomised clinical trials, conducted by MacIntyre and Chughtai, on the use of respiratory protection by healthcare workers, sick patients and individuals randomly selected from the population, the findings suggest a benefit of wearing a mask superior to that associated with hand hygiene alone (results were here reported according to the PRISMA criteria)47. In the population, in community settings with conditions highly favourable to transmissions, such as households or colleges, the practice of mask use and hand hygiene together would further increase the protective effect. Randomised controlled trials among healthcare workers indicate that respirators, when worn throughout a shift, are effective, but not when worn intermittently. Medical masks are not sufficiently effective for this group of workers, while homemade tissue masks are even less effective47. Moreover, it appears that the effectiveness of homemade masks is strongly linked to several factors, such as the nature of the fabric, the design and their washing capacity, even suggesting, but without generalising, a risk of increased infections caused by the homemade masks themselves. In short, homemade masks are not a recommended option for healthcare workers47. None of these trials, however, have examined the combined or unique effect of visors alone or in conjunction with other protective equipment or practice in the population.
5. Clinical features of COVID-19 and treatment strategies
To date, to the best of our knowledge, there is no effective vaccine developed or recognised by the WHO against SARS-CoV-2. However, in order to better synthesize such treatment, different animal models have been developed to study the pathogenicity of the virus on organs, similar to what we could observe in clinical cases in humans. These include hACE2 transgenic mice in which symptoms of pneumonia were reproduced with SARS-CoV-2; that is, weight loss and intestinal pneumonia comparable to the initial clinical reports of pneumonia observed in COVID-1948. The main clinical signs of the disease in humans, listed in Table 1, as recently reported in the literature, include fever, cough, dyspnea, dysgeusia and hyposmia49. It is obviously not possible to diagnose COVID-19 on the basis of these symptoms alone, but they should, when they occur, lead to a serious examination of the condition of the potentially infected patient.
Table 1. Symptoms of COVID-19 from British Medical Journal49.
| Factor | Prevalence (%) | Characteristics of symptoms | ||
|---|---|---|---|---|
| Key diagnostic | Common | Uncommon | ||
| Fever | 78 | X | X | |
| Cough | 57 | X | X | |
| Dyspnea | 31-40 | X | X | |
| Dysgeusia/Hyposmia | 38 / 41 | X | X | |
| Fatigue | 31 | X | ||
| Arthralgia or Myalgia | 11-17 | X | ||
| Expectoration | 23.7 | X | ||
| Chest tightness | 22.9 | X | ||
| Sore throat | 12 | X | ||
| Gastrointestinal.symptoms | 20 | X |
5.1 Current vaccine studies
In this ongoing effort to understand the disease and develop a vaccine against it, as of August 2020, the global COVID-19 vaccine research and development landscape includes a large number of candidates, of which many are currently at exploratory or preclinical stages. Table 2 lists some of the most actual advanced projects in the clinical development phases, although other initiatives exploiting innovative technology platforms are continuously announced by academic and industrial institutions. The technology landscape presented in Table 2 is intended to illustrate, while inviting the reader not to be limited to it, the variety of potential targets that can be exploited.
Table 2. Ongoing clinical trials for potential vaccines against Sars-CoV-2.
| Vaccine prospect | Developer | Technology | Evolution of trial | ||
|---|---|---|---|---|---|
| Phase | Number of Participants | Duration (MM/YY) | |||
| NVX-CoV2373 50 | Novavax | recombinant spike protein | I | 131 | 05/20 - 07/21 |
| SCB-2019 51 | Clover Biopharm | spike protein trimeric subunit | I | 150 | 06/20 - 03/21 |
| AG0301-COVID19 52 | AnGes Inc. | DNA plasmid | I - II | 30 | 06/20 - 07/21 |
| Ad5-nCoV 53 | CanSinoBiologics | recombinant adenovirus vector | II | 382 | 03/20 - 12/20 |
| AZD1222 54 | Univ. Of Oxford | adenovirus vector | II - III | 10 260 | 05/20 - 08/21 |
| BNT162 55 | BioNTech | RNA | II - III | 30 000 | 04/20 - 05/21 |
| CoronaVac 56 | Sinovac Biotech | inactivated SARS-CoV-2 virus | III | 10 490 | 04/20 - 12/20 |
| BBIBP-CorV 57 | Sinopharm | inactivated SARS-CoV-2 virus | III | 15 000 | 07/20 - 07/21 |
| mRNA-1273 58 | Moderna | nanoparticle | III | 30 000 | 07/20 - 10/21 |
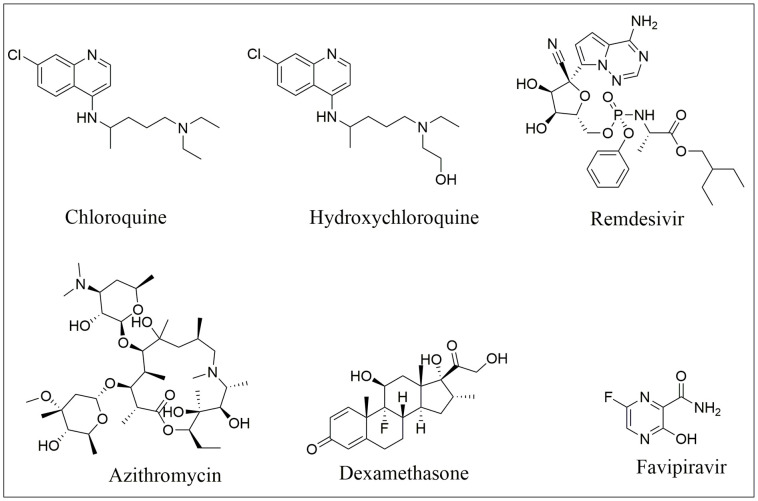
Nonetheless, the discovery of a vaccine is not the only possible and feasible avenue we should emphasise for prevention and treatment of the disease. Molecules capable of directly attacking the virus are being tested (Figure 3). That said, despite more than 200 clinical trials, no curative compound has been proven, by broad consensus, to be effective against SARS-CoV-2. Studies at this level have focused primarily on antivirals, antibiotics combinations and immunomodulators. Among the many proposals in the literature, our review has focused on approaches that have appeared to achieve a degree of agreement and that would allow access to the highest possible number of individuals, taking into account production costs, availability and the therapeutic profile.
Figure 3. Structures of antimalarials chloroquine and hydroxychloroquine, antivirals remdesivir and favipiravir, corticosteroid dexamethasone and antibiotic azithromycin.
5.2 Remdesivir
A monophosphate derivative of an adenine nucleoside analogue, remdesivir was initially developed to treat Ebola virus disease and Marburg virus infections2,59. Its antiviral action targets a wide range of RNA viruses, including potentially SARS/MERS-CoV, thus presumably SARS-CoV-2. As such, Wang et al. have reported an inhibitory action of remdesivir on human Huh-7 cancerous liver cells known to be susceptible to SARS-CoV-260. In addition, more recently, Pruijssers et al. demonstrated strong inhibition of SARS-CoV-2 replication in cultures of human lung cells and primary human airway epithelium in the presence of remdesivir (EC50= 0.01 µM)61. This is consistent with the ability of these cells to metabolize the prodrug, in contrast to infected Vero E6 cells, which have a significantly lower sensitivity to remdesivir (EC50= 1.65 µM). In vivo results in mice infected with a chimeric SARS-CoV virus also demonstrated an improvement in lung function, accompanied by a decrease in viral load61. Other as yet preliminary studies also address this promising avenue, indicating a reduction in hospitalization time, with remdesivir62.
5.3 Favipiravir
Favipiravir, or T-705, is an organofluorinated pyrazine that is believed to act by selective inhibition of RNA-dependent RNA polymerase from RNA viruses (RdRp), since it does not interact with DNA transcription to RNA or DNA replication in mammalian cells63. Approved since 2014, in Japan, to treat influenza strains that cause more severe disease and resistant to existing antiviruses, its action has been studied in February 2020 against SARS-CoV-264,65. It appears to be more effective than the lopinavir/ritonavir combination according to a preliminary study carried out on 80 patients66.
5.4 Immunomodulators
The inflammatory and immune dimension of COVID-19 is another aspect that one can address, through the beneficial effects of interleukin inhibitors and anti-inflammatory drugs. Increasing evidence have suggested a correlation between the SARS-CoV-2 infection and interleukin-6 (IL-6) production67-70. This massive induction of interleukin, generating a "cytokine storm", is believed to be responsible for the severe inflammatory pulmonary response in infected patients. The interest of interleukin inhibitors is, therefore, to reduce this pulmonary inflammatory response. A very early study has demonstrated the 45% reducing effect of tocilizumab on the risk of mortality compared to a control group receiving only respiratory assistance. However, tocilizumab was associated in patients in this study (n=78) with an increased incidence of superinfections (54% vs 26%; P<0.001), although this does not appear to significantly affect the long-term fatality rate between tocilizumab-treated patients with superinfection versus those without superinfection (22% vs 15%; P=0.42)71. Interestingly, another IL-6 inhibitor, sarilumab, have not shown notable benefit on clinical outcomes in the preliminary phase II analysis, conducted by Sanofi and Regeneron, when comparing "severe + critical" groups versus placebo. The discrepancy between these two treatments, in an effort to treat COVID-19, is not well understood or explained in the literature at this time and more evidence remains to be generated.
Besides, in light of the current knowledge of the beneficial effects of both the immunomodulators and the antiviral remdesivir discussed earlier, a randomized controlled clinical trial was initiated to evaluate the safety of a regimen of remdesivir with the immunomodulator interferon beta-1a in patients with COVID-1972. One of the reasons for this initiative is that, at present, an improvement in median recovery time of approximately 4 days is observed in subjects receiving remdesivir with a mortality rate of 7.1% versus 11.9% for the placebo group62,73. In order to significantly improve this mortality rate, a research run by the U.S. National Institute of Allergy and Infectious Diseases (NIAID) has therefore proposed a combination with other therapeutic agents73. In addition, the inhibitory effect of interferon type 1 was observed in the laboratory on the SARS-CoV, SARS-CoV-2 and MERS-CoV coronaviruses72. As the combination of remdesivir and interferon (beta-1a) has never been evaluated in a large randomized trial, this information opened the door to a probable new treatment alternative. As a result, the Adaptive COVID-19 Treatment Trial 3 (ACTT 3), sponsored by NIAID and which began on February 21, 2020, plans to recruit 1,000 adults with COVID-19. The goal of this controlled clinical trial is to study, in its methodology, the effect of remdesivir plus interferon beta-1a versus remdesivir alone on the duration of hospitalization and ultimately mortality of COVID-19 subjects. Preliminary results will be available by fall 202072.
5.5 Dexamethasone
In the same spirit of studying the consequences of "cytokine storm", it has been observed that the severe form of COVID-19 is associated with interstitial pneumonia and then with alveolar damage that can precipitate acute respiratory distress syndrome (ARDS)74. Due to their anti-inflammatory and immunomodulatory properties, inducing the activation of the endothelial nitric oxide synthase (eNOS)75, corticosteroids have been the subject of studies evaluating their ability to reduce systemic and pulmonary damage in patients with an ARDS. Therefore, dexamethasone presented itself as a suitable candidate. Indeed, it is currently recommended for many conditions such as inflammatory problems, dermatological affections, allergies, respiratory diseases etc. Horby et al. published the preliminary results of a treatment arm of the national open-label study Recovery which is evaluating the efficacy and safety of several potential treatments of COVID-1976. Among the possible treatment proposals, subjects treated with dexamethasone had a statistically significantly lower incidence of mortality compared to the standard care group receiving invasive mechanical ventilation (29.3% vs. 41.4%; ratio, 0.64; 95% CI, 0.51-0.81)76. The effect was less marked compared to subjects under oxygenation without invasive mechanical ventilation (23.3% vs. 26.2%; ratio, 0.82; 95% CI, 0.72-0.94), whereas no significant effect was observed in patients not requiring any oxygenation support (17.8% vs. 14.0%; ratio, 1.19; 95% CI, 0.91-1.55)76. In addition, dexamethasone provides this major element in that, although remdesivir may shorten the recovery time in hospitalised patients, no therapeutic agent has so far been shown to be capable of reducing significantly and unequivocally mortality like dexamethasone. That being said, it should be borne in mind that, by its very nature, dexamethasone has typical side effects associated with corticosteroids, in addition to being recommendable, depending on the situation, only at the severe stage of the disease, as defined by the Infectious Diseases Society of America (IDSA)77.
5.6 Chloroquine and its derivatives
In this category, chloroquine is among the first molecules to have aroused the interest of researchers. Commonly used as an affordable antimalarial and immunosuppressant, chloroquine is believed to act by alkalinising the endosomal pH required for virus infection while interfering with the glycosylation of the cellular receptors for SARS-CoV78. Chloroquine would presumably be effective against the SARS-CoV-2 virus at EC50 concentrations of 1.13 µM associated with CC50 toxicity at concentrations of 100 µM, or almost two orders of magnitude higher79. Its use, however, also remains off-label for potential treatment of the disease80. Moreover, according to several authors, it is important to remain cautious and closely monitor the condition of patients receiving chloroquine2,81. In order to reduce the risks associated with the side effects to be monitored, other alternatives to chloroquine have been proposed, such as hydroxychloroquine, which is a derivative of chloroquine bearing an hydroxyl group (Figure 3)82.
5.7 Azithromycin/Hydroxychloroquine combination
Although it may seem counterintuitive to use antibiotics in the treatment of a viral disease, we have long known that the risk of bacterial superinfection plays a role in the severity of the influenza virus infection83. It is therefore not uncommon to combine antibiotics with antivirals in order to improve the synergistic effect and reduce the prognosis of complications. Thus, based on proposals submitted by recent papers reporting an inhibitor effect of chloroquine on the growth of SARS-CoV-2 in vitro60 and in vivo79, Raoult et al. reported in March 2020 that the combination of hydroxychloroquine (HCQ) and azithromycin allowed them to treat COVID-1984. As mentioned above for its analogue, the chloroquine, precautions should be taken with hydroxychloroquine regarding its use and monitoring of the patient's cardiac, hepatic and renal condition is recommended. Although the risks remain low at the recommended daily doses (< 1000 mg) of HCQ85,86. This notwithstanding, based on the balance of risks versus benefits, the use of chloroquine and hydroxychloroquine has been revoked by the FDA, as part of the emergency use authorisation (EUA), to treat certain patients hospitalised with COVID-19 when no clinical trial is in place87. According to the agency, the legal criteria for decreeing such an emergency authorisation are no longer met. A decision by the American College of Physicians is also in the same vein regarding the use of hydroxychloroquine, alone or in combination with azithromycin, as a preventative or for the treatment of coronavirus disease88.
6. Conclusion
This article reviews several published prevention and treatment approaches against the new coronavirus. The SARS-CoV-2 virus has demonstrated that it can be fatal, especially for the patients belonging to one of the risk groups, with a transmission potential that should not be overlooked. To date, more than 23 million confirmed cases and 800 909 deaths have been recorded worldwide, hence the importance of taking this pandemic seriously and focusing efforts to find a cure and prevent its spreading. By first comparing SARS-CoV and MERS-CoV, it is possible to establish similarities that can guide potentially effective therapies. That said, no vaccine or antivirus has yet been recognised and supported by conclusive data as being fully effective against SARS-CoV-2. Already known compounds, such as anti-inflammatory steroid dexamethasone and the antiviral medication remdesivir, either in combination or alone, appear to be a fast and appealing route of treatment as several encouraging results suggest that COVID-19 can possibly be treated with the medical arsenal we already have at our disposal.
Acknowledgments
The author is grateful to Université de Saint-Boniface for financial support, Mr. Jean-Michel Martin, apparitor in the department of experimental sciences, for technical strategic assistance and to the reviewers for their helpful comments on the manuscript.
Footnotes
Conflict of interests: The authors declare no conflicts of interest.
Angiotensin-2 Conversion Enzyme (ACE2); Coronavirus Disease 19 (COVID-19); Fatal Risk of Infection (IFR); Human Coronavirus (HCoV); Hydroxychloroquine (HCQ); International Council of Nurses (ICN); International Committee on Taxonomy of Viruses (ICTV); Middle East Respiratory Syndrome-related Coronavirus (MERS-CoV); Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2); World Health Organization (WHO).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.World Health Organization Naming the coronavirus disease (COVID-19) and the virus that causes it. World Health Organization. Accessed: 2020, June 20. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
- 2.Emerging Therapeutic Strategies for COVID-19 patients. Zhu Shudong, Guo Xialing, Geary Kyla, Zhang Dianzheng. Discoveries (Craiova) 2020;8(1):e105. doi: 10.15190/d.2020.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Chan Jasper Fuk-Woo, Yuan Shuofeng, Kok Kin-Hang, To Kelvin Kai-Wang, Chu Hin, Yang Jin, Xing Fanfan, Liu Jieling, Yip Cyril Chik-Yan, Poon Rosana Wing-Shan, Tsoi Hoi-Wah, Lo Simon Kam-Fai, Chan Kwok-Hung, Poon Vincent Kwok-Man, Chan Wan-Mui, Ip Jonathan Daniel, Cai Jian-Piao, Cheng Vincent Chi-Chung, Chen Honglin, Hui Christopher Kim-Ming, Yuen Kwok-Yung. Lancet (London, England) 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghebreyesus TA. WHO Director-General's opening remarks at the media briefing on COVID-19. World Health Organization. Speeches 2020. Accessed: 2020 June 20. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 5.WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. Accessed: 2020 August 24. 2020. https://covid19.who.int/ https://covid19.who.int/
- 6.International Council of Nurses calls for data on healthcare worker infection rates and deaths. International Council of Nurses. Accessed: 2020 June 25. 2020. https://www.icn.ch/sites/default/files/inline-files/PR_20_Infections%20and%20deaths%20from%20COVID-19%20among%20nurses.pdf https://www.icn.ch/sites/default/files/inline-files/PR_20_Infections%20and%20deaths%20from%20COVID-19%20among%20nurses.pdf
- 7.Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. Chan Jasper Fuk-Woo, Yip Cyril Chik-Yan, To Kelvin Kai-Wang, Tang Tommy Hing-Cheung, Wong Sally Cheuk-Ying, Leung Kit-Hang, Fung Agnes Yim-Fong, Ng Anthony Chin-Ki, Zou Zijiao, Tsoi Hoi-Wah, Choi Garnet Kwan-Yue, Tam Anthony Raymond, Cheng Vincent Chi-Chung, Chan Kwok-Hung, Tsang Owen Tak-Yin, Yuen Kwok-Yung. Journal of clinical microbiology. 2020;58(5) doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long-term Positivity to SARS-CoV-2: A Clinical Case of COVID-19 with Persistent Evidence of Infection. D'Ardes Damiano, Boccatonda Andrea, Rossi Ilaria, Pontolillo Michela, Cocco Giulio, Schiavone Cosima, Santilli Francesca, Guagnano Maria Teresa, Bucci Marco, Cipollone Francesco. European journal of case reports in internal medicine. 2020;7(6):001707. doi: 10.12890/2020_001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Wu Canrong, Liu Yang, Yang Yueying, Zhang Peng, Zhong Wu, Wang Yali, Wang Qiqi, Xu Yang, Li Mingxue, Li Xingzhou, Zheng Mengzhu, Chen Lixia, Li Hua. Acta pharmaceutica Sinica. B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W. The Coronavirus Prevention Handbook: 101 Science-Based Tips That Could Save Your Life. Skyhorse Publishing: United States of America; 2020. [Google Scholar]
- 11.Ultrastructural characterization of SARS coronavirus. Goldsmith Cynthia S, Tatti Kathleen M, Ksiazek Thomas G, Rollin Pierre E, Comer James A, Lee William W, Rota Paul A, Bankamp Bettina, Bellini William J, Zaki Sherif R. Emerging infectious diseases. 2004;10(2):320–6. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modern uses of electron microscopy for detection of viruses. Goldsmith Cynthia S, Miller Sara E. Clinical microbiology reviews. 2009;22(4):552–63. doi: 10.1128/CMR.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The life cycle of SARS coronavirus in Vero E6 cells. Qinfen Zhang, Jinming Cui, Xiaojun Huang, Huanying Zheng, Jicheng Huang, Ling Fang, Kunpeng Li, Jingqiang Zhang. Journal of medical virology. 2004;73(3):332–7. doi: 10.1002/jmv.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Architecture of SARS-CoV-2 Transcriptome. Kim Dongwan, Lee Joo-Yeon, Yang Jeong-Sun, Kim Jun Won, Kim V Narry, Chang Hyeshik. Cell. 2020;181(4):914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Identification of Diverse Bat Alphacoronaviruses and Betacoronaviruses in China Provides New Insights Into the Evolution and Origin of Coronavirus-Related Diseases. Han Yelin, Du Jiang, Su Haoxiang, Zhang Junpeng, Zhu Guangjian, Zhang Shuyi, Wu Zhiqiang, Jin Qi. Frontiers in microbiology. 2019;10:1900. doi: 10.3389/fmicb.2019.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lu Roujian, Zhao Xiang, Li Juan, Niu Peihua, Yang Bo, Wu Honglong, Wang Wenling, Song Hao, Huang Baoying, Zhu Na, Bi Yuhai, Ma Xuejun, Zhan Faxian, Wang Liang, Hu Tao, Zhou Hong, Hu Zhenhong, Zhou Weimin, Zhao Li, Chen Jing, Meng Yao, Wang Ji, Lin Yang, Yuan Jianying, Xie Zhihao, Ma Jinmin, Liu William J, Wang Dayan, Xu Wenbo, Holmes Edward C, Gao George F, Wu Guizhen, Chen Weijun, Shi Weifeng, Tan Wenjie. Lancet (London, England) 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID-19 pneumonia: what has CT taught us? Lee Elaine Y P, Ng Ming-Yen, Khong Pek-Lan. The Lancet. Infectious diseases. 2020;20(4):384–385. doi: 10.1016/S1473-3099(20)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coronavirus virulence genes with main focus on SARS-CoV envelope gene. DeDiego Marta L, Nieto-Torres Jose L, Jimenez-Guardeño Jose M, Regla-Nava Jose A, Castaño-Rodriguez Carlos, Fernandez-Delgado Raul, Usera Fernando, Enjuanes Luis. Virus research. 2014;194:124–37. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Ahmed Syed Faraz, Quadeer Ahmed A, McKay Matthew R. Viruses. 2020;12(3) doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Xu Xintian, Chen Ping, Wang Jingfang, Feng Jiannan, Zhou Hui, Li Xuan, Zhong Wu, Hao Pei. Science China. Life sciences. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Functional assessment of cell entry and receptor usage for lineage B β-coronaviruses, including 2019-nCoV. Letko Michael, Munster Vincent. bioRxiv : the preprint server for biology. 2020 doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. Hoffmann Markus, Kleine-Weber Hannah, Krüger Nadine, Müller Marcel, Drosten Christian, Pöhlmann Stefan. 2020 [Google Scholar]
- 23.Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Lam Tommy Tsan-Yuk, Jia Na, Zhang Ya-Wei, Shum Marcus Ho-Hin, Jiang Jia-Fu, Zhu Hua-Chen, Tong Yi-Gang, Shi Yong-Xia, Ni Xue-Bing, Liao Yun-Shi, Li Wen-Juan, Jiang Bao-Gui, Wei Wei, Yuan Ting-Ting, Zheng Kui, Cui Xiao-Ming, Li Jie, Pei Guang-Qian, Qiang Xin, Cheung William Yiu-Man, Li Lian-Feng, Sun Fang-Fang, Qin Si, Huang Ji-Cheng, Leung Gabriel M, Holmes Edward C, Hu Yan-Ling, Guan Yi, Cao Wu-Chun. Nature. 2020;583(7815):282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 24.Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. Li Xingguang, Zai Junjie, Zhao Qiang, Nie Qing, Li Yi, Foley Brian T, Chaillon Antoine. Journal of medical virology. 2020;92(6):602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An Extensive Meta-Metagenomic Search Identifies SARS-CoV-2-Homologous Sequences in Pangolin Lung Viromes. Wahba Lamia, Jain Nimit, Fire Andrew Z, Shoura Massa J, Artiles Karen L, McCoy Matthew J, Jeong Dae-Eun. mSphere. 2020;5(3) doi: 10.1128/mSphere.00160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Author Correction: A new coronavirus associated with human respiratory disease in China. Wu Fan, Zhao Su, Yu Bin, Chen Yan-Mei, Wang Wen, Song Zhi-Gang, Hu Yi, Tao Zhao-Wu, Tian Jun-Hua, Pei Yuan-Yuan, Yuan Ming-Li, Zhang Yu-Ling, Dai Fa-Hui, Liu Yi, Wang Qi-Min, Zheng Jiao-Jiao, Xu Lin, Holmes Edward C, Zhang Yong-Zhen. Nature. 2020;580(7803):E7. doi: 10.1038/s41586-020-2202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Zhang Tao, Wu Qunfu, Zhang Zhigang. Current biology : CB. 2020;30(7):1346–1351.e2. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A pneumonia outbreak associated with a new coronavirus of probable bat origin. Zhou Peng, Yang Xing-Lou, Wang Xian-Guang, Hu Ben, Zhang Lei, Zhang Wei, Si Hao-Rui, Zhu Yan, Li Bei, Huang Chao-Lin, Chen Hui-Dong, Chen Jing, Luo Yun, Guo Hua, Jiang Ren-Di, Liu Mei-Qin, Chen Ying, Shen Xu-Rui, Wang Xi, Zheng Xiao-Shuang, Zhao Kai, Chen Quan-Jiao, Deng Fei, Liu Lin-Lin, Yan Bing, Zhan Fa-Xian, Wang Yan-Yi, Xiao Geng-Fu, Shi Zheng-Li. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.COVID-19: Review of Epidemiology and Potential Treatments Against 2019 Novel Coronavirus. Jan Hasnain, Faisal Shah, Khan Ayyaz, Khan Shahzar, Usman Hazrat, Liaqat Rabia, Shah Sajjad Ali. Discoveries (Craiova) 2020;8(2):e108. doi: 10.15190/d.2020.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Presumed Asymptomatic Carrier Transmission of COVID-19. Bai Yan, Yao Lingsheng, Wei Tao, Tian Fei, Jin Dong-Yan, Chen Lijuan, Wang Meiyun. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Shiu Eunice Y C, Leung Nancy H L, Cowling Benjamin J. Current opinion in infectious diseases. 2019;32(4):372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 32.Recognition of aerosol transmission of infectious agents: a commentary. Tellier Raymond, Li Yuguo, Cowling Benjamin J, Tang Julian W. BMC infectious diseases. 2019;19(1):101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Airborne spread of infectious agents in the indoor environment. Wei Jianjian, Li Yuguo. American journal of infection control. 2016;44(9 Suppl):S102–8. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Transmission of Viruses in indoor Air: HVAC System Protection Options. Quality FICfIA. Agency EP, editor. USA. 2009.
- 35.Perspectives on the basic reproductive ratio. Heffernan J M, Smith R J, Wahl L M. Journal of the Royal Society, Interface. 2005;2(4):281–93. doi: 10.1098/rsif.2005.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The basic reproduction number (R0) of measles: a systematic review. Guerra Fiona M, Bolotin Shelly, Lim Gillian, Heffernan Jane, Deeks Shelley L, Li Ye, Crowcroft Natasha S. The Lancet. Infectious diseases. 2017;17(12):e420–e428. doi: 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 37.COVID-19, SARS and MERS: are they closely related? Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. Mahase Elisabeth. BMJ (Clinical research ed.) 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 39.Geographic and Genomic Distribution of SARS-CoV-2 Mutations. Mercatelli Daniele, Giorgi Federico M. Frontiers in microbiology. 2020;11:1800. doi: 10.3389/fmicb.2020.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. Zhang Lizhou, Jackson Cody B, Mou Huihui, Ojha Amrita, Rangarajan Erumbi S, Izard Tina, Farzan Michael, Choe Hyeryun. bioRxiv : the preprint server for biology. 2020 [Google Scholar]
- 41.The Rate of Underascertainment of Novel Coronavirus (2019-nCoV) Infection: Estimation Using Japanese Passengers Data on Evacuation Flights. Nishiura Hiroshi, Kobayashi Tetsuro, Yang Yichi, Hayashi Katsuma, Miyama Takeshi, Kinoshita Ryo, Linton Natalie M, Jung Sung-Mok, Yuan Baoyin, Suzuki Ayako, Akhmetzhanov Andrei R. Journal of clinical medicine. 2020;9(2) doi: 10.3390/jcm9020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Li Ruiyun, Pei Sen, Chen Bin, Song Yimeng, Zhang Tao, Yang Wan, Shaman Jeffrey. Science (New York, N.Y.) 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coronavirus disease 2019 in pregnancy: early lessons. Breslin Noelle, Baptiste Caitlin, Miller Russell, Fuchs Karin, Goffman Dena, Gyamfi-Bannerman Cynthia, D'Alton Mary. American journal of obstetrics & gynecology MFM. 2020;2(2):100111. doi: 10.1016/j.ajogmf.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. Zou Lirong, Ruan Feng, Huang Mingxing, Liang Lijun, Huang Huitao, Hong Zhongsi, Yu Jianxiang, Kang Min, Song Yingchao, Xia Jinyu, Guo Qianfang, Song Tie, He Jianfeng, Yen Hui-Ling, Peiris Malik, Wu Jie. The New England journal of medicine. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virological assessment of hospitalized patients with COVID-2019. Wölfel Roman, Corman Victor M, Guggemos Wolfgang, Seilmaier Michael, Zange Sabine, Müller Marcel A, Niemeyer Daniela, Jones Terry C, Vollmar Patrick, Rothe Camilla, Hoelscher Michael, Bleicker Tobias, Brünink Sebastian, Schneider Julia, Ehmann Rosina, Zwirglmaier Katrin, Drosten Christian, Wendtner Clemens. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 46.It is Time to Address Airborne Transmission of COVID-19. Morawska Lidia, Milton Donald K. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. MacIntyre C Raina, Chughtai Abrar Ahmad. International journal of nursing studies. 2020;108:103629. doi: 10.1016/j.ijnurstu.2020.103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Bao Linlin, Deng Wei, Huang Baoying, Gao Hong, Liu Jiangning, Ren Lili, Wei Qiang, Yu Pin, Xu Yanfeng, Qi Feifei, Qu Yajin, Li Fengdi, Lv Qi, Wang Wenling, Xue Jing, Gong Shuran, Liu Mingya, Wang Guanpeng, Wang Shunyi, Song Zhiqi, Zhao Linna, Liu Peipei, Zhao Li, Ye Fei, Wang Huijuan, Zhou Weimin, Zhu Na, Zhen Wei, Yu Haisheng, Zhang Xiaojuan, Guo Li, Chen Lan, Wang Conghui, Wang Ying, Wang Xinming, Xiao Yan, Sun Qiangming, Liu Hongqi, Zhu Fanli, Ma Chunxia, Yan Lingmei, Yang Mengli, Han Jun, Xu Wenbo, Tan Wenjie, Peng Xiaozhong, Jin Qi, Wu Guizhen, Qin Chuan. Nature. 2020;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 49.Coronavirus disease 2019 (COVID-19) History and exam. BMJ. 2020. Accessed: 2020 August 21. 2020. https://bestpractice.bmj.com/topics/engb/3000168/history-exam https://bestpractice.bmj.com/topics/engb/3000168/history-exam
- 50.Novavax. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine With/Without Matrix-M Adjuvant. ClinicalTrials.gov ( NCT04368988). Accessed: 2020 July 27. 2020. https://ClinicalTrials.gov/show/NCT04368988 https://ClinicalTrials.gov/show/NCT04368988
- 51.Ltd CBAP. SCB-2019 as COVID-19 Vaccine. ClinicalTrials.gov ( NCT04405908). Accessed: 2020 July 27. 2020. https://ClinicalTrials.gov/show/NCT04405908 https://ClinicalTrials.gov/show/NCT04405908
- 52.AnGes I. Study of COVID-19 DNA Vaccine (AG0301-COVID19) ClinicalTrials.gov ( NCT04463472).Accessed: 2020, August 24. 2020. https://ClinicalTrials.gov/show/NCT04463472 https://ClinicalTrials.gov/show/NCT04463472
- 53.Institute of Biotechnology AoMMS, PLA of China A Phase II Clinical Trial to Evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector). ClinicalTrials.gov ( NCT04341389). Accessed: 2020 July 27. https://ClinicalTrials.gov/show/NCT04341389 https://ClinicalTrials.gov/show/NCT04341389
- 54.Oxford Uo. Investigating a Vaccine Against COVID-19. ClinicalTrials.gov ( NCT04400838). Accessed: 2020 July 27. 2020. https://ClinicalTrials.gov/show/NCT04400838 https://ClinicalTrials.gov/show/NCT04400838
- 55.SE B. Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Adults. ClinicalTrials.gov ( NCT04368728). Accessed: 2020 July 27. 2020. https://ClinicalTrials.gov/show/NCT04368728 https://ClinicalTrials.gov/show/NCT04368728
- 56.Sinovac Research and Development Co. L. Safety and Immunogenicity Study of Inactivated Vaccine for Prevention of SARS-CoV-2 Infection (COVID-19) ClinicalTrials.gov ( NCT04383574). Accessed: 2020 July 27. 2020. https://ClinicalTrials.gov/show/NCT04383574 https://ClinicalTrials.gov/show/NCT04383574
- 57.Chen W. A Phase III clinical trial for inactivated novel coronavirus pneumonia (COVID-19) vaccine (Vero cells) Chinese Clinical Trial Registry. Accessed: 2020 July 27. 2020. http://www.chictr.org.cn/showprojen.aspx?proj=56651 http://www.chictr.org.cn/showprojen.aspx?proj=56651
- 58.ModernaTX I. A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19. ClinicalTrials.gov ( NCT04470427). ccessed: 2020 July 27. 2020. https://ClinicalTrials.gov/show/NCT04470427 https://ClinicalTrials.gov/show/NCT04470427
- 59.Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Warren Travis K, Jordan Robert, Lo Michael K, Ray Adrian S, Mackman Richard L, Soloveva Veronica, Siegel Dustin, Perron Michel, Bannister Roy, Hui Hon C, Larson Nate, Strickley Robert, Wells Jay, Stuthman Kelly S, Van Tongeren Sean A, Garza Nicole L, Donnelly Ginger, Shurtleff Amy C, Retterer Cary J, Gharaibeh Dima, Zamani Rouzbeh, Kenny Tara, Eaton Brett P, Grimes Elizabeth, Welch Lisa S, Gomba Laura, Wilhelmsen Catherine L, Nichols Donald K, Nuss Jonathan E, Nagle Elyse R, Kugelman Jeffrey R, Palacios Gustavo, Doerffler Edward, Neville Sean, Carra Ernest, Clarke Michael O, Zhang Lijun, Lew Willard, Ross Bruce, Wang Queenie, Chun Kwon, Wolfe Lydia, Babusis Darius, Park Yeojin, Stray Kirsten M, Trancheva Iva, Feng Joy Y, Barauskas Ona, Xu Yili, Wong Pamela, Braun Molly R, Flint Mike, McMullan Laura K, Chen Shan-Shan, Fearns Rachel, Swaminathan Swami, Mayers Douglas L, Spiropoulou Christina F, Lee William A, Nichol Stuart T, Cihlar Tomas, Bavari Sina. Nature. 2016;531(7594):381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Wang Manli, Cao Ruiyuan, Zhang Leike, Yang Xinglou, Liu Jia, Xu Mingyue, Shi Zhengli, Hu Zhihong, Zhong Wu, Xiao Gengfu. Cell research. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remdesivir Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. Pruijssers Andrea J, George Amelia S, Schäfer Alexandra, Leist Sarah R, Gralinksi Lisa E, Dinnon Kenneth H, Yount Boyd L, Agostini Maria L, Stevens Laura J, Chappell James D, Lu Xiaotao, Hughes Tia M, Gully Kendra, Martinez David R, Brown Ariane J, Graham Rachel L, Perry Jason K, Du Pont Venice, Pitts Jared, Ma Bin, Babusis Darius, Murakami Eisuke, Feng Joy Y, Bilello John P, Porter Danielle P, Cihlar Tomas, Baric Ralph S, Denison Mark R, Sheahan Timothy P. Cell reports. 2020;32(3):107940. doi: 10.1016/j.celrep.2020.107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maffei D. Gilead Presents Additional Data on Investigational Antiviral Remdesivir for the Treatment of COVID-19. Gilead Sciences I, editor. FOSTER CITY, California, USA: Gilead Sciences, Inc.; 2020. [Google Scholar]
- 63.Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Furuta Yousuke, Komeno Takashi, Nakamura Takaaki. Proceedings of the Japan Academy. Series B, Physical and biological sciences. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Influenza virus polymerase inhibitors in clinical development. Hayden Frederick G, Shindo Nahoko. Current opinion in infectious diseases. 2019;32(2):176–186. doi: 10.1097/QCO.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Li Guangdi, De Clercq Erik. Nature reviews. Drug discovery. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 66.Discovering drugs to treat coronavirus disease 2019 (COVID-19). Dong Liying, Hu Shasha, Gao Jianjun. Drug discoveries & therapeutics. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 67.Effective treatment of severe COVID-19 patients with tocilizumab. Xu Xiaoling, Han Mingfeng, Li Tiantian, Sun Wei, Wang Dongsheng, Fu Binqing, Zhou Yonggang, Zheng Xiaohu, Yang Yun, Li Xiuyong, Zhang Xiaohua, Pan Aijun, Wei Haiming. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Ruan Qiurong, Yang Kun, Wang Wenxia, Jiang Lingyu, Song Jianxin. Intensive care medicine. 2020;46(6):1294–1297. doi: 10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Qin Chuan, Zhou Luoqi, Hu Ziwei, Zhang Shuoqi, Yang Sheng, Tao Yu, Xie Cuihong, Ma Ke, Shang Ke, Wang Wei, Tian Dai-Shi. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Chen Xiaohua, Zhao Binghong, Qu Yueming, Chen Yurou, Xiong Jie, Feng Yong, Men Dong, Huang Qianchuan, Liu Ying, Yang Bo, Ding Jinya, Li Feng. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Somers Emily C, Eschenauer Gregory A, Troost Jonathan P, Golob Jonathan L, Gandhi Tejal N, Wang Lu, Zhou Nina, Petty Lindsay A, Baang Ji Hoon, Dillman Nicholas O, Frame David, Gregg Kevin S, Kaul Dan R, Nagel Jerod, Patel Twisha S, Zhou Shiwei, Lauring Adam S, Hanauer David A, Martin Emily, Sharma Pratima, Fung Christopher M, Pogue Jason M. medRxiv : the preprint server for health sciences. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.NIH clinical trial testing remdesivir plus interferon beta-1a for COVID-19 treatment begins. US National Institute of Health. Accessed in August 2020. 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-testing-remdesivir-plus-interferon-beta-1a-covid-19-treatment-begins https://www.nih.gov/news-events/news-releases/nih-clinical-trial-testing-remdesivir-plus-interferon-beta-1a-covid-19-treatment-begins
- 73.Remdesivir for the Treatment of Covid-19 - Preliminary Report. Reply. Beigel John H, Tomashek Kay M, Dodd Lori E. The New England journal of medicine. 2020;383(10):994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 74.Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Yang Xiaobo, Yu Yuan, Xu Jiqian, Shu Huaqing, Xia Jia'an, Liu Hong, Wu Yongran, Zhang Lu, Yu Zhui, Fang Minghao, Yu Ting, Wang Yaxin, Pan Shangwen, Zou Xiaojing, Yuan Shiying, Shang You. The Lancet. Respiratory medicine. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Hafezi-Moghadam Ali, Simoncini Tommaso, Yang Zequan, Limbourg Florian P, Plumier Jean-Christophe, Rebsamen Michael C, Hsieh Chung-Ming, Chui Dao-Shan, Thomas Kennard L, Prorock Alyson J, Laubach Victor E, Moskowitz Michael A, French Brent A, Ley Klaus, Liao James K. Nature medicine. 2002;8(5):473–9. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. Horby Peter, Lim Wei Shen, Emberson Jonathan R, Mafham Marion, Bell Jennifer L, Linsell Louise, Staplin Natalie, Brightling Christopher, Ustianowski Andrew, Elmahi Einas, Prudon Benjamin, Green Christopher, Felton Timothy, Chadwick David, Rege Kanchan, Fegan Christopher, Chappell Lucy C, Faust Saul N, Jaki Thomas, Jeffery Katie, Montgomery Alan, Rowan Kathryn, Juszczak Edmund, Baillie J Kenneth, Haynes Richard, Landray Martin J. The New England journal of medicine. 2020 [Google Scholar]
- 77.Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Bhimraj Adarsh, Morgan Rebecca L, Shumaker Amy Hirsch, Lavergne Valery, Baden Lindsey, Cheng Vincent Chi-Chung, Edwards Kathryn M, Gandhi Rajesh, Muller William J, O'Horo John C, Shoham Shmuel, Murad M Hassan, Mustafa Reem A, Sultan Shahnaz, Falck-Ytter Yngve. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Vincent Martin J, Bergeron Eric, Benjannet Suzanne, Erickson Bobbie R, Rollin Pierre E, Ksiazek Thomas G, Seidah Nabil G, Nichol Stuart T. Virology journal. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Gao Jianjun, Tian Zhenxue, Yang Xu. Bioscience trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 80.Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. Kalil Andre C. JAMA. 2020;323(19):1897–1898. doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 81.[Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2020;43(3):185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Liu Jia, Cao Ruiyuan, Xu Mingyue, Wang Xi, Zhang Huanyu, Hu Hengrui, Li Yufeng, Hu Zhihong, Zhong Wu, Wang Manli. Cell discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Influenza and Bacterial Superinfection: Illuminating the Immunologic Mechanisms of Disease. Rynda-Apple Agnieszka, Robinson Keven M, Alcorn John F. Infection and immunity. 2015;83(10):3764–70. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Gautret Philippe, Lagier Jean-Christophe, Parola Philippe, Hoang Van Thuan, Meddeb Line, Mailhe Morgane, Doudier Barbara, Courjon Johan, Giordanengo Valérie, Vieira Vera Esteves, Tissot Dupont Hervé, Honoré Stéphane, Colson Philippe, Chabrière Eric, La Scola Bernard, Rolain Jean-Marc, Brouqui Philippe, Raoult Didier. International journal of antimicrobial agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Raoult D, Houpikian P, Tissot Dupont H, Riss J M, Arditi-Djiane J, Brouqui P. Archives of internal medicine. 1999;159(2):167–73. doi: 10.1001/archinte.159.2.167. [DOI] [PubMed] [Google Scholar]
- 86.Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Singh Awadhesh Kumar, Singh Akriti, Shaikh Altamash, Singh Ritu, Misra Anoop. Diabetes & metabolic syndrome. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. USA FDA. Accessed: 2020 August 21. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
- 88.Update Alert 2: Should Clinicians Use Chloroquine or Hydroxychloroquine Alone or in Combination With Azithromycin for the Prophylaxis or Treatment of COVID-19? Living Practice Points From the American College of Physicians. Qaseem Amir, Yost Jennifer, Etxeandia-Ikobaltzeta Itziar, Humphrey Linda L. Annals of internal medicine. 2020;173(5):W88–W89. doi: 10.7326/L20-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]