Abstract
Objective
To assess the effect of a pharmacist-driven, polymerase chain reaction (PCR)−based nasal screening protocol for methicillin-resistant Staphylococcus aureus (MRSA) on vancomycin therapy duration and on rates of adverse drug events and 30-day hospital readmission.
Patients and Methods
From July 8, 2017, through January 31, 2019, we performed a retrospective, multicenter, preimplementation-postimplementation study. Patients with a vancomycin order to treat lower respiratory tract infection (LRTI) underwent MRSA PCR screening; tests were ordered by health care providers, including physicians, physician assistants, and advanced practice registered nurses. During the preimplementation period (July 8, 2017, through September 30, 2018), pharmacists could order MRSA PCR screening only after receiving a verbal order from a health care provider. During the postimplementation period (October 1, 2018, through January 31, 2019), a collaborative practice agreement allowed pharmacists to order MRSA PCR screening tests.
Results
The preimplementation group included 241 patients, and the postimplementation group included 74 patients. Of these patients, 124 in the preimplementation group and 62 in the postimplementation group received MRSA PCR screening. Twenty patients (16.1%) in the preimplementation group and 9 (14.5%) in the postimplementation group had a positive MRSA PCR screening test result (between-group difference, 1.6%; P=.80). Duration of therapy was significantly shorter in the postimplementation group (median [interquartile range], 14.3 [5.0-28.6] hours vs 24.0 [12.4-47.0] hours; P<.001).
Conclusion
Vancomycin therapy carries a risk of adverse events and may increase health care costs. A pharmacist-driven protocol for MRSA nasal swab PCR screening effectively reduces the duration of vancomycin therapy for patients with lower respiratory tract infection.
Abbreviations and Acronyms: AKI, acute kidney injury; CPA, collaborative practice agreement; EHR, electronic health record; LRTI, lower respiratory tract infection; MRSA, methicillin-resistant Staphylococcus aureus; NPV, negative predictive value; PCR, polymerase chain reaction
Methicillin-resistant Staphylococcus aureus (MRSA) is part of the normal microbiota of humans and is a common pathogen implicated in lower respiratory tract, bloodstream, skin, and soft tissue infections.1 Vancomycin therapy is often initiated empirically for MRSA-suspected lower respiratory tract infections (LRTIs) despite evidence that the incidence of LRTIs caused by MRSA is declining.2 Administration and monitoring of anti-MRSA antibiotic agents, particularly vancomycin, has a substantial health care cost,3 and treatment with vancomycin also carries an elevated risk of nephrotoxicity, thereby further increasing health care cost and hospital length of stay.4,5
Numerous studies have evaluated the performance of MRSA nasal swab polymerase chain reaction (PCR) or culture screening for LRTI and have reported negative predictive values (NPVs) of 95.2% to 99.2%.6, 7, 8, 9, 10 Because of the high reported NPVs, a quick screening tool, such as one that determines MRSA nasal carriage status, may allow for rapid de-escalation of empirical MRSA-targeted therapy in patients with suspected MRSA LRTI. Several studies11, 12, 13 have examined the utility of allowing pharmacists to order MRSA nasal PCR screening at initiation of anti-MRSA therapy with the aim of de-escalating therapy if a patient presumed to have a MRSA LRTI has a negative result. Dunaway et al11 showed an approximately 40-hour reduction in duration of vancomycin therapy after implementing a protocol that included automatic MRSA nasal screening via order sets for hospital-acquired pneumonia. Pharmacists were also able to independently discontinue vancomycin therapy when negative MRSA screening test results were obtained. Baby et al12 highlighted a 46.6-hour reduction in the mean duration of anti-MRSA therapy (vancomycin or linezolid) for patients who primarily had hospital-acquired pneumonia. Willis et al13 showed a similar reduction in the duration of vancomycin therapy (by ∼50 hours) in patients who received empirical vancomycin for suspected pneumonia and exacerbation of acute chronic obstructive pulmonary disease. In two of these previous studies,12,13 pharmacists were permitted to independently order the MRSA screening test when receiving orders for anti-MRSA antibiotic drug therapy in patients with suspected pneumonia. This previous work effectively showed that pharmacist-driven protocols for ordering MRSA nasal PCR screening reduced the duration of vancomycin therapy by approximately 36 to 50 hours.
A recent unpublished internal review at our institution suggested that pharmacist-driven MRSA nasal swab culture screening effectively reduced the duration of vancomycin therapy. However, different types of MRSA nasal swab screening tests are used at different centers within our health system. For example, at Mayo Clinic in Rochester, Minnesota, nasal cultures are obtained, but Mayo Clinic Health System–Southwest Wisconsin Region uses MRSA nasal swab PCR assays.
Herein we aimed to build on our previous internal results by replacing culture screening of MRSA nasal swabs with PCR screening. The PCR screening test is performed at our institution at all hours of the day. Its turnaround time is approximately 2 hours after specimen collection, which is considerably faster than culture screening. We designed this study to determine whether pharmacist-ordered MRSA nasal swab PCR screening for LRTI in patients receiving parenteral vancomycin reduced the duration of vancomycin therapy given the shorter turnaround time of PCR screening.
Methods
This study was approved by the Mayo Clinic Institutional Review Board. We performed this retrospective, multicenter preimplementation and postimplementation study at the 2 Mayo Clinic Health System–Southwest Wisconsin Region hospitals: a tertiary care hospital in La Crosse, Wisconsin, and a critical access hospital in Sparta, Wisconsin. The study examined the effects of a collaborative practice agreement (CPA) that allowed pharmacists to order a MRSA nasal swab PCR screening test for any patient on initiation of empirical intravenous vancomycin therapy for the indication of LRTI. Although this CPA allowed pharmacists to order MRSA nasal swab screening independent of health care provider contact, it did not allow pharmacists to order the screening test for vancomycin indications other than LRTI or to independently discontinue vancomycin therapy if a negative screening result was obtained. Clinical pharmacists reviewed MRSA nasal swab PCR screening results as part of the preexisting vancomycin workflow. The pharmacy resident (N.L.W.) was responsible for the development and implementation of the CPA protocol as part of a longitudinal research project, including presenting to and obtaining approval from the institutional medication oversight group, a pharmacist education committee for workflow changes and data collection and analysis.
This study was conducted from July 8, 2017, through January 31, 2019. The preimplementation period spanned from July 8, 2017, through September 30, 2018, during which time no CPA was in place allowing pharmacists to order MRSA nasal swab PCR screening. All of the tests were ordered by health care providers, including physicians, physician assistants, and advanced practice registered nurses, but pharmacists could order MRSA PCR screening after receiving a verbal order from one of these providers. The start date of the preimplementation period was defined by our institution’s transition to a new electronic health record (EHR) vendor, and the end date was defined as the last day before implementation of the CPA. The postimplementation period spanned from October 1, 2018, through January 31, 2019. The start date of the postimplementation period was defined by the onset of the CPA, and the end date was chosen to facilitate project completion within the academic year for the pharmacy resident.
We queried the EHR system to identify all of the patients with an order for parenteral vancomycin during the preimplementation and postimplementation periods. We included patients if they were 18 years or older and had parenteral vancomycin ordered for an indication of a respiratory tract infection or if a respiratory tract infection was included in the differential diagnosis. We excluded patients with vancomycin orders for other indications (eg, skin and soft tissue infection, central nervous system infection, bloodstream infection, febrile neutropenia, perioperative prophylaxis) and those with repeated vancomycin courses. The statistical analysis assumed independence of therapy events. Therefore, only the first course of vancomycin treatment was included for patients who had multiple courses of therapy during the preimplementation or postimplementation period, regardless of whether additional courses were administered during the same or a subsequent hospitalization. Patient characteristics and clinical data were abstracted from the EHRs, including serum creatinine levels, evidence of ototoxicity, and 30-day hospital readmission due to LRTIs caused by MRSA. We screened for ototoxicity by searching for the term ototoxicity in the EHRs.
The primary outcome was the difference in duration of vancomycin therapy between the preimplementation and postimplementation groups. Duration of vancomycin therapy was calculated as the time from the initial vancomycin order to the final order for discontinuation, as documented in the EHRs. Secondary outcomes included the differences in rates of acute kidney injury (AKI), ototoxicity, and 30-day hospital readmission due to LRTIs caused by MRSA. We defined AKI as an increase in serum creatinine level of at least 0.3 mg/dL (to convert to μmol/L, multiply by 88.4) or at least a 50% increase compared with baseline levels (as measured ≤90 days before initiation of vancomycin therapy) within a 48-hour period during the first 3 days of therapy.
Baseline continuous variables were compared between groups using the Wilcoxon rank sum test, and categorical variables were compared using the Pearson χ2 test. The primary outcome of duration of vancomycin therapy was compared between groups using the Wilcoxon rank sum test. For secondary outcomes, the 30-day hospital readmission rate due to MRSA was compared between groups using the Fisher exact test, and rate of AKI within 3 days was compared using the Pearson χ2 test. A P<.05 was considered statistically significant. SAS software, version 9.4 (SAS Institute Inc) was used for all the analyses.
Results
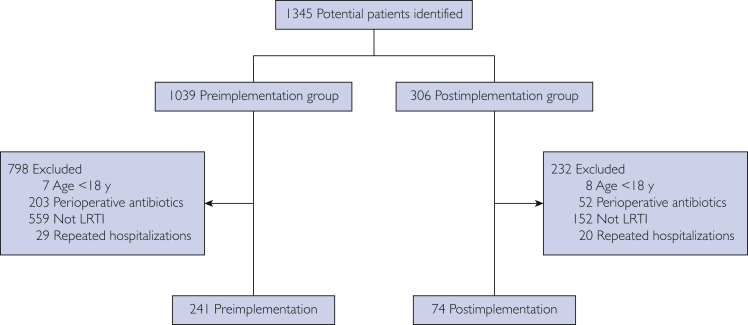
We identified 1345 potentially eligible hospital admissions. From these, 315 patients were included in the final study cohort, with 241 in the preimplementation group and 74 in the postimplementation group (Figure 1). Except for body mass index, no baseline characteristics were significantly different between groups (Table 1).
Figure 1.
Patient enrollment. LRTI = lower respiratory tract infection.
Table 1.
| Characteristic | Preimplementation group (n=241) | Postimplementation group (n=74) | P value |
|---|---|---|---|
| Age (y), mean ± SD | 68.9 ± 15.8 | 67.7 ± 17.8 | .85 |
| Male sex (No. [%]) | 132 (54.8) | 45 (60.8) | .36 |
| Height (cm), mean ± SD | 169.5 ± 11.6 | 170.6 ± 10.4 | .56 |
| Body weight (kg), mean ± SD | 87.0 ± 27.0 | 81.5 ± 28.0 | .07 |
| Body mass index, mean ± SD | 30.3 ± 8.7 | 27.9 ± 8.7 | .02 |
| History of MRSA colonization before the study period (No. [%]) | 45 (18.7) | 14 (18.9) | .96 |
| Baseline serum creatinine (mg/dL), median (IQR) | 1.0 (0.8-1.2) | 1.0 (0.7-1.2) | .93 |
IQR = interquartile range; MRSA = methicillin-resistant Staphylococcus aureus.
SI conversion factor: To convert creatinine values to μmol/L, multiply by 88.4.
Twenty of 124 patients (16.1%) who received MRSA PCR screening in the preimplementation group had a positive test result vs 9 of 62 (14.5%) in the postimplementation period (between-group difference, 1.6%; P=.80). During the preimplementation period, pharmacists ordered 11 of 124 MRSA nasal swab PCR screening tests (8.9%); during the postimplementation period, they ordered 35 of 62 tests (56.5%). Of these, 1 test (9.1%) was positive in the preimplementation group vs 4 (11.4%) in the postimplementation group (between-group difference, 2.3%; P=.83). The median time from PCR order entry to test result was 0.65 hours longer in the preimplementation group than in the postimplementation group (median [interquartile range], 4.3 [2.7-6.5] hours vs 3.6 [2.2-7.9] hours; P=.17).
The median duration of vancomycin therapy in the preimplementation group was 9.7 hours longer than that in the postimplementation group (median [interquartile range], 24.0 [12.4-47.0] hours vs 14.3 [5.0-28.6] hours; P<.001) (Figure 2). Patients in the preimplementation group received a mean of 2.95 vancomycin doses per patient, and those in the postimplementation group received a mean of 2.31 vancomycin doses per patient. For 10 patients in the postimplementation group, the initial MRSA nasal swab PCR screening test had a negative result and the order for vancomycin was discontinued before any doses were administered. Regardless of the number of vancomycin doses administered, duration of vancomycin therapy was calculated as the time from the initial vancomycin order to the final order for discontinuation. The rates of adverse drug events and 30-day hospital readmission for LRTI due to MRSA were not significantly different between groups (Table 2).
Figure 2.
Duration of vancomycin therapy. The horizontal line in the middle of each box indicates the median; bottom border, 25th percentile; top border, 75th percentile; bottom whisker, minimum value less than the 25th percentile and within 1.5 × interquartile range; top whisker, maximum value greater than the 75th percentile and within 1.5 × interquartile range; open circles, individual outliers. The duration of vancomycin therapy was significantly shorter in the postimplementation group (P<.001).
Table 2.
Secondary Outcomesa
| Outcome | Preimplementation group (No. [%]) (n=241) | Postimplementation group (No. [%]) (n=74) | P value |
|---|---|---|---|
| Hospital readmission due to MRSA within 30 d | 1 (0.4) | 1 (1.4) | .42 |
| Ototoxicity | 0 | 0 | NA |
| Acute kidney injury within 3 db | 52 (23.1) | 14 (20.9) | .70 |
MRSA = methicillin-resistant Staphylococcus aureus; NA = not applicable.
Data were missing for 16 patients in the preimplementation group and 7 patients in the postimplementation group.
Discussion
To date, available clinical practice guidelines for hospital-acquired and ventilator-associated pneumonia do not address how to use negative MRSA nasal swab screening results to guide de-escalation of anti-MRSA therapy for LRTIs.14,15 The clinical guidelines for community-acquired pneumonia16 recommend nasal culture or PCR screening for inpatients with severe pneumonia and for inpatients with nonsevere pneumonia and a history of respiratory isolation for MRSA. In either case, a negative MRSA nasal screening test result can guide the decision to withhold or discontinue empirical anti-MRSA therapy. Despite the information provided by the community-acquired pneumonia guidelines, empirical therapy for MRSA is frequently administered to patients with suspected LRTI, and identification of patients for appropriate de-escalation of anti-MRSA antibiotic drug therapy remains difficult. Numerous studies have shown that nasal swab screening for MRSA colonization has a reliably high NPV and effectively identifies patients at low risk for LRTIs caused by MRSA.6, 7, 8, 9, 10
The present study builds on previous data by showing a significant reduction in vancomycin therapy duration in the period after implementation of the CPA allowing pharmacists to order MRSA nasal swab PCR screening, with no significant between-group differences in median time from PCR order to result or MRSA nasal swab positivity rates. Several studies have shown a decrease in duration of therapy with implementation of pharmacist-driven screening test protocols.11, 12, 13 Willis et al13 showed a reduction in vancomycin therapy duration from a median of 100.8 hours to 50.4 hours with a pharmacist-driven screening test program. We were able to use the rapid turnaround time of the MRSA PCR screening test to significantly reduce vancomycin therapy duration from a median of 24.0 hours to 14.3 hours, indicating that a pharmacist-driven CPA can effectively reduce duration of therapy, even when the baseline duration is already relatively short (compared with previous studies). In fact, orders for vancomycin therapy were discontinued for 10 patients before any doses were administered as a result of the expedient use of MRSA nasal swab PCR screening tests. This reduction in duration of therapy and, in some cases, complete avoidance of unnecessary antimicrobial drug therapy represent core goals of antimicrobial stewardship programs.17 The data presented herein reinforce that pharmacist-driven MRSA nasal screening protocols can reduce unnecessary antibiotic drug exposure for patients. Dunaway et al11 showed a greater reduction in therapy duration from a median of 48 hours to 18 hours. However, their intervention involved the automatic addition of a MRSA nasal swab PCR test to the order sets for community-acquired and health care–associated pneumonia, which may have expedited screening; furthermore, patients were excluded if a MRSA test was not performed within 24 hours after admission. Pharmacists were also able to independently discontinue vancomycin therapy in the event of a negative MRSA test result; this option was not included in the CPA of our health system.
In this study, the significant decrease in vancomycin therapy duration was attained without increased rates of adverse clinical outcomes. The rates of AKI were similar between groups. No instances of vancomycin-induced ototoxicity occurred in either group. The rates of 30-day hospital readmission due to LRTIs caused by MRSA were similar between groups. This lack of a significant difference in 30-day readmission rates supports the conclusion that vancomycin was not prematurely discontinued on the basis of false-negative MRSA nasal swab PCR screening results for patients who ultimately had an LRTI caused by MRSA.
The rates of vancomycin-induced AKI observed in this study are similar to published rates.18, 19, 20 Although this study was not a priori powered to detect differences in secondary outcomes, no differences in the rates of AKI were detected between groups. This phenomenon is likely due to the fact that the duration of vancomycin therapy was less than 48 hours for 75% of patients in the preimplementation group and was further reduced to less than 30 hours for 75% of patients in the postimplementation group. Vancomycin-induced nephrotoxicity typically does not develop until therapy duration extends beyond the durations observed in this study.19
This study is not without limitations. It was conducted retrospectively and depended on the accuracy of documentation in the EHRs. In addition, detection of ototoxicity-related adverse events depended on health care providers documenting these events by using wording that exactly matched the specific term used in this study to search patient records, and it also depended on the accuracy of the search function of the EHR system.
Conclusion
This study reports a significant reduction in duration of vancomycin therapy with implementation of a pharmacist-driven CPA for MRSA nasal swab PCR screening. This reduction did not increase the 30-day hospital readmission rate for LRTIs caused by MRSA. These results contribute to data indicating that use of a pharmacist-driven CPA for MRSA nasal screening is an effective strategy for reducing the duration of vancomycin therapy in patients with LRTI without increasing adverse clinical outcomes.
Acknowledgments
Editing, proofreading, and reference verification were provided by Scientific Publications, Mayo Clinic.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Lowy F.D. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Jones B.E., Jones M.M., Huttner B., et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403–1410. doi: 10.1093/cid/civ629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffres M.N. The whole price of vancomycin: toxicities, troughs, and time. Drugs. 2017;77(11):1143–1154. doi: 10.1007/s40265-017-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kullar R., Davis S.L., Levine D.P., Rybak M.J. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 5.Jeffres M.N., Isakow W., Doherty J.A., Micek S.T., Kollef M.H. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–1115. doi: 10.1016/j.clinthera.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Dangerfield B., Chung A., Webb B., Seville M.T. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58(2):859–864. doi: 10.1128/AAC.01805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson J.A., Wright M.E., Sheperd L.A., Musher D.M., Dang B.N. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19(1):34–36. doi: 10.7812/TPP/14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith M.N., Erdman M.J., Ferreira J.A., Aldridge P., Jankowski C.A. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168–171. doi: 10.1016/j.jcrc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Giancola S.E., Nguyen A.T., Le B., et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86(3):307–310. doi: 10.1016/j.diagmicrobio.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Tilahun B., Faust A.C., McCorstin P., Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24(1):8–12. doi: 10.4037/ajcc2015102. [DOI] [PubMed] [Google Scholar]
- 11.Dunaway S., Orwig K.W., Arbogast Z.Q., Myers Z.L., Sizemore J.A., Giancola S.E. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40(3):526–532. doi: 10.1007/s11096-018-0647-3. [DOI] [PubMed] [Google Scholar]
- 12.Baby N., Faust A.C., Smith T., Sheperd L.A., Knoll L., Goodman E.L. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61(4):e02432–e02516. doi: 10.1128/AAC.02432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis C., Allen B., Tucker C., Rottman K., Epps K. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health Syst Pharm. 2017;74(21):1765–1773. doi: 10.2146/ajhp160964. [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Bayer A., Cosgrove S.E., et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 15.Kalil A.C., Metersky M.L., Klompas M., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metlay J.P., Waterer G.W., Long A.C., et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html
- 18.Hazlewood K.A., Brouse S.D., Pitcher W.D., Hall R.G. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? Am J Med. 2010;123(2):182.e1–182.e7. doi: 10.1016/j.amjmed.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meaney C.J., Hynicka L.M., Tsoukleris M.G. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34(7):653–661. doi: 10.1002/phar.1423. [DOI] [PubMed] [Google Scholar]
- 20.Cano E.L., Haque N.Z., Welch V.L., et al. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther. 2012;34(1):149–157. doi: 10.1016/j.clinthera.2011.12.013. [DOI] [PubMed] [Google Scholar]