Abstract
Objectives
To develop methods guidance to support the conduct of rapid reviews (RRs) produced within Cochrane and beyond, in response to requests for timely evidence syntheses for decision-making purposes including urgent health issues of high priority.
Study Design and Setting
Interim recommendations were informed by a scoping review of the underlying evidence, primary methods studies conducted, and a survey sent to 119 representatives from 20 Cochrane entities, who were asked to rate and rank RR methods across stages of review conduct. Discussions among those with expertise in RR methods further informed the list of recommendations with accompanying rationales provided.
Results
Based on survey results from 63 respondents (53% response rate), 26 RR methods recommendations are presented for which there was a high or moderate level of agreement or scored highest in the absence of such agreement. Where possible, how recommendations align with Cochrane methods guidance for systematic reviews is highlighted.
Conclusion
The Cochrane Rapid Reviews Methods Group offers new, interim guidance to support the conduct of RRs. Because best practice is limited by the lack of currently available evidence for some RR methods shortcuts taken, this guidance will need to be updated as additional abbreviated methods are evaluated.
Keywords: Rapid review, Systematic review, Evidence synthesis, Decision-making
What is new?
Key findings
-
•
This article offers new, interim guidance composed of 26 specific recommendations to support the conduct of rapid reviews produced within Cochrane, and beyond.
-
•
Guidance emphasizes the involvement of key stakeholders throughout the stages of the rapid review, and promotes a flexible, iterative approach that can be tailored for various urgent and emergent health decision-making scenarios.
What this adds to what is known:
-
•
This article presents specific guidance on the steps and considerations regarding accelerating each part of the review process.
-
•
Guidance was developed within the context of Cochrane and is focused on the conduct of rapid reviews to address urgent and high priority questions to inform decision-making.
What is the implication, what should change now?
-
•
Although this RR guidance was developed for Cochrane, the recommended methods are widely applicable for anyone conducting a rapid review.
-
•
We recommend that rapid review authors adopt and use this guidance with the aim to improve the utility and robustness of rapid review results as a useful evidence synthesis tool for timely decision-making in health care.
1. Introduction
1.1. Context for rapid reviews
In health care, systematic reviews (SRs) are highly valued evidence syntheses that inform decisions. However, the methodological rigor and process that makes SR evidence trustworthy often take one to 2 years to complete [[1], [2], [3], [4]] and limit their utility to meet the time-sensitive needs of stakeholders. For example, given the onset of coronavirus, decision makers have an urgent need for evidence that cannot be met using traditional SR methods. Rapid reviews (RRs) have emerged as an efficient tool to get evidence to decision makers more quickly and are now considered part of the knowledge synthesis family [5]. Although published descriptions of RRs date back nearly a decade [6,7], a standard or consensus definition does not exist. However, RRs have been described as a type of knowledge synthesis in which SR methods are streamlined and processes are accelerated to complete the review more quickly [1,2,7,8]. Evidence suggests that policymakers are increasingly using RRs in their daily decision-making [[9], [10], [11], [12]]. Respected national and international health agencies [13,14] are also using RRs, including to inform guideline recommendations in urgent and emergent public health settings [15,16].
1.2. Cochrane's involvement with rapid reviews
Cochrane, a global leader in the production of high-quality SRs and methodological guidance, has taken important steps to foster engagement in RRs. In 2015, the Cochrane Rapid Reviews Methods Group (RRMG) was established [17] and has been involved in related methods research including developing standards for the reporting of RRs [18]. In 2018, Cochrane's Content Strategy identified the need to explore and, if appropriate, produce RRs [19]. To inform this work, the RRMG conducted several research activities that culminated in the development of interim RR methods recommendations. Although a formal implementation strategy for Cochrane RRs had been planned, due to the onset of the coronavirus disease 2019 (COVID-19) pandemic, Cochrane supported the early release of this guidance, so it was available to Cochrane's network of reviewers and others in the research community conducting RRs for urgent, highest priority topics.
1.3. Objectives
The objectives of this manuscript are to present features that define a Cochrane RR and to provide interim methodological recommendations for the development of RRs. While there are examples of RR guidance [16,20,21], to our knowledge, this is the first that provides clear, actionable recommendations and minimum standards based on up to date empirical evidence evaluating RR methods.
2. Methods
Recommendations were informed by a suite of related methodological work briefly described below, that included two scoping reviews, two primary methods studies, and a survey of Cochrane stakeholders.
2.1. Underlying evidence and primary studies for RR defining features and methods
We conducted a systematic scoping review and thematic analysis of definitions and defining characteristics of RRs and proposed a definition of RR that covers the most common themes that were identified in approximately 50% or more of the 216 RRs and 90 methods articles included [22]. We also undertook a second scoping review that identified 14 empirical studies that evaluated RR methods, which we mapped to stages of review conduct [23]. The RRMG also led two methodological studies: one that assessed the impact of limiting inclusion criteria solely to English language publications [24] and one that assessed the accuracy of single-reviewer screening vs. dual-reviewer screening as part of an online parallel group randomized controlled trial using the Cochrane Crowd platform [25]. Collectively, this work formed the evidentiary base for the subsequent RR methods options survey. Details of these publications are reported elsewhere [[23], [24], [25]].
2.2. Cochrane rapid review methods options survey
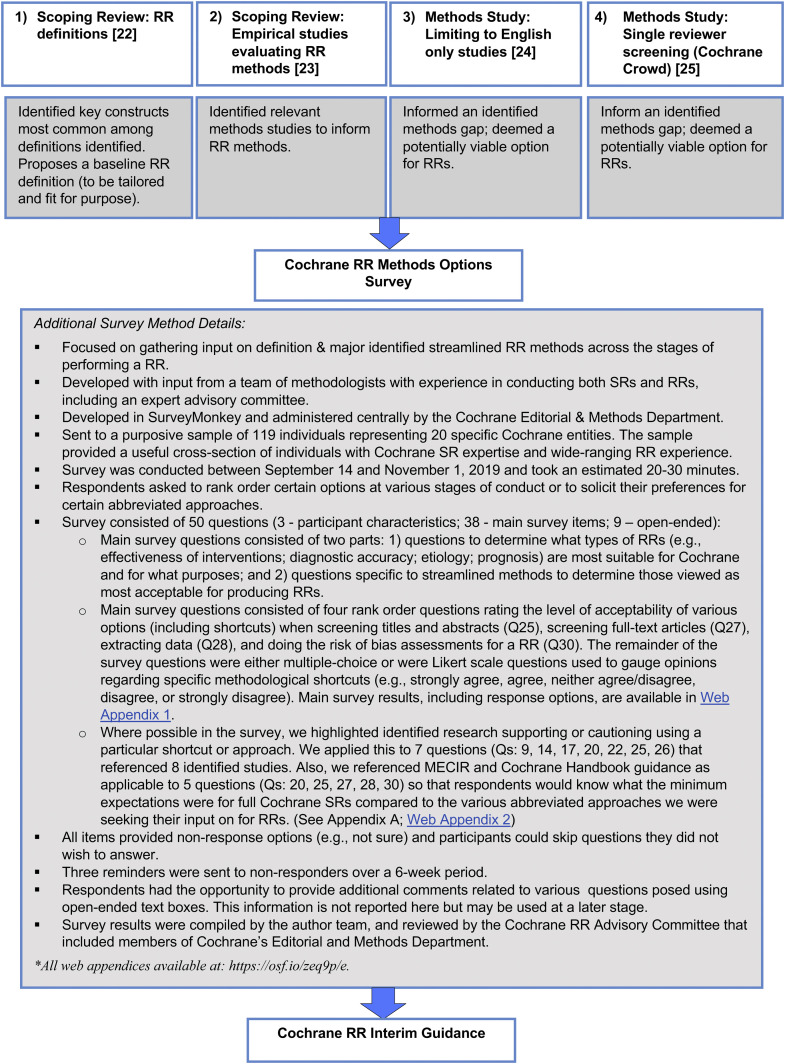
The survey was intended to elicit input on the major identified streamlined RR methods across the stages of performing an RR and was developed following recommended survey methods [26]. Where possible, for certain questions, we highlighted identified research supporting or cautioning the use of a particular shortcut or approach (Appendix A & Web Appendix 2). Surveys were sent to 119 individuals from 20 specific Cochrane entities in September 2019. The results were analyzed using descriptive statistics with details reported in the text and summarized in tabular form. Given this was an internal organizational survey, formal ethics approval was not sought but informed consent was required to participate. See Fig. 1 for further survey methods details.
Fig. 1.
Suite of methodological research to inform the definition and methods for Cochrane rapid reviews.
2.3. Deriving interim guidance for Cochrane rapid review methods
As a preliminary approach, the RRMG elected to adopt recommendations based on survey items for which there was a high or moderate level of agreement using the following as a guide: high-level (endorsed by ≥ 70% of respondents); moderate-level (endorsed by ≥50–69% of respondents); and low-level (endorsed by <50% of respondents). If, for a particular question, a response set only reached “low-level agreement,” we still recommended the most endorsed response, so that each stage of conduct had an accompanying recommendation.
The guidance also incorporated information as to how endorsed items mapped to Cochrane Methodological Expectations of Cochrane Intervention Reviews (MECIR) guidance for SRs. Further discussion among RRMG convenors informed the agreed list of recommendations presented along with the accompanying rationales provided to support and provide further explanation and context for each recommendation with supporting evidence cited if available. Although we did not follow a formal recommendation process, we used a deliberate and systematic approach to develop this interim guidance as per Web Appendix 3.
3. Results
3.1. Results of the survey
The overall survey response rate was 53% (n = 63) with a completed response rate of 46% (n = 53). Respondents were from 19 of 20 entities approached. Of those that responded, a large proportion were either extremely/very (38%; n = 24) or somewhat (38%; n = 24) familiar with RRs. Further, 62% (n = 39) had previously participated in an RR in various capacities (See Appendix B. Participant Characteristics). Overall, there was general approval for Cochrane implementing RRs as a product (only one of 59 respondents indicated Cochrane should not undertake RRs). The label Cochrane RR was strongly endorsed as a sufficient term (43/57; 75%) for use within Cochrane. Overall, we included twenty-six items that attained a moderate to a high level of agreement or that ranked first as an approach across the stages of conduct (See Appendix C. Recommendations, Level of Agreement, and Relation to MECIR Guidance). See Web Appendix 1 (https://osf.io/zeq9p/) for main survey results.
3.2. Cochrane Rapid Reviews–defining features
We recommend that Cochrane adopts the following definition of an RR: “A rapid review is a form of knowledge synthesis that accelerates the process of conducting a traditional systematic review through streamlining or omitting various methods to produce evidence for stakeholders in a resource-efficient manner.” [22] Further, RRs should be driven by the need for timely evidence for decision-making purposes including to address urgent and emergent health issues and questions deemed to be of high priority.
3.3. Cochrane rapid review methods—interim recommendations
The Cochrane RRMG has put forth a list of twenty-six recommendations for Cochrane RRs as outlined in Table 1 , with rationales for the recommendations provided in the following:
Table 1.
Cochrane rapid review methods recommendations
| Setting the research question—topic refinement |
|
| Setting eligibility criteria |
|
| Searching |
|
| Study selection |
Title and abstract screening
|
| Data extraction |
|
| Risk of bias assessment |
|
| Synthesis |
|
| Other considerations for Cochrane RRs |
| RRs should be preceded by a protocol submitted to and approved by Cochrane (R23); the protocol should be published (e.g., PROSPERO or Open Science Framework) (R24); allow for post hoc changes to the protocol (eligibility criteria etc.) as part of an efficient and iterative process (R25); document all post hoc changes; and incorporate use of online SR software (e.g., Covidence, DistillerSR, and EPPI-Reviewer) to streamline the process (R26). |
To be considered a systematic review (SR) for screening purposes, studies need to clearly report inclusion/exclusion criteria; search at least two databases; conduct risk of bias assessment; and provide a list and synthesis of included studies.
3.3.1. Recommendation 1: setting the research question (topic refinement)
Evidence from one study suggests that knowledge brokering of proposals improved the perceived clarity of information provided to policymakers; reasons for commissioning the RR; and context for the questions, scope, methods, and report conclusions. Further, it improved the confidence of reviewers to meet the policymakers’ needs [27]. It is also important to develop a protocol that includes the review questions using a question framework (e.g., PICOs) and that details the eligibility criteria.
3.3.2. Recommendations 2–9: setting eligibility criteria
The various limits to eligibility criteria are important to ensure RRs are manageable and timely. Such restrictions need to be considered together with stakeholders. Limits include clearly defining the population, intervention, and comparator. If the findings of an RR are to influence practice and policy, then outcome selection needs to be relevant to clinicians, policymakers, and patients. Although exploring the full range of outcomes at the outset of the project is good practice, the list of outcomes needs to be carefully condensed given time and resource limitations of RRs. Author teams, therefore, must judiciously select the outcomes to consider. Prioritizing outcomes will depend on the needs of stakeholders, who should be involved in the selection process.
If limiting the inclusion criteria by study date of publication, evidence suggests that limiting the search to a set number of years may lead to a loss of studies and a change in the results of meta-analyses [28]. In this study, most effect size changes were small but moderate, and significant changes were relatively common. Therefore, although setting a date restriction is a pragmatic shortcut, this needs to be carefully considered for each topic. Language restriction (i.e., English-only studies) is also practical when conducting RRs for conventional interventions. A recent study assessed whether limiting inclusion criteria solely to English language publications affected the reviews' overall conclusions. Findings suggest that exclusion of non-English publications from SRs on clinical interventions had a minimal effect on overall conclusions and can be a viable methodological shortcut for RRs [24]. However, we do not recommend restricting to English-only publications if the expectation is that relevant studies may be published in languages other than English. For example, if RRs are related to COVID-19, or involve complementary and alternative medicine therapies, it is expected that studies would emerge in languages other than English.
Consider a stepwise approach for the inclusion of evidence, emphasizing synthesized research (e.g., SRs) first, where available, then on higher quality designs for primary studies. Emphasizing locating and summarizing evidence first from relevant, higher quality study designs will assist in streamlining available evidence.
3.3.3. Recommendations 10–13: searching
Searching for RRs needs to involve an experienced information specialist. Further, the selection of databases to search will depend on the topic under review and access to them. Two key studies helped inform the recommendation for searching major databases [28,29]. In making our recommendation, we have adopted a conventional approach involving CENTRAL, MEDLINE, and Embase (if available), which also conforms to Cochrane MECIR guidance.
Although Peer Review of Electronic Search Strategies [30] is not part of current MECIR guidance, we recommend its use for Cochrane RRs. Evidence suggests that the absence of search strategy peer review often results in many missed studies not retrieved. Unless captured in an accompanying gray literature search, these records would not have appeared in the published RRs, thus reducing the integrity of reports [31].
We also recommend limiting gray literature and supplemental searching. If it is warranted, consider limiting searches to clinical trial registries, the scanning of SR bibliographies and reference lists of included studies to identify potentially relevant studies. In most cases, gray literature searching should be performed after the abstract and full-text screening is completed. Screening reference lists can detect missed studies when searching electronic databases or eligible studies excluded in error during screening.
3.3.4. Recommendation 14: study selection—title & abstract screening
One reviewer to include and two reviewersto exclude at title and abstract screening for Cochrane RRs was the highest ranked among survey respondents. By comparison, currently, Cochrane allows for both dual and single title and abstract screening for Cochrane Reviews, although MECIR states that it is desirable to use two screeners working independently. For RRs, it is important that a standardized title and abstract form is used, and that before the start of screening, a pilot exercise is conducted using a minimum of the same 30–50 abstracts screened by the entire team to calibrate and test the review form. At the outset, two reviewers should be used to dual screen at least 20% of abstracts with conflicts resolved. This should be followed by one reviewer screening the remaining abstracts and a second reviewer screening all excluded abstracts.
Although single reviewer screening may be a practical solution for certain RRs, we do not recommend this for RRs at this time. Findings from two recent studies indicate that single screening of the titles and abstracts is not equivalent to dual screening, as more studies are missed [25,32]. Further, this approach ranked as least acceptable among Cochrane survey respondents. Nonetheless, forthcoming advances in automation and crowdsourcing have the potential to reduce the time spent screening when conducting RRs.
3.3.5. Recommendation 15: study selection - full-text screening
The recommendation (i.e., one reviewer to include and two reviewers to exclude) represents a methodological shortcut for RRs, when compared with full-text screening for Cochrane Reviews (i.e., two independent reviewers using full text to determine if the study meets eligibility criteria). As with title and abstract screening, a standardized full-text form should be used. A pilot exercise is recommended using the same 5–10 full-text articles for the entire screening team to calibrate and test the review form. Dual, independent screening of full-text articles ranked second as most acceptable. It fully adheres to Cochrane guidance and may be used if time and resources are available. Review teams should also use SR software to make the screening, tracking, and documentation more efficient whenever possible.
3.3.6. Recommendations 16-18: data extraction
The recommended approach for data extraction for RRs was the highest ranked among survey respondents but deviates from Cochrane Reviews whereby data extraction may be separated into two parts: i) extracting study characteristics for which Cochrane allows for both dual and single extraction although in duplicate is highly desired and ii) extracting outcomes data, which Cochrane makes mandatory to extract in duplicate.
Data for RRs should be extracted using a pilot-tested form. It will be important that data are efficiently abstracted using concise descriptions of the participant, intervention and comparator characteristics, and outcomes assessed. Although a recently published study that found the experience of data abstractors may matter less than initially thought and that adjudication is what leads to reduced errors [33], skilled extractors will be key to minimizing error rates for RRs.
3.3.7. Recommendations 19–20: risk of bias assessment (RoB)
The recommended approach that ranked highest in the survey for RRs is less stringent than for Cochrane Reviews, but still involves one reviewer to do RoB with another reviewer to verify all judgments. To effectively manage this section of the RR, it is important to limit the RoB ratings to the primary outcomes included in the summary of findings tables and to use, if possible, a valid RoB assessment tool specific to the study design(s) included in the RR.
3.3.8. Recommendations 21–22: synthesis
RR teams need to develop an appropriate analysis plan in advance. At the outset of the synthesis stage, providing a descriptive summary of the included studies helps confirm if they are similar and reliable enough to synthesize and if it is possible to pool results. Reviewers need to decide how to group and tabulate data based on the RR question, type of data included, and what they planned for in the protocol to the extent possible. Beyond a simple descriptive summary, for all RRs, a narrative synthesis of findings from multiple studies should be conducted. Even if a meta-analysis is possible, a narrative synthesis is needed to interpret the collective evidence fully.
Reviewers should organize the narrative synthesis around the Population, Intervention, Comparison, and Outcomes (PICO) question framework elements with findings grouped by key question(s), interventions, then by comparisons, followed by outcomes. A narrative synthesis is also an important step in determining if meta-analysis seems appropriate on the surface. The standards for conducting a meta-analysis for an SR equally apply to an RR and that meta-analysis will depend on the nature of the data and information provided in the individual studies. Involve a statistician, who is familiar with SRs and meta-analysis. Depending on the volume and nature of included studies, the depth and details of analysis will vary across RRs. At times, RR authors may require more time to understand studies better vs. making a quick decision as to whether they are similar enough to pool. It may also be that in the essence of time, author teams are only able to report overall findings and are not able to go into depth exploring variability among the findings.
Cochrane Reviews incorporate the Grading of Recommendations Assessment, Development and Evaluation approach [34] for grading certainty of the evidence, and therefore, we also recommend its use for Cochrane RRs. When grading, reviewers should ideally present results in a Summary of Findings table for each intervention and comparator and are advised to include only those outcomes most important to stakeholders, typically the primary outcomes. Because grading is an involved process and takes time, for RRs, we recommend limiting to the main intervention and comparator, and to those outcomes deemed most critical to decision-making set out in advance, inclusive of harms. If using this grading system, at minimum, the reasons for uprating or downrating should be transparently described in conjunction with evidence in tabular or narrative form.
3.3.9. Recommendations 23–26: other considerations
To minimize duplication, and to ensure relevancy and transparency, author teams should confer with Cochrane or other registries (e.g., the international Prospective Register of Systematic Reviews [PROSPERO] and Open Science Framework [OSF]) before starting an RR. Specific to Cochrane, authors will be required to submit a completed protocol that will undergo editorial and methodological checks. For transparency, authors will need to register the Cochrane RR protocols with PROSPERO or the OSF prespecifying the methods to be used. Cochrane currently has a streamlined intake process for COVID-19 priority topics and provides access to several resources (e.g., protocol and review templates) to support the review process. In future, we expect Cochrane will expand this workflow to accommodate RRs across other priority topics.
Sometimes changes to the protocol are necessary once the RR has started. For example, search parameters may be expanded or limited depending on the search yields, or eligibility criteria may need to be tweaked after preliminary screening. Therefore, the RR process should allow for post hoc changes. Significant changes should be discussed with the stakeholders involved, and any amendments tracked and reported. Moreover, authors should seek stakeholder feedback throughout the process to ensure the RR meets their information needs.
We strongly encourage the use of software in the production of RRs. Online systematic review software enhances collaboration by allowing for real-time project management and multiuser participation across geographic boundaries. Importantly, it enables members of the RR team to work in parallel across all stages of the review and provides a fully transparent process. It also facilitates the incorporation of protocol amendments and other changes to questions and forms often common to RRs. It also improves the data quality and efficiency through the automated collation of the screening results (inclusions/exclusions). Those undertaking RRs and other types of syntheses should look to ways to harness innovation, using software and adopting automation tools that reliably assist in expediting stages of review conduct.
Importantly, given the methodological modifications inherent to RRs, authors must be transparent in reporting their methods and results. Although an extension to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for RRs is underway [18], until it is officially completed, we suggest authors use the general PRISMA statement to the extent possible and adapt it accordingly.
4. Discussion
This article presents 26 recommendations as part of interim guidance developed by the Cochrane RRMG to standardize the conduct of RRs. Having specific guidance will improve the utility and robustness of the results of Cochrane RRs and more broadly contributes to the ongoing progression of RR methodology as a synthesis tool to be used more widely for timely evidence-based decision-making in health care.
A strength of this guidance is that recommendations were informed by a review of related RR methods studies across stages of conduct, two metaepidemiological studies conducted by the RRMG, through gathering the opinions and preferences from a wider group of researchers involved in the Cochrane community using survey methods, and the involvement of RR methodology experts. Notably, this guidance provides actionable recommendations and minimum standards yet promotes a flexible and iterative RR process that supports a tailored approach for each review. For example, although survey respondents agreed Cochrane RRs should take no longer than 6 months, some of the shortcuts could be modified further or additional shortcuts taken to reflect very compressed timelines (e.g., <2 weeks) in circumstances when there is more urgent need for evidence.
Importantly, this interim guidance is based on what is currently known about RR methods and is being actively used in the development of early Cochrane RR products to address pressing clinical questions posed by international stakeholders in response to COVID-19 [35,36]. As such, its use is encouraged although we recognize that further enhancements and fine-tuning are needed. In terms of next steps, we plan to collect feedback on the guidance's perceived utility as applied in urgent, real-time RR scenarios. We also intend to adapt the guidance beyond interventions of effectiveness to other review types (e.g., RRs of diagnostic test accuracy or screening). Certain recommendations (e.g., involving stakeholders and involving an information specialist) will be applicable across other types of RRs, whereas some may not be (e.g., English-only inclusion may not be appropriate in some cases and database selection will potentially differ). Further, specific RR types will pose unique methodological issues [37]. There are many challenges to the conduct of RRs that further merit discussion including how to establish a manageable set of rank-ordered outcomes of most importance [38]. We also need to develop criteria for the appropriateness of undertaking RRs vs. traditional SRs or living SRs. We intend to refine specific recommendations as new evidence emerges, building on work completed to date.
What sets our RR methods apart from other RR guidance is that it was developed within the context of Cochrane and focuses on RRs of interventions. Other RR guides focus on health policy and systems research, public health, or WHO guideline development in the face of public health emergencies [16,20,21]. The focus of our guide is on the conduct of RRs, not on planning, packaging, or dissemination. Recommendations for every step of the review are very specific, compared with other guides that rather provide an overview of common RR practices. Although other RR guides are evidence informed too, our guide is based on up-to-date empirical evidence evaluating RR methods complemented by an expert survey. Cochrane RRs are recommended to address only urgent and high priority questions explicitly requested by decision makers, whereas other guides do not necessarily limit RR conduct to urgent questions only [20] (Web Appendix 4).
In spite of the survey response rate being somewhat lower than those typically obtained from Internet surveys (58%) [26], it did include participation from representatives across 19 of 20 Cochrane groups approached to take part in this survey. Although not all stages of an RR had corresponding evidence to inform our survey, we know this is similar for SR methods in that many established steps in the process are based on limited, outdated, or no available evidence. Importantly, the body of work underlying this guidance is mutually beneficial to informing both RRs and SRs.
Given RRs serve an important purpose for certain stakeholders, ongoing interest in them is expected to grow. Beyond COVID, this guidance will also be applicable for future circumstances when decisions need to be made in a period of weeks to a few months, and for which the traditional SR timeline does not meet the timeframe for urgent decision-making. Although the rationale and the context for this guidance is heavily posited within the Cochrane landscape, the recommended methods are widely applicable for anyone conducting an RR. As a leader in methods guidance on SRs, Cochrane is well positioned as an organization to not only produce RRs but with input of the Cochrane RRMG, to also advise on strengths and limitations of abbreviated methods and their potential impact on decision-making to minimize compromising validity. Endorsing an RR approach alongside interim methods guidance, demonstrates Cochrane's ability to respond quickly as a world leader in knowledge synthesis.
5. Conclusion
This article offers new, interim guidance composed of 26 methods recommendations to support the conduct of RRs produced within Cochrane and beyond. We hope this guidance will encourage thoughtful use of abbreviated SR practices for RRs and fosters further development through deliberation and formal assessment. Application of the proposed methods in exemplar RRs must be closely studied to understand methodological choices made and the challenges experienced.
Acknowledgments
The Cochrane RRMG would like to acknowledge the support of Ella Flemyng (Cochrane Methods Implementation Coordinator) for facilitating survey administration. A further thanks to Cochrane's Editorial and Methods Department including Karla Soares-Weiser (Editor-in-Chief) and Toby Lasserson (Deputy Editor-in-Chief) for their support provided throughout development of this guidance. We would also like to extend thanks to the members of the Cochrane Rapid Reviews Advisory Committee for their input on the survey findings. This includes: Drs. Andrea Tricco, Jos Kleijnen, Mala Mann, David Moher, Lisa Hartling, Marguerite Koster, Elie Akl, Craig Umscheid, and Holger Schünemann.
Footnotes
Funding source: This work was supported by funding provided by the Cochrane Collaboration (United Kingdom) to the Cochrane Rapid Reviews Methods Group and in-kind support from the following institutions: Ottawa Hospital Research Institute (OHRI); Cochrane Austria, Danube University Krems; CADTH; The Center for Evidence-based Policy (CEBP), Oregon Health & Science University.
Conflict of interest: None of the authors report any conflicts of interest with respect to this manuscript.
Authors' contributions: CG: Conceptualization, Funding acquisition, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing - review & editing. GG: Conceptualization, Funding acquisition, Investigation, Methodology, Writing - review & editing. BNS: Conceptualization, Funding acquisition, Investigation, Methodology, Writing - review & editing. VJK: Conceptualization, Funding acquisition, Methodology, Writing - review & editing. CH: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing - review & editing. CK: Conceptualization, Funding acquisition, Methodology, Writing - review & editing. LA: Investigation, Validation, Writing - review & editing. AS: Conceptualization, Funding acquisition, Methodology, Writing - review & editing.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinepi.2020.10.007.
Supplementary data
References
- 1.Ganann R., Ciliska D., Thomas H. Expediting systematic reviews: methods and implications of rapid reviews. Implement Sci. 2010;5:56. doi: 10.1186/1748-5908-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khangura S., Konnyu K., Cushman R., Grimshaw J., Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1:10. doi: 10.1186/2046-4053-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bero L., Busuttil G., Farquhar C., Koehlmoos T.P., Moher D., Nylenna M. Measuring the performance of the Cochrane library. Cochrane Database Syst Rev. 2012;12:ED000048. doi: 10.1002/14651858.ED000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsafnat G., Glasziou P., Choong M.K., Dunn A., Galgani F., Coiera E. Systematic review automation technologies. Syst Rev. 2014;3:74. doi: 10.1186/2046-4053-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D., Stewart L., Shekelle P. All in the family: systematic reviews, rapid reviews, scoping reviews, realist reviews, and more. Syst Rev. 2015;4:183. doi: 10.1186/s13643-015-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant M.J., Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 7.Tricco A.C., Antony J., Zarin W., Strifler L., Ghassemi M., Ivory J. A scoping review of rapid review methods. BMC Med. 2015;13:224. doi: 10.1186/s12916-015-0465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tricco A.C., Zarin W., Ghassemi M., Nincic V., Lillie E., Page M.J. Same family, different species: methodological conduct and quality varies according to purpose for five types of knowledge synthesis. J Clin Epidemiol. 2018;96:133–142. doi: 10.1016/j.jclinepi.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Moore G., Redman S., Rudge S., Haynes A. Do policy-makers find commissioned rapid reviews useful? Health Res Policy Syst. 2018;16:17. doi: 10.1186/s12961-018-0293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mijumbi-Deve R., Rosenbaum S.E., Oxman A.D., Lavis J.N., Sewankambo N.K. Policymaker experiences with rapid response briefs to address health-system and technology questions in Uganda. Health Res Policy Syst. 2017;15:37. doi: 10.1186/s12961-017-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartling L., Guise J.-M., Hempel S., Featherstone R., Mitchell M.D., Motu’apuaka M.L. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6:32. doi: 10.1186/s13643-017-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson K., Floyd N., Ferguson L., Christensen V., Helfand M. User survey finds rapid evidence reviews increased uptake of evidence by Veterans Health Administration leadership to inform fast-paced health-system decision-making. Syst Rev. 2016;5:132. doi: 10.1186/s13643-016-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thigpen S., Puddy R.W., Singer H.H., Hall D.M. Moving knowledge into action: developing the rapid synthesis and translation process within the interactive systems framework. Am J Community Psychol. 2012;50:285–294. doi: 10.1007/s10464-012-9537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patnode C.D., Eder M.L., Walsh E.S., Viswanathan M., Lin J.S. The use of rapid review methods for the U.S. Preventive services task force. Am J Prev Med. 2018;54:S19–S25. doi: 10.1016/j.amepre.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Hersi M., Stevens A., Quach P., Hamel C., Thavorn K., Garritty C. Effectiveness of personal protective equipment for healthcare workers caring for patients with filovirus disease: a rapid review. PLoS One. 2015;10:e0140290. doi: 10.1371/journal.pone.0140290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garritty C.M., Norris S.L., Moher D. Developing WHO rapid advice guidelines in the setting of a public health emergency. J Clin Epidemiol. 2017;82:47–60. doi: 10.1016/j.jclinepi.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garritty C., Stevens A., Gartlehner G., King V., Kamel C., On behalf of the Cochrane Rapid Reviews Methods Group Cochrane Rapid Reviews Methods Group to play a leading role in guiding the production of informed high-quality, timely research evidence syntheses. Syst Rev. 2016;5:184. doi: 10.1186/s13643-016-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens A., Garritty C., Hersi M., Moher D. The Equator Network; 2018. Developing PRISMA-RR, a reporting guideline for rapid reviews of primary studies (Protocol) p. 12.https://www.equator-network.org/wp-content/uploads/2018/02/PRISMA-RR-protocol.pdf Available at. [Google Scholar]
- 19.Cochrane Strategy to 2020. https://indd.adobe.com/embed/4869de43-450e-44e9-ac51-e8c7c5f45c08?startpage=1&allowfullscreen=true Available at.
- 20.Dobbins M. National Collaborating Centre for Methods and Tools; Hamilton, ON: 2017. Rapid Review Guidebook.https://www.nccmt.ca/capacity-development/rapid-review-guidebook Available at. [Google Scholar]
- 21.Tricco A.C., Langlois E.V., Straus S.E., World Health Organization . World Health Organization; 2017. Alliance for Health Policy and Systems Research. Rapid reviews to strengthen health policy and systems: a practical guide.http://apps.who.int/iris/bitstream/10665/258698/1/9789241512763-eng.pdf Available at. [Google Scholar]
- 22.Hamel C., Michaud A., Thaku M., Skidmore B., Stevens A., Nussbaumer-Streit B. Defining rapid reviews: a systematic scoping review and thematic analysis of definitions and defining characteristics of rapid reviews. J Clin Epidemiol. 2020;129:74–85. doi: 10.1016/j.jclinepi.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Hamel C., Michaud A., Thuku M., Affengruber L., Skidmore B., Nussbaumer-Streit B. Few evaluative studies exist examining rapid review methodology across stages of conduct: a systematic scoping review. J Clin Epidemiol. 2020;126:131–140. doi: 10.1016/j.jclinepi.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 24.Nussbaumer-Streit B., Klerings I., Dobrescu A.I., Persad E., Stevens A., Garritty C. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42–54. doi: 10.1016/j.jclinepi.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Gartlehner G., Affengruber L., Titscher V., Noel-Storr A., Dooley G., Ballarini N. Single-reviewer abstract screening missed 13 percent of relevant studies: a crowd-based, randomized controlled trial. J Clin Epidemiol. 2020;121:20–28. doi: 10.1016/j.jclinepi.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Dillman D.A. 2nd ed. John Wiley & Sons Inc; Hoboken, NJ: 2007. Mail and internet surveys: The tailored design method. [Google Scholar]
- 27.Moore G., Redman S., D’Este C., Makkar S., Turner T. Does knowledge brokering improve the quality of rapid review proposals? A before and after study. Syst Rev. 2017;6:23. doi: 10.1186/s13643-017-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall I.J., Marshall R., Wallace B.C., Brassey J., Thomas J. Rapid reviews may produce different results to systematic reviews: a meta-epidemiological study. J Clin Epidemiol. 2019;109:30–41. doi: 10.5281/zenodo.1447087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nussbaumer-Streit B., Klerings I., Wagner G., Heise T.L., Dobrescu A.I., Armijo-Olivo S. Abbreviated literature searches were viable alternatives to comprehensive searches: a meta-epidemiological study. J Clin Epidemiol. 2018;102:1–11. doi: 10.1016/j.jclinepi.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 30.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Spry C., Mierzwinski-Urban M. The impact of the peer review of literature search strategies in support of rapid review reports. Res Synth Methods. 2018;9:521–526. doi: 10.1002/jrsm.1330. [DOI] [PubMed] [Google Scholar]
- 32.Waffenschmidt S., Knelangen M., Sieben W., Bühn S., Pieper D. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol. 2019;19:132. doi: 10.1186/s12874-019-0782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jian-Yu E., Saldanha I.J., Canner J., Schmid C.H., Le J.T., Li T. Adjudication rather than experience of data abstraction matters more in reducing errors in abstracting data in systematic reviews. Res Synth Methods. 2020;1 doi: 10.1002/jrsm.1396. [DOI] [PubMed] [Google Scholar]
- 34.Schünemann H., Brojek J., Guyatt G., Oxman A., editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. https://www.gradeworkinggroup.org/ [Google Scholar]
- 35.Nussbaumer-Streit B., Mayr V., Dobrescu A.I., Chapman A., Persad E., Klerings I. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paludan-Müller A.S., Boesen K., Klerings I., Jørgensen K.J., Munkholm K. Hand cleaning with ash for reducing the spread of viral and bacterial infections: a rapid review. Cochrane Database Syst Rev. 2020:1–56. doi: 10.1002/14651858.CD013597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arevalo-Rodriguez I., Tricco A.C., Steingart K.R., Nussbaumer-Streit B., Kaunelis D., Alonso-Coello P. Challenges of rapid reviews for diagnostic test accuracy questions: a protocol for an international survey and expert consultation. Diagn Progn Res. 2019;3:7. doi: 10.1186/s41512-019-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tricco A.C., Garritty C.M., Boulos L., Lockwood C., Wilson M., McGowan J. Rapid review methods more challenging during COVID-19: commentary with a focus on 8 knowledge synthesis steps. J Clin Epidemiol. 2020;126:177–183. doi: 10.1016/j.jclinepi.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.