Abstract
Purpose
A recently published randomized controlled trial has demonstrated that in patients with endometrial cancer with high-risk features, the addition of chemotherapy to radiation therapy, compared with radiation therapy alone, resulted in a significant improvement in failure-free survival. However, in the study, the effect of chemotherapy was limited to stage III patients, and the benefit was less pronounced in stage I and II patients. Our study aims to investigate the current practice of treatment and clinical outcomes in stage I high-risk endometrioid-type endometrial cancer.
Methods and Materials
A single-center retrospective study was conducted on patients with stage I high-risk endometrioid-type endometrial cancer without serous or clear cell features who have undergone hysterectomy between 1998 and 2015. Data on patients, tumor, and treatments were collected and correlated with clinical outcomes.
Results
A total of 1,572 patients with stage I disease were identified and 46 patients who met the inclusion criteria were selected for final analysis. The median age at diagnosis was 63 years (range, 49-86 years) and median follow-up was 5.9 years. Among the entire cohort, 40 (87.0%) patients underwent adjuvant radiation therapy, of which 36 (78.2%) patients underwent external beam radiation therapy and 4 (8.7%) patients underwent vaginal brachytherapy. Two of the 40 patients who received adjuvant radiation therapy also received adjuvant chemotherapy. Six (13.0%) patients received no adjuvant treatment. Of the 46 patients, the cumulative risk of distant recurrence was 19.6%, and only 1 patient (2.2%) recurred within pelvis (perirectal lymph node). Five-year disease-free survival and overall survival rates were 73.1% and 80.1%, respectively.
Conclusions
Adjuvant radiation therapy in stage I endometrioid-type endometrial cancer patients with high-risk features resulted in high rates of locoregional disease control, and most recurrences occurred at distant sites. Effective systemic therapy may be indicated in this patient population to further reduce the risk of distant relapses and improve survival.
Introduction
Endometrial cancer is the most common gynecologic malignancy in North America.1,2 The standard of care for non-metastatic disease is upfront total hysterectomy and bilateral salpingo-oophorectomy with proper surgical staging, followed by observation, chemotherapy and/or radiation therapy depending on the patients’ surgical stage assignment.3 Adjuvant treatments are planned based on the estimated risk and patterns of recurrence, which depend on multiple prognostic factors, such as age, degree of myometrial involvement, tumor grade, lymph node involvement, and lymphovascular space invasion, among many others.4, 5, 6, 7, 8, 9
Previous studies have reported no survival benefit from adjuvant treatment after hysterectomy in low- and intermediate-risk patients with endometrial cancer.10, 11, 12, 13 However, evidence suggests potential benefit of locoregional disease control in the intermediate-risk patients with the addition of adjuvant radiation therapy, especially vaginal brachytherapy.14 For the patients with high-risk features, multiple studies have investigated different adjuvant therapies that not only would reduce the risk of recurrence, both locoregional and distant, but also maximize survival benefits and maintain the quality of life. A recently published randomized controlled study, Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC-3) has examined the benefit of adding chemotherapy to radiation therapy in the adjuvant setting in the high-risk population, and found a significant improvement in the 5-year failure-free survival with the addition of chemotherapy.15 However, the benefit of improved failure-free survival was more pronounced in stage III high-risk patients, whereas the benefit was limited in stage I and II patients.
In this retrospective study, we aimed to investigate the patterns of current practice of adjuvant treatment for stage I high-risk endometrioid-type endometrial cancer and correlate to oncologic outcomes, then compare the outcomes with that of the published literature.
Methods and Materials
Institutional Review Board approval was obtained before commencement of the study. A single-center retrospective study was conducted on patients with endometrioid-type endometrial cancer with International Federation of Gynecology and Obstetrics (FIGO 2009) stage I and high-risk features, according to PORTEC-3 criteria15 (grade 3 with deep myometrial invasion or lymph-vascular space invasion, or both), who have undergone total hysterectomy and bilateral salpingo-oophorectomy in 1998 to 2007 and 2010 to 2015. Patients with low- or intermediate-risk features, stage II and higher on surgical pathology, serous or clear cell histology, uterine sarcoma, or positive lymph node involvement were excluded. Patients with previous history of malignancy, radiation therapy, hormone therapy, or chemotherapy were also excluded. All patients underwent preoperative staging investigations with routine clinical examination, transvaginal or transabdominal ultrasound of the pelvis and chest x-rays. For select patients with higher risk of disease, computed tomography scans of the chest, abdomen, and pelvis were obtained. Pelvic or para-aortic lymphadenectomy was not required but was performed at the discretion of the surgeon. No routine postoperative preadjuvant therapy restaging investigations were performed before the adjuvant therapy.
Data on patient demographics, tumor characteristics (grade, extent of myometrial involvement, lymphovascular space invasion), and treatment (surgery, radiation therapy, chemotherapy) were collected and correlated to the oncologic outcomes. Disease-free survival was defined as the time interval between the treatment completion and the clinical or radiologic evidence of recurrence, or death, whichever occurred first, disease-specific survival was defined as the time interval between the treatment completion and the clinical or radiologic evidence of recurrence (noncancer-related deaths were censored), and overall survival was defined as the time interval between the treatment completion and death from any cause. Statistical analyses were performed using IBM SPSS Statistics 25.0 (Armonk, NY). Survival outcomes were calculated using the Kaplan-Meier method, and the log-rank test was used to compare survival outcomes between different variables for risk factors.
Results
A total of 1,572 patients with stage I endometrial cancer were identified from our institution surgical database (Fig 1), and 46 patients who met the inclusion criteria were selected for final analysis. The patient demographics and tumor characteristics are summarized in Table 1. Among the selected 46 patients, the median age at diagnosis was 63 years (range, 49-86 years), and the median follow-up was 5.9 years (range, 1.4-18.8 years). There were 35 patients (76.1%) with 50% or higher myometrial involvement (FIGO stage IB) and 11 patients (23.9%) with less than 50% myometrial involvement (FIGO stage IA). Positive lymphovascular space invasion was found in 32 (69.6%) patients.
Figure 1.
Consolidated Standard of Reporting Trials diagram for stage I high-risk endometrioid-type endometrial cancer patients and types of adjuvant treatment. Abbreviation: LVSI = lymphovascular space invasion.
Table 1.
Patient demographics, tumor characteristics and oncologic outcomes for stage I high-risk endometrioid-type endometrial cancer patients
| All (n = 46) | Stage IA with LVSI (n = 11) | Stage IB (n = 35) | |
|---|---|---|---|
| Age, median (range), y | 63 (49-86) | 57 (49-67) | 65 (50-86) |
| <60 y, n (%) | 19 (41.3) | 7 (63.6) | 12 (34.3) |
| ≥60 y, n (%) | 27 (58.7) | 4 (36.4) | 23 (65.7) |
| Myometrial invasion, n (%) | |||
| <50% | 11 (23.9) | 11 (100) | 0 (0) |
| >50% | 35 (76.1) | 0 (0) | 35 (100) |
| LVSI, n (%) | |||
| Yes | 32 (69.6) | 11 (100) | 21 (60.0) |
| No | 14 (30.4) | 0 (0) | 14 (40.0) |
| Type of surgery, n (%) | |||
| Laparotomy | 35 (76.1) | 7 (63.6) | 28 (80.0) |
| Minimally invasive surgery | 11 (23.9) | 4 (36.4) | 7 (20.0) |
| Lymphadenectomy, n (%) | |||
| Yes | 38 (82.6) | 9 (81.8) | 29 (82.9) |
| No | 8 (17.4) | 2 (18.2) | 6 (17.1) |
| Adjuvant treatment, n (%) | |||
| Any | 40 (87.0) | 10 (90.9) | 30 (85.7) |
| Radiation therapy | 40 (87.0) | 10 (90.9) | 30 (85.7) |
| EBRT | 36 (78.3) | 7 (63.6) | 29 (82.9) |
| VBT | 4 (8.7) | 3 (27.3) | 1 (2.9) |
| Chemotherapy∗ | 2 (4.3) | 1 (9.1) | 1 (2.9) |
| No adjuvant treatment | 6 (13.0) | 1 (9.1) | 5 (14.3) |
| Recurrence, n (%)† | |||
| Any | 10 (21.7) | 3 (27.3) | 7 (20.0) |
| Locoregional | 1 (2.2) | 1 (9.1) | 0 (0) |
| Distant | 9 (19.6) | 2 (18.2) | 7 (20.0) |
| Death, n (%)† | |||
| Any cause | 15 (32.6) | 3 (27.3) | 12 (34.3) |
| Disease-specific | 7 (15.2) | 2 (18.2) | 5 (14.3) |
| 5-y disease control rate, % | |||
| Any | 78.4 | 71.6 | 80.8 |
| Locoregional | 97.8 | 90.9 | 100 |
| Distant | 80.2 | 78.8 | 80.8 |
| 5-y disease-specific survival,‡ % | 78.4 | 71.6 | 80.8 |
| 5-year disease-free survival, % | 73.1 | 63.6 | 76.1 |
| 5-year overall survival, % | 80.1 | 72.7 | 79.5 |
Abbreviations: EBRT = external beam radiation therapy; LVSI = lymphovascular space invasion; VBT = vaginal brachytherapy.
Patients also received adjuvant radiation therapy.
Cumulative incidence at the time of analysis.
Disease-specific survival indicates the proportion of patients without evidence of disease and noncancer-related death is censored.
Pelvic lymphadenectomy was performed on the majority of patients (38 patients, 82.6%), and the median number of lymph node sampled was 8 (range, 2-23). Thirty-five (76.1%) and 11 (23.9%) patients underwent laparotomy and minimally invasive surgery, respectively. The majority of patients (40 patients, 87.0%) underwent adjuvant radiation therapy, among which 36 (78.3%) patients received external beam radiation therapy to the pelvis (45 Gy in 1.8 Gy fractions), and 4 (8.7%) patients received vaginal brachytherapy (30 Gy in 10 Gy weekly high-dose-rate fractions delivered to the upper 5 cm of the vagina, dose prescribed to the surface of the applicator). No patient received both the external beam radiation therapy and brachytherapy. The external beam radiation therapy was delivered using a conformal 4-field technique before 2013, but the intensity modulated radiation therapy technique was more commonly used after 2013. Of the 40 patients who underwent the adjuvant radiation therapy, 2 patients also received adjuvant chemotherapy consisting of 6 cycles of 3-weekly carboplatin and paclitaxel in addition to the adjuvant radiation therapy; 1 patient received the chemotherapy followed by radiation therapy, and the other patient received the radiation therapy followed by chemotherapy. The median time interval between the surgery and the start of adjuvant radiation therapy was 67 days (range, 42-173 days). Six (13.0%) patients declined the adjuvant radiation therapy and received no adjuvant treatment.
Of the entire cohort of 46 patients, 11 (23.9%) patients had FIGO stage IA disease with lymphovascular space invasion and 35 (76.1%) patients had FIGO stage IB disease with or without lymphovascular space invasion. External beam radiation therapy was more commonly used for patients with stage IB disease (82.9%) than with stage IA disease and lymphovascular space invasion (63.6%), but the use of vaginal brachytherapy was more common with stage IA disease and lymphovascular space invasion (27.3%) than with stage IB disease (2.9%). Clinical outcomes were similar between the 2 groups (Table 1).
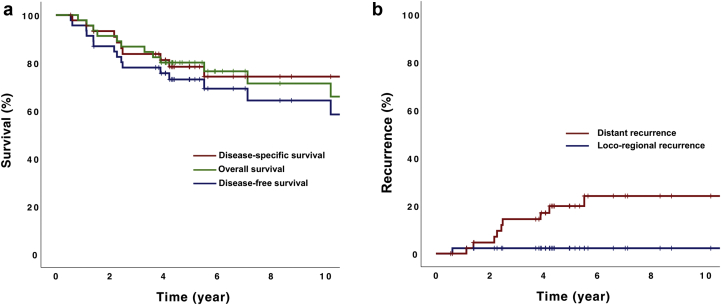
The 5-year disease-specific, disease-free survival and overall survival rates were 78.4%, 73.1%, and 80.1%, respectively (Fig 2a). At the time of analysis, 10 (21.7%) patients recurred among the entire cohort. All the recurrences were found in patients who have received the adjuvant radiation therapy only. Of the 10 patients with recurrent disease, the majority of recurrences were at distant sites only (9 patients) and only 1 patient recurred at a regional site only (a perirectal lymph node; Fig 2b). The most common sites for distant recurrence are lungs (50%), liver (50%), and bone (30%), and no para-aortic nodal recurrences were observed. This translated into the 5-year locoregional control rate of 97.8%. Majority of the recurrences (70%) occurred within 3 years, and the median time to recurrence was 2.4 years (range, 0.6-5.5 years). Among the entire cohort of 46 patients, 15 (32.6%) patients died, of which 7 (15.2%) deaths were related to endometrial cancer. No treatment-related death was observed.
Figure 2.
(a) Kaplan-Meier survival curve for disease-specific survival, overall survival, and disease-free survival. Five-year disease-specific survival, disease-free survival. and overall survival rates were 78.4%, 73.1%, and 80.1%, respectively. (b) Cumulative incidence of distant and locoregional disease relapses. Five-year distant and locoregional recurrence rates were 19.8% and 2.2%, respectively.
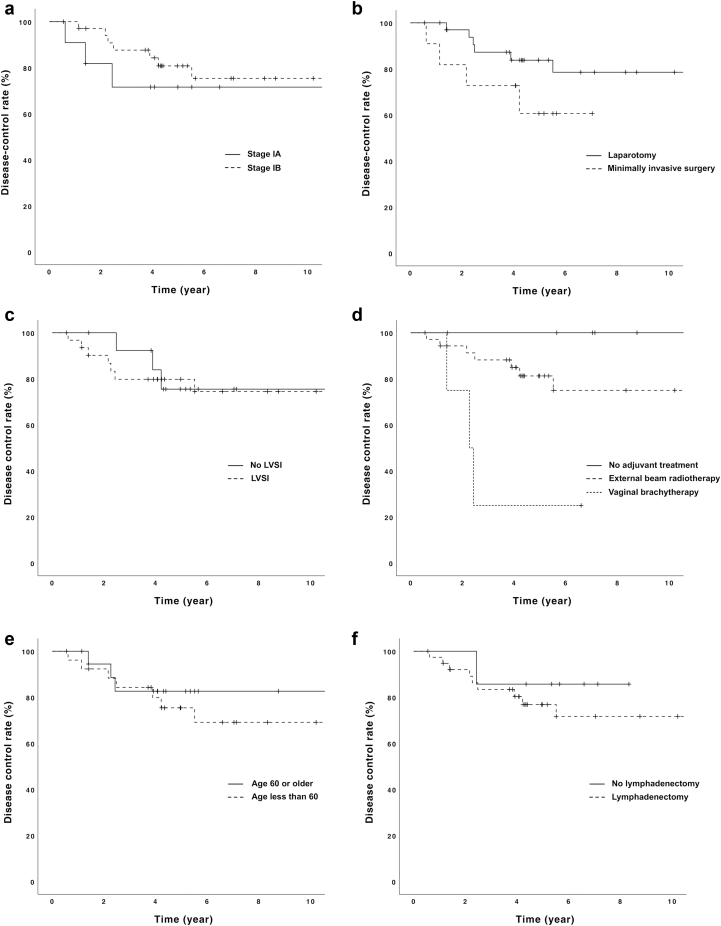
Log-rank test identified the type of adjuvant radiation therapy (no treatment vs external beam radiation therapy vs vaginal brachytherapy) as a prognostic factor for the risk of disease recurrence (P = .005; Table 2, Fig 3). Minimally invasive surgery, compared with laparotomy, was associated with a higher rate of disease-specific survival, but this was not statistically significant (P = .16). Other variables, including FIGO stage (IA vs IB), lymphovascular space invasion, age (<60 vs ≥60), and pelvic lymphadenectomy were not associated with the risk of disease recurrence.
Table 2.
Five-year disease-control rate of various risk groups
| 5-y disease-control rate, % | Plog-rank | |
|---|---|---|
| FIGO stage | 0.50 | |
| Stage IA∗ (n = 11) | 71.6 | |
| Stage IB (n = 35) | 80.8 | |
| Type of surgery | 0.16 | |
| Laparotomy (n = 35) | 83.8 | |
| Minimally invasive surgery (n = 11) | 60.6 | |
| LVSI | 0.87 | |
| Yes (n = 32) | 75.5 | |
| No (n = 14) | 79.8 | |
| Radiation therapy type | 0.005 | |
| EBRT (n = 36) | 81.2 | |
| VBT (n = 4) | 25.0 | |
| No treatment (n = 6) | 100 | |
| Age | 0.49 | |
| <60 y (n = 19) | 75.4 | |
| ≥60 y (n = 27) | 82.6 | |
| Lymphadenectomy | 0.51 | |
| Yes (n = 38) | 76.8 | |
| No (n = 8) | 85.7 |
Abbreviations: EBRT = external beam radiation therapy; FIGO = International Federation of Gynecology and Obstetrics; LVSI = lymphovascular space invasion; VBT = vaginal brachytherapy.
Stage IA patient with LVSI.
Figure 3.
Various risk factors for disease recurrence: (a) International Federation of Gynecology and Obstetrics stage, (b) type of surgery, (c) lymphovascular space invasion, (d) type of radiation therapy, (e) age, and (f) lymphadenectomy. Only the type of radiation therapy was associated with disease recurrence (P = .005), and other risk factors were not associated with disease recurrence. Abbreviation: LVSI = lymphovascular space invasion.
Discussion
Our study demonstrates that adjuvant radiation therapy, especially external beam radiation therapy, without chemotherapy, is the most common form of adjuvant therapy for stage I high-risk endometrioid-type endometrial cancer at our institution. The treatments resulted in a high rate of locoregional disease control with only one patient recurring locoregionally among the total of 46 patients (2.2%), although a significant proportion of patients (19.6%) recurred at distant sites.
In our study, all the patients were offered the adjuvant radiation therapy after their surgery and the majority of them (87.0%) completed the adjuvant treatment, and 6 (13.0%) patients declined and did not receive any adjuvant treatment. Despite the conflicting evidence on the survival benefit of adjuvant treatment with pelvic external beam radiation therapy in the high-risk group,16,17 and vaginal brachytherapy achieving comparable locoregional disease control compared with external beam radiotherapy,18,19 these patients with high-risk features in the modern era generally undergo adjuvant pelvic radiation therapy, mostly external beam radiation therapy.20 Such practice pattern stems from multiple studies demonstrating that the adjuvant radiation therapy significantly decreases the risk of locoregional recurrences with an acceptable side effect profile in the intermediate-risk patients,12,13 who carry a lower risk of recurrence than the high-risk group patients. The evidence is supported by a report by Mundt and colleagues on 43 high-risk patients with endometrial cancer treated with adjuvant chemotherapy and no radiation therapy.21 The study demonstrated a high rate of 3-year locoregional recurrence (46.5%), highlighting that the adjuvant radiation therapy plays an important role in this particular patient population and that the radiation therapy is an indispensable treatment in the adjuvant setting. Similar findings have been reported by Secord et al on stage IIIc endometrial cancer patients.22
Despite the known efficacy of the adjuvant radiation therapy for locoregional control in endometrial cancer, distant recurrences remain a challenging problem. Maggi et al in their randomized clinical trial involving 345 patients compared adjuvant radiation therapy alone to adjuvant chemotherapy alone in high-risk endometrial cancer patients.23 Although there was no survival difference between these 2 groups, a trend toward the lower rate of distant metastasis was observed with the use of adjuvant chemotherapy, although the adjuvant radiation therapy alone was associated with improved locoregional disease control.
Multiple randomized clinical trials have confirmed survival benefit of the addition of chemotherapy to adjuvant radiation therapy. Pooled results from 2 large randomized clinical trials, The Nordic Society of Gynecologic Oncology/European Organization for the Research and Treatment of Cancer (NSGO-EC-9501/EORTC-55991) and Gynecologic Oncology Group at the Mario Negri Institute (MaNGO ILIADE-III) involving 534 patients have shown that the addition of chemotherapy to adjuvant radiation therapy was associated with a significant improvement in the progression-free survival (hazard ratio 0.63, P = .009), and a trend toward the improved overall survival was observed (P = .07).24 The recently published PORTEC-3 study has shown similar results: 5-year failure-free survival of 75.5% with concurrent chemoradiotherapy, compared with 68.6% with radiation therapy alone (P = .022).15 In addition, Aoki et al have shown in their retrospective review of stage I and II endometrial cancer patients with high-risk features a significant survival benefit from reduced distant recurrences with the addition of adjuvant chemotherapy and suggested that adjuvant treatment should include chemotherapy, although no patient received adjuvant radiation therapy in the study.25
The PORTEC-3 trial demonstrated that the benefit of adjuvant chemotherapy was mainly found in stage III patients, although the benefit was less obvious in stage I and II patients. Given the smaller benefit from chemotherapy, significant toxicity and potential effect on quality of life in stage I and II patients, the authors have suggested that further study is required and advised against the routine use of adjuvant chemotherapy in these patients. However, Greven et al in their phase II study Radiation Therapy Oncology Group (RTOG 9708) on 46 patients have demonstrated that chemoradiotherapy (combined external beam radiation therapy and vaginal brachytherapy with 2 cycles of concurrent cisplatin and 4 additional cycles of cisplatin and paclitaxel) was well tolerated with minimal toxicity.26 In addition, in the PORTEC-3 study, no significant differences in the grade 3 adverse events were found between the 2 groups by 24 months, despite the higher rate of toxicities from the chemoradiotherapy group at 12 months compared with the radiation therapy only group.27 Therefore, the controversy continues on the routine use of adjuvant chemotherapy in stage I and II high-risk patients.
Perhaps the best evidence comes from the PORTEC trial conducted by Creutzberg et al on 104 patients with inclusion criteria similar to that of our study: stage IC, grade 3 endometrial cancer.28 In their study, similar to our study, the majority of the recurrences were found at distant sites, which translated into an overall survival rate of 58%, and the survival rates were largely driven by the increased relapse rate at distant sites (31%). In the setting of the high distant relapse rate in the stage I high-risk patients, the question on the use of chemotherapy was raised by the authors, but no recommendation was made in the absence of conclusive data. A recently published randomized phase III trial Gynecologic Oncology Group (GOG 249) on 601 patients with early stage high-risk features (stage I with high-intermediate features, stage II or stage I-II with serous or clear cell features) compared the combined treatment of vaginal brachytherapy with chemotherapy versus external beam radiation therapy.29 With a median follow-up of 53 months, the study demonstrated that the external beam radiation therapy and the combined treatment of vaginal brachytherapy with chemotherapy resulted in comparable disease control rates and survival outcomes. It is interesting to note that the external beam radiation therapy, without chemotherapy, resulted in a similar distant disease control of 18% to vaginal brachytherapy with chemotherapy.
In contrast to local disease relapses, where various salvage options of radiation therapy, systemic therapy or surgery are available with relatively high rates of disease control, distant disease relapses pose more management challenges and many patients are left with only limited treatment options, mostly in the palliative setting. This further supports the use of effective chemotherapy in adjuvant treatment in this particular patient population to further reduce the rate of distant recurrences. However, in the absence of level I evidence from randomized controlled trials and conflicting results on the use of chemotherapy in the early stage high-risk group, the routine use of chemotherapy cannot be recommended for all early stage high-risk patients.
Multiple studies have recently highlighted the heterogeneous nature of endometrial cancer that are both prognostic and predictive of response to treatment. The Cancer Genome Atlas in their comprehensive analysis of 373 endometrial cancer has characterized gene mutations and molecular markers that carry potential therapeutic implications.30 In their analysis, 4 mutation classes have been identified: POLE (ultramuted), microsatellite instability (MSI) (hypermutated), copy-number low (endometrioid), and copy-number high (serous-like). Bosse and colleagues, using this molecular classification, have investigated survival outcomes of grade 3 endometrioid-type endometrial cancers and has identified the POLE mutation to be associated with better prognosis than the p53 abnormal, loss of mismatch repair protein expression (MMRd), and no specific molecular profile groups.31 In addition, several other biomarkers and their molecular pathways, including HER2/neu,32 ARID1A,33, 34, 35 FGFR2,36,37 CTNNB1,38 and PIK3CA pathway,39, 40, 41 have been identified as potential therapeutic targets in endometrial cancer. Pembrolizumab, an immune checkpoint programmed cell death protein 1 inhibitor commonly used to treat metastatic lung cancer with positive programmed cell death protein 1 mutation, has been shown to be safe and effective in treatment of advanced endometrial cancer,42 and the drug has been granted an accelerated Food and Drug Administration approval for patients with unresectable or metastatic solid tumors, including endometrial cancer, that are MSI-high or MMRd (the approval has been extended to patients who are not MSI-high or MMRd recently43). More importantly, the PORTEC-4a trial currently in its recruitment phase is investigating individualized adjuvant treatment in high-intermediate-risk endometrial cancer patients with a specific molecular-integrated risk profile based on the 4 aforementioned mutations.44 We remain hopeful that the recent rapid advancement in the molecular genomics of endometrial cancer can provide guidance on selecting the right patients for appropriate adjuvant therapy and potentially avoiding unnecessary treatments and related toxicity in the early stage high-risk patients with endometrial cancer.
It is interesting to note that the log-rank analysis from our study demonstrated that the type of radiation therapy (external beam radiation therapy vs vaginal brachytherapy vs no adjuvant treatment) was significantly associated with the risk of disease recurrence (P = .005). However, owing to the small number of patients who received either no adjuvant treatment (n = 6) or vaginal brachytherapy (n = 4), and the majority of patients received external beam radiation therapy (n = 36), a great caution must be exercised when interpreting these results. Similarly, a trend toward the higher rate of disease recurrence was found with the laparotomy compared with the minimally invasive surgery (P = .16). A recently published report demonstrated a significantly higher risk of disease recurrence associated with the minimally invasive surgery in the intermediate-risk patient population.45 Several variables have been proposed as potential risk factors for the higher recurrence in the minimally invasive surgery: increased time interval between the surgery and adjuvant treatment,46 routine use of the uterine manipulator,47 and carbon dioxide insufflation.48, 49, 50 Additional prospective studies would be necessary to confirm the association between the patterns of disease recurrences and minimally invasive surgery.
Our study has a number of limitations. The retrospective nature of the study introduces potential selection bias. The significance of the study is also weakened by the small sample size and the limited number of events. However, owing to the low incidence of stage I endometrioid-type endometrial cancer with high-risk features, these shortcomings could not have been avoided. In addition, data were collected by 2 separate groups during 2 study periods (1998-2007 and 2010-2015), introducing potential data collection bias. More importantly, unlike the patients in the PORTEC-3 study, patients with serous or clear cell features were not included in our study and thus the results from our study cannot be applied to patients with such features. In contrast, despite the small number of patients in our study, narrow inclusion criteria were applied, resulting in a homogeneous population with similar tumor characteristics and prognosis. In addition, a large proportion of patients (82.6%) underwent surgical staging with lymphadenectomy, thus excluding patients with positive lymph node involvement. Lastly, the study period spans more than 16 years, while including all patients who have been treated at our institution, indicating little treatment variability among the patients.
Conclusions
Oncologic outcomes in stage I high-risk endometrioid-type endometrial cancer treated with external beam radiation therapy remain comparable with historic controls with a high rate of locoregional disease control. However, distant disease relapses remain a challenging problem in this population. Effective chemotherapy in the adjuvant setting may be considered for stage I high-risk patients to further reduce distant recurrences. Future prospective studies are required to validate the hypothesis and confirm the results of our study.
Footnotes
Sources of support: none.
Disclosures: none.
The data sets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.United States Cancer Statistics (USCS). Site recode ICD-O-3/WHO 2008. U.S. Data Variable Definition and Frequency. Available at: https://www.cdc.gov/cancer/uscs/public-use/dictionary/site-recode-ICD-O-3-WHO-2008.htm. Accessed February 20, 2020.
- 2.Canadian Cancer Society. Canadian cancer statistics. A 2018 special report on cancer incidence by stage. Available at: https://www.cancer.ca/∼/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2018-EN.pdf?la=en. Accessed February 20, 2020.
- 3.National Comprehensive Cancer Network. Uterine neoplasms. NCCN clinical practice guidelines in oncology (NCCN guidelines). Version 2.2018. May 25, 2018. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed February 20, 2020.
- 4.Aalders J., Abeler V., Kolstad P. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 40 patients. Obstet Gynecol. 1980;56:419–427. [PubMed] [Google Scholar]
- 5.Thomas A., Chandy R., Sebastian A. Surgical outcomes and patterns of recurrence in endometrial cancers. J Gynecol Surg. 2017;33:97–104. [Google Scholar]
- 6.Morrow C.P., Bundy B.N., Kurman R.J. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto T., Nanjyo H., Fukuda J. Endometrioid uterine cancer: Histopathological risk factors of local and distant recurrence. Gynecol Oncol. 2009;112:342–347. doi: 10.1016/j.ygyno.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Greven K.M., Lanciano R.M., Corn B. Pathologic stage III endometrial carcinoma. Prognostic factors and patterns of recurrence. Cancer. 1993;71:3697–3702. doi: 10.1002/1097-0142(19930601)71:11<3697::aid-cncr2820711137>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Cusano E., Myers V., Samant R. Prognostic significance of lymphovascular space invasion in the absence of lymph node metastases in early-stage endometrial cancer. Int J Gynecol Cancer. 2018;28:890–894. doi: 10.1097/IGC.0000000000001229. [DOI] [PubMed] [Google Scholar]
- 10.Sorbe B., Nordström B., Mäenpää J. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: A controlled randomized study. Int J Gynecol Cancer. 2009;19:873–878. doi: 10.1111/IGC.0b013e3181a6c9df. [DOI] [PubMed] [Google Scholar]
- 11.Sorbe B., Straumits A., Karlsson L. Intravaginal high-dose-rate brachytherapy for stage I endometrial cancer: A randomized study of two dose-per-fraction levels. Int J Radiat Oncol Biol Phys. 2005;62:1385–1389. doi: 10.1016/j.ijrobp.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 12.Creutzberg C.L., van Putten W.L., Koper P.C. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomized trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 13.Keys H.M., Roberts J.A., Brunetto V.L. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Nout R.A., Smit V.T., Putter H. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 15.de Boer S.M., Powell M.E., Mileshkin L. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicenter, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ASTEC/EN.5 Study Group. Blake P., Swart A.M., Orton J. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onstad M., Ducie J., Fellman B.M. Adjuvant therapy for grade 3, deeply invasive endometrioid adenocarcinoma of the uterus. Int J Gynecol Cancer. 2020;30:485–490. doi: 10.1136/ijgc-2019-000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang M., English D.P., Kidd E.A. Defining the survival benefit of adjuvant pelvic radiotherapy and chemotherapy versus chemotherapy alone in stages III-IVA endometrial carcinoma. Gynecol Oncol. 2019;154:487–494. doi: 10.1016/j.ygyno.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Hochreiter A., Kelly J.R., Young M.R. Outcomes and relapse patterns of stage IB grade 2 or 3 endometrial cancer treated with adjuvant vaginal brachytherapy. Int J Gynecol Cancer. 2020;30:48–55. doi: 10.1136/ijgc-2019-000675. [DOI] [PubMed] [Google Scholar]
- 20.Klopp A., Smith B.D., Alektiar K. The role of postoperative radiation therapy for endometrial cancer: Executive summary of an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2014;4:137–144. doi: 10.1016/j.prro.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundt A.J., McBride R., Rotmensch J. Signifiacnt pelvic recurrence in high-risk pathologic stage I-IV endometrial carcinoma patients after adjuvant chemotherapy alone: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:1145–1153. doi: 10.1016/s0360-3016(01)01566-8. [DOI] [PubMed] [Google Scholar]
- 22.Secord A.A., Geller M.A., Broadwater G. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013;128:65–70. doi: 10.1016/j.ygyno.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Maggi R., Lissoni A., Spina F. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: Results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogberg T., Signorelli M., de Oliveira C.F. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer—results from two randomised studies. Eur J Cancer. 2010;46:2422–2431. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki Y., Watanabe M., Amikura T. Adjuvant chemotherapy as treatment of high-risk stage I and II endometrial cancer. Gynecol Oncol. 2004;94:333–339. doi: 10.1016/j.ygyno.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 26.Greven K., Winter K., Underhill K. Final analysis of RTOG 9708: Adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006;103:155–159. doi: 10.1016/j.ygyno.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 27.de Boer S.M., Powell M.E., Mileshkin L. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): An open-label, multicenter, randomised, phase 3 trial. Lancet Oncol. 2016;17:1114–1126. doi: 10.1016/S1470-2045(16)30120-6. [DOI] [PubMed] [Google Scholar]
- 28.Creutzberg C.L., van Putten W.L.J., Wárlám-Rodenhuis C.C. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: The postoperative radiation therapy in endometrial carcinoma trial. J Clin Oncol. 2004;22:1234–1241. doi: 10.1200/JCO.2004.08.159. [DOI] [PubMed] [Google Scholar]
- 29.Randall M.E., Filiaci V., McMeekin D.S. Phase III trial: Adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early sage endometrial cancer. J Clin Oncol. 2019;37:1810–1818. doi: 10.1200/JCO.18.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research Network. Kandoth C., Schultz N. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:63–67. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosse T., Nout R.A., McAlpine J.N. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol. 2018;42:561–568. doi: 10.1097/PAS.0000000000001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fader A.N., Roque D.M., Siegel E. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol. 2018;36:2044–2051. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y., Malouf G.G., Zhang J. Long non-coding RNA profiling links subgroup classification of endometrioid endometrial carcinomas with trithorax and polycomb complex aberrations. Oncotarget. 2015;6:39865–39876. doi: 10.18632/oncotarget.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda T., Banno K., Okawa R. ARID1A gene mutation in Ovarian and endometrial cancers (review) Oncol Rep. 2016;35(2):607–613. doi: 10.3892/or.2015.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J., Roh J.W., Bandyopadhyay S. Overexpression of enhancer of Zeste Homolog 2 (EZH2) and focal adhesion kinase (FAK) in high grade endometrial carcinoma. Gynecol Oncol. 2013;128:344–348. doi: 10.1016/j.ygyno.2012.07.128. [DOI] [PubMed] [Google Scholar]
- 36.Byron S.A., Gartside M., Powell M.A. FGFR2 point mutations in 466 endometrioid endometrial tumors: Relationship with MSI, KRAS, PIK3CA, CTNNB1 mutations and clinicopathological features. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konecny G.E., Finkler N., Garcia A.A. Second-line Dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: A non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 2015;16:686–694. doi: 10.1016/S1470-2045(15)70159-2. [DOI] [PubMed] [Google Scholar]
- 38.Levine D.A., Dizon D.S., Carlson J.W. Predictive biomarkers of endometrial cancer response: Result from NRG Oncology/Gynecologic Oncology Group study 86P [abstract] Gynecol Oncol. 2018;149(Suppl 1):12–13. [Google Scholar]
- 39.Slomovitz B.M., Lu K.H., Johnston T. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everlolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slomovitz B.M., Jiang Y., Yates M.S. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. 2015;33:930–936. doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oza A.M., Elit L., Tsao M.S. Phase II study of temsirolimus inw omen with recurrent or metastatic endometrial cancer: A trial of the NCIC Clinicals Trials Group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott P.A., Bang Y.J., Berton-Rigaud D. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535–2541. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 43.US. Food & Drug Administration Simultaneous review decisions for pembrolizumab plus lenvatinib in Australia, Canada and US. https://www.fda.gov/drugs/resources-information-approved-drugs/simultaneous-review-decisions-pembrolizumab-plus-lenvatinib-australia-canada-and-us Available at:
- 44.Wortman B.G., Bosse T., Nout R.A. Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: Evaluation of the pilot phase of the PORTEC-4a trial. Gynecol Oncol. 2018;151:69–75. doi: 10.1016/j.ygyno.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Song J., Le T., Hopkins L. A comparison of disease recurrence between robotic versus laparotomy approach in patients with intermediate-risk endometrial cancer. Int J Gynecol Cancer. 2020;30:160–166. doi: 10.1136/ijgc-2019-000838. [DOI] [PubMed] [Google Scholar]
- 46.Fabrini M.G., Gadducci A., Perrone F. Relationship between interval from surgery to radiotherapy and local recurrence rate in patients with endometrioid-type endometrial cancer: A retrospective mono-institutional Italian study. Anticancer Res. 2012;32:169–173. [PubMed] [Google Scholar]
- 47.Sonoda Y., Zebre M., Smith A. High incidence of positive peritoneal cytology in low-risk endometrial cancer treated by laparoscopically assisted vaginal hysterectomy. Gynecol Oncol. 2001;80:378–382. doi: 10.1006/gyno.2000.6079. [DOI] [PubMed] [Google Scholar]
- 48.Gupta An, Watson D.I., Ellis T., Jamieson G.G. Tumour implantation following laparoscopy using different insufflation gases. ANZ J Surg. 2002;72:254–257. doi: 10.1046/j.1445-2197.2002.02385.x. [DOI] [PubMed] [Google Scholar]
- 49.Volz J., Köster S., Sacek Z., Paweletz N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer. 1999;86:770–774. [PubMed] [Google Scholar]
- 50.Lin F., Pan L., Li L. Effects of a simulated CO2 pneumoperitoneum environments on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med Sci Monit. 2014;20:2497–2503. doi: 10.12659/MSM.891179. [DOI] [PMC free article] [PubMed] [Google Scholar]