Abstract
Purpose
Our purpose was to assess the clinical outcomes and target positioning accuracy of frameless linear accelerator single-isocenter multiple-target (SIMT) dynamic conformal arc (DCA) stereotactic radiosurgery (SRS) for multiple brain metastases (BM).
Methods and Materials
Between October 2016 and September 2018, 31 consecutive patients ≥18 years old with 204 BM <3 cm in maximum size receiving SIMT DCA SRS were retrospectively evaluated. All plans were created using a dedicated automated treatment planning software (Brainlab, Munich, Germany), and treatments were performed with a Truebeam STx or a Novalis Tx (Brainlab and Varian Medical Systems, CA). The accuracy of setup and interfraction patient repositioning was assessed by Brainlab ExacTrac radiograph 6-dimensional image system and the risk of compromised target dose coverage evaluated. Brain control and overall survival were estimated by Kaplan-Meier method calculated from the time of SRS.
Results
Fourteen patients were treated for 4 to 6 and 17 patients for 7 to 10 BM. The mean gross tumor volume (GTV) was 0.65 cm3 and the mean planning target volume (PTV) was 0.89 cm3. Mean V95 (the volume of the PTV covered by 95% of the prescription dose) and D95 (the prescription dose covering 95% of the PTV) were 99.5% and 21.1 Gy, respectively. With a median clinical follow-up of 11 months (range, 4-26 months), the 1-year survival was 68% and local control was 89%. As a consequence of plan isocenter residual errors, a loss of target coverage, defined as V95 < 95%, occurred in 28 PTVs (10 patients); using a 1 mm GTV-to-PTV margin, adequate dose coverage was maintained for all lesions.
Conclusions
SIMT DCA SRS represents a fast and effective approach for patients with up to 10 BM. The dosimetric effects of residual set-up and intrafraction positioning errors are modest, although a GTV-to-PTV margin of 1 mm is recommended.
Introduction
The clinical management of patients with brain metastases (BM) has changed substantially in the last few years, with a shift away from whole brain radiation therapy (WBRT) to stereotactic radiosurgery (SRS). Based on randomized studies, SRS has become the recommended treatment for patients with 1 to 4 BM, yielding an equivalent survival but a lower risk of long-term neurocognitive decline compared with SRS plus WBRT.1,2 Notably, similar survival and preservation of neurocognitive function has been demonstrated in patients receiving SRS for more than 5 BM.3, 4, 5
When treating multiple BM with frameless linear accelerator-based SRS, typically 1 isocenter is placed at each lesion, which is treated separately. Highly conformal radiation doses are usually employed using either dynamic conformal arc (DCA) or volumetric modulated arc therapy (VMAT); however, single-target SRS approaches may require several sessions and long treatment times, ranging from about 20 minutes for a single lesion to hours for 5 to 10 lesions.
More recently, single-isocenter multiple-target (SIMT) SRS techniques have been used for the simultaneous treatment of multiple lesions. Using SIMT SRS, all arc groups share a single isocenter located at the geometric center of all lesions, and each metastasis is treated by 1 group of volumetric arcs in a single session. Dose delivery accuracy and conformality are achieved through the use of noncoplanar arcs and simultaneous variation of multileaf collimator leaf positions.6, 7, 8 Several studies demonstrated that SIMT SRS techniques, either DCA or VMAT, provide excellent plan dosimetry and conformity consistent with those achieved with single-target SRS,9, 10, 11, 12, 13 although data related to patient outcomes and repositioning accuracy when treating multiple BM are scarce. The major concern is that rotational and translational deviations of the plan isocenter, usually chosen as the geometric center of all target volumes, may result in a significant compromised target coverage for lesions at greater distance from the isocenter.14, 15, 16, 17 In a series of 50 SIMT VMAT SRS plans for intracranial lesions, Roper et al14 showed no significant loss of target volume coverage when a plan isocenter rotational error of 0.5° was simulated, although rotational errors up to 2° resulted in significant loss of target coverage in more than one-third of cases, especially in smaller targets distant from the isocenter. Similar results have been reported in a few other studies assessing the plan quality of single-isocenter SRS,15, 16, 17 suggesting that larger gross tumor volume (GTV)-to-planning target volume (PTV) margins would be required with SIMT SRS compared with single-target SRS.16,17
In the present study, we report our initial clinical experience in patients treated with SIMT DCA SRS for up to 10 BM using a dedicated automated treatment planning software. Clinical outcomes and target positioning accuracy have been evaluated. In addition, we assess the risk of compromised target coverage due to residual isocenter errors.
Methods and Materials
Between October 2016 and September 2018, 31 consecutive patients ≥18 years old with 4 to 10 BM <3 cm in maximum size receiving SIMT DCA SRS at UPMC Hillman Cancer Center San Pietro Hospital, Rome, and IRCCS Sacro Cuore Don Calabria Hospital, Negrar, were retrospectively evaluated. Radiographic and pathologic information was drawn from a prospectively maintained database of patients with brain tumors treated at our institution. All lesions were treated with frameless linear accelerator-based SRS using a commercial stereotactic mask fixation system (Brainlab, Feldkirchen, Germany). The GTV was contoured on postcontrast thin-slice (0.6-1 mm) gadolinium-enhanced T1-weighted axial magnetic resonance imaging (MRI) sequences fused to the treatment planning computed tomography (CT), which was acquired at 0.625 mm slice spacing. To compensate for uncertainties, the PTV was generated by the geometric expansion of GTV plus 1 (n = 176) or 2 (n = 28) mm.
After treatment, all patients were clinically examined every 2 months. At each visit, the neurologic status and the severity of complications were rated according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 5.18 MRI was performed every 2 months in the first year after treatment and then every 3 to 4 months or as appropriate. Complete and partial responses were defined according to the Response Assessment in Neuro-Oncology criteria.19
SRS procedure
SIMT DCA SRS plans were created using commercial software (Brainlab Elements Multiple Brain Mets SRS, version 1.5). Software specifics have been described in detail elsewhere.20 In brief, the software automatically creates an inversely optimized conformal SRS plan to target multiple BM with a single isocenter using an enhanced multiple DCA technique, with each arc delivering dose to a subgroup of targets. No monitor unit or gantry speed modulation is applied. After selection of an arc geometry template consisting of 10 noncoplanar DCA beams at 5 preset couch positions, the isocenter location is automatically determined as the center of mass of all target volumes. The start and stop angles of each arc are first set to default values (10°~170° when couch angle ranges from 0°~90° and 190°~350° when couch angle ranges from 270°~360° per International Eectrotechnical Commission 61217 convention) and then automatically modified during optimization. Metastases lining up in the direction of leaf-motion are not treated simultaneously to restrict normal tissue exposure. An inverse planning algorithm is used for optimizing beam margins, gantry span, multileaf collimator angles, and apertures with respect to target coverage and critical organ sparing, dose conformity, and monitor unit efficiency.
Prescribed doses were 20 to 22 Gy for lesions <2 cm and 16 to 18 Gy for those ≥2 cm in size or in close proximity to sensitive brain regions, for example, brain stem or the optic chiasm. Each PTV was usually encompassed by at least 98% of the prescribed dose while keeping the maximum dose <120%. Maximum doses to the brain stem, optic apparatus, and lens were 12, 8, and 2 Gy, respectively.
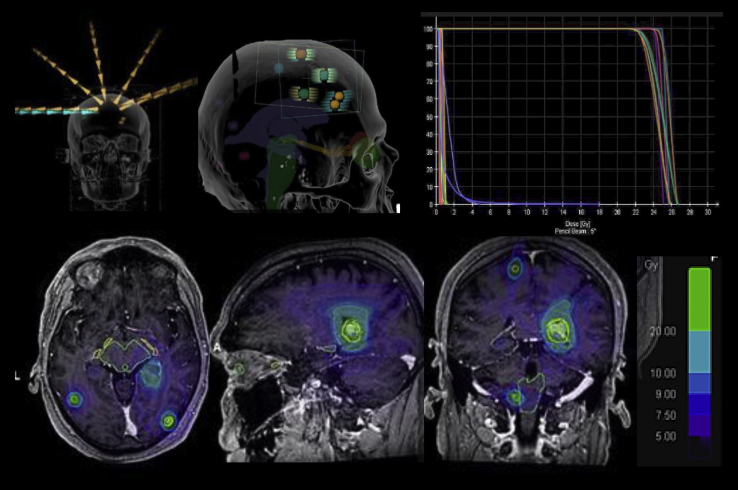
An example of SIMT DCA SRS treatment in a patient presenting with 10 BM is shown in Figure 1. The treatment was performed with a TrueBeam STx (Varian Medical Systems, Paolo Alto, CA) or a Novalis Tx (Brainlab and Varian Medical Systems) equipped with 120 leaves with a leaf width of 5 mm or 2.5 mm in the central 8 cm and capable of performing couch adjustments in 6 degrees of freedom.
Figure 1.
Example of a treatment plan using single-isocenter multitarget (SIMT) dynamic conformal arc (DCA) stereotactic radiosurgery (SRS) in a patient with 9 brain lesions. The total volume of brain disease was 2.1 cm3. A gross tumor volume (GTV)-to-planning target volume (PTV) margin of 1 mm was used, with 22 Gy prescribed to each target in a single session. Treatment was delivered with 5 dynamic conformal arcs.
During each treatment, the accuracy of patient set-up was assessed with the Brainlab stereoscopic ExacTrac kV X-Ray 6-dimensional (6D) image guided radiation therapy system (version 5.5). Patient setup was automatically obtained by correlating the position of the planning CT isocenter with the position of the infrared markers located on the cranial reference array using the 3-dimensional coordinates generated by the frameless stereotactic localizer during CT simulation. Then, positioning accuracy was achieved by matching radiograph images with reference digitally reconstructed radiographies generated from the simulation CT data set. After applying the translational and rotational shifts, a second set of radiograph verification images was taken to confirm the accuracy of the isocenter position. If the residual errors after corrections resulted within a tolerance limit of 0.5 mm for translational shifts and 0.5° for rotational shift, the treatment was delivered without further correction. In addition, a kilo-voltage cone beam CT was acquired to assess offline the accuracy of dose distribution in the target volumes (see the following discussion). During the treatment, stereoscopic radiograph imaging was repeated at different times to verify and correct the isocenter position.
SRS accuracy and data analysis
The accuracy of target geometry and dosimetry was evaluated using Velocity AI software (version 3.0.2, Varian Medical Systems), where the cone beam CT was fused offline to the planning CT. After applying the residual isocenter translations and rotations, the V95 (the volume of the PTV covered by 95% of the prescription dose) and the D95 (the prescription dose that covers 95% of the PTV) were assessed for each target. The V95 data were categorized at levels above and below 95%. Time-to-event analyses were estimated using the Kaplan-Meier method from the date of SRS. The log rank test and Cox regression model were used to assess the effects of clinical/treatment variables on outcomes at univariate analysis. The 3-dimensional displacement, determined as the square root of the sum of the squares of the displacements in all 3 directions, was calculated. The Fisher exact test was performed for comparison of results between 2 groups or variables. The Spearmen’s rank test was used for test of correlation. Statistical significance was considered at P values of <.05. A standard software was used for statistical analysis (XLSTAT software).
Results
Patient characteristics
Thirty-one patients (15 men and 16 women) with a total of 204 BM who underwent SIMT DCA SRS between October 2016 and September 2018 were analyzed. Patient and tumor characteristics are listed in Table 1. The median age at the time of SRS was 60 years (range, 39-78). Twenty patients were treated for 4 to 7 and 11 patients for 8 to 10 BM. The median GTV was 0.40 cm3 and the median PTV was 0.68 cm3. Median conformity index (volume covered by reference dose/PTV) and gradient index (volume irradiated by 50%/volume irradiated by 100% of the prescribed dose) were 1.3 and 3.9, respectively. With a median distance of 39 mm from the planning isocenter for all patients, mean V95 and D95 were 99.5% and 21.1 Gy, respectively. Individual treatment and dosimetric characteristics are summarized in Table E1. Systemic therapy, immunotherapy (n = 4), or molecular targeted agents (n = 5) were given at the time of SRS in 9 patients. Data were reported to September 2019. At this time, 13 (42%) patients are alive.
Table 1.
Summary of patients' characteristics and treatment parameters
| Parameter | No. |
|---|---|
| Number of patients | 31 |
| Median age | 60 |
| Sex (F/M) | 16/15 |
| Histology | |
| Lung | 14 |
| Breast | 5 |
| Melanoma | 8 |
| Ovary | 1 |
| Kidney | 3 |
| No. of lesions per patient | |
| 4-7 lesions | 20 |
| 8-10 lesions | 11 |
| Tumor location | |
| Frontal | 60 (29.4%) |
| Parietal | 42 (20.6%) |
| Temporal | 41 (20%) |
| Cerebellar | 40 (19.7%) |
| Occipital | 21 (10.3%) |
| SRS dose | |
| 22 Gy | 71 (34.8%) |
| 20 Gy | 115 (56.3%) |
| 16/18 Gy | 18 (8.9%) |
| GTV (cm3) | |
| Mean (SD) | 0.65 (0.40) |
| Median (range) | 0.40 (0.07-3.8) |
| Mean total GTV (SD) | 3.9 (1.93) |
| Median total GTV (range) | 3.8 (1.4-9.9) |
| PTV (cm3) | |
| Mean (SD) | 0.89 (0.42) |
| Median | 0.68 (0.18-6.1) |
| Mean total PTV | 6.6 (2.5) |
| Median total PTV | 6.5 (2.6-13.2) |
| Distance from isocenter | |
| Median (range) | 39 (0.6-77) |
| Mean (SD) | 38.6 (7.5) |
| Conformity index | |
| Median (range) | 1.32 (1.1-1.6) |
| Mean (SD) | 1.3 (0.05) |
| Gradient index | |
| Median (range) | 3.94 (2.78-5.60) |
| Mean (SD) | 3.8 (0.27) |
| D95 (Gy) | |
| Mean (SD) | 21.1 (0.34) |
| Median (range) | 20.8 (17.6-24.1) |
| V95 (%) | |
| Mean (SD) | 99.5 (0.3) |
| Median (range) | 99.0 (92.4-100) |
Abbreviations: GTV = gross tumor volume; PTV = planning target volume; SD = standard deviation; SRS = stereotactic radiosurgery.
Clinical outcomes and toxicity
With a median clinical follow-up of 11 months (range, 4-26 months), the 1-year and 2-year local control rates were 89% and 77%, respectively. A local failure of at least 1 of the treated BM occurred in 4 (13%) patients at a median time of 10 months. Seventy-six (37.3%) lesions had a complete response, 68 (33.3%) had a partial response, 49 (24%) remained stable, and 11 (5.4%) recurred. For all lesions, the estimated 1-year and 2-year local control rates were 94% and 86%, respectively. The median survival time was 16.1 months; the 1-year and 2-year survival rates were 68% and 40%; the median distant brain failure time and 1-year rates were 8 months and 38%. In cases showing brain progression, salvage WBRT was performed in 3 patients and further SRS in 15 patients. Controlled extracranial disease (P = .027) and concomitant systemic therapy (P = .033) were significantly correlated with longer survival; cumulative intracranial tumor volume and the Karnofsky performance status (KPS) were of borderline significance (P = .07 and P = .09). No factors were associated with local and distant brain failure; however, combining systemic treatment and SRS showed a trend throughout of better distant brain control (P = .07).
At a median time of 2 months after the treatment, a clinical improvement was recorded in 6 out of 8 patients with pre-SRS neurologic symptoms. KPS scores improved, remained stable, or worsened in 10, 14, and 2 patients, respectively. The treatment was well tolerated with no grade 3 or 4 acute or long-term toxicities after SRS. Radiologic changes suggestive of brain necrosis of at least 1 lesion, as suggested by MR and positron emission tomography-CT imaging, occurred in 5 (16.1%) patients at a median time of 10 months after the treatment, and were associated with grade 2 motor deficits in 2 patients. For all lesions, the estimated risk of radionecrosis was 11% at 12 months; individual V12Gy > 10 cm3 was significantly correlated with the risk (P = .01). In contrast, number of lesions, target volumes, cumulative intracranial total volumes, and the total volume of normal brain (brain volume minus cumulative GTVs) exposed to doses of 8 to 16 Gy were not a predictor of radionecrosis.
Geometric and dosimetric deviations of target volumes
The mean residual translational and rotational shifts of the treatment isocenter position after matching pretreatment radiograph verification images with the digitally reconstructed radiographies are shown in Table 2. The mean residual rotational errors were –0.13° for the vertical rotation (pitch), 0.1° for the longitudinal rotation (roll), and –0.08° for the lateral rotation (jaw). The mean translational displacements were –0.09 mm in the lateral (x) direction, 0.15 mm in the vertical (y) direction, and –0.18 mm in the longitudinal (z) direction. The maximum translational and rotational residual errors were 0.44 mm and 0.4° seen in the longitudinal direction and z-axis, respectively. The mean 3-dimensional residual error determined by the square root of the sum of the squares of all translations was 0.34 mm (range, 0.1-0.53).
Table 2.
Mean residual planning isocenter setup errors and their effect on target volume geometric and dosimetric deviations
| Parameter | Mean distance from isocenter | X (lateral) | y (vertical) | z (longitudinal) | 3D vector | Pitch (x) | Yaw (y) | Roll (z) | Mean nonoverlapping GTV % | Mean nonoverlapping PTV % | Mean V95 variation % | Mean D95 variation % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 34.7 | –0.09 | 0.15 | –0.18 | 0.34 | –0.13 | –0.08 | 0.2 | 12.6∗ | 6.1† | 1.34‡ | 1.5§ |
| SD | 17.1 | 0.18 | 0.2 | 0.23 | 0.30 | 0.12 | 0.16 | 0.18 | 19 | 10 | 2.3 | 2.6 |
| Range | 0.22-76.3 | –0.31 to 0.28 | –0.36 to 0.37 | –0.36 to 0.44 | 0.1-0.53 | –0.22 to 0.28 | –0.33 to 0.32 | –0.35 to 0.42 | 0-58 | 0-44 | 0-9.2 | 0-24.5 |
Abbreviations: 3D = 3-dimensional; GTV = gross tumor volume; PTV = planning target volume; SD = standard deviation.
>10% in 84 lesions.
>10% in 48 lesions.
>5% in 22 PTVs.
>5% in 25 PTVs.
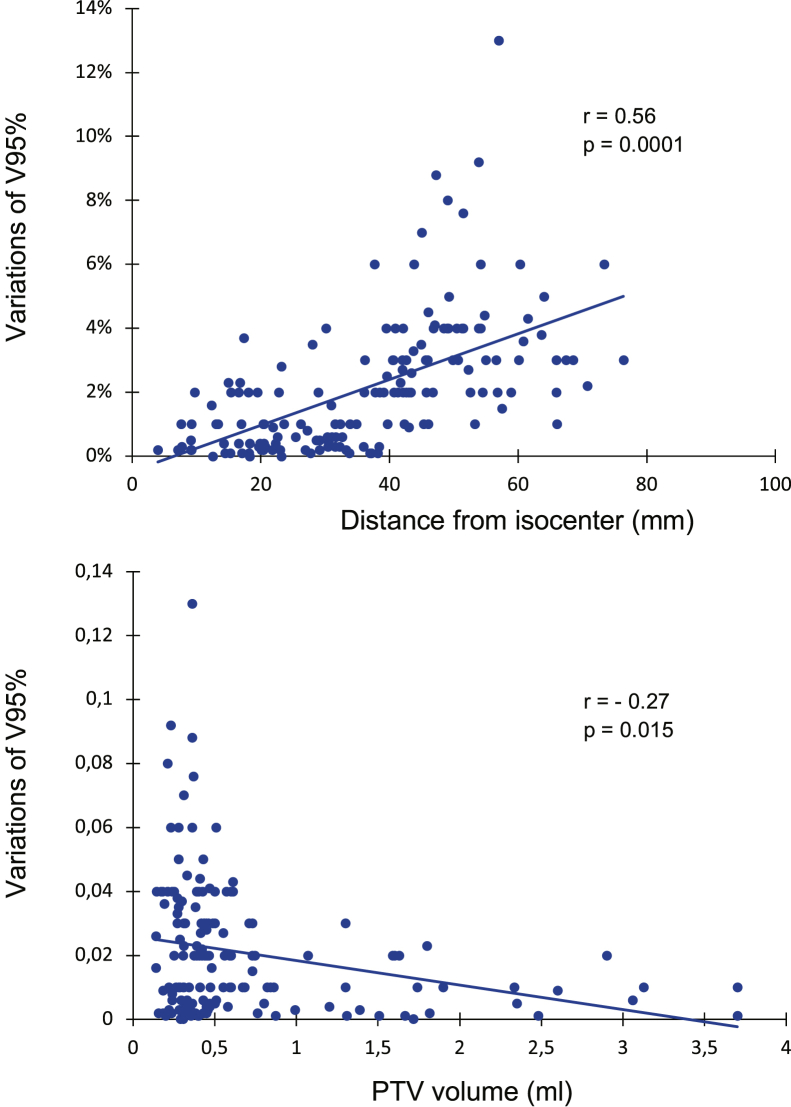
As a consequence of plan isocenter residual errors, mean geometric deviations of PTV and GTV were 6.1% and 12.6%, respectively. Nonoverlapping GTV and PTV >10% were observed in 84 (41%) and 48 (23.5%) targets, respectively. With a mean variation of V95 of 1.34% for all targets, a significant loss of target coverage (V95 < 95%) occurred in 28 targets (12 patients), and this was associated with a variation of D95 > 5% in 22 targets. Factors associated with target coverage were the volume of lesions and the distance from isocenter (Fig 2). With a median distance of 39 mm from the plan isocenter, a loss of target coverage was seen in 7 and 21 lesions located at a distance of <39 mm and ≥39 mm, respectively (P = .0001). According to the target volumes, a loss of target dose coverage was seen in 20 lesions with a target volume <0.40 cm3 and 8 lesions with a target volume ≥0.40 cc (P = .015).
Figure 2.
Spearman correlation showing a significant correlation between variation of V95 and size of lesion and distance from isocenter.
To define the optimal margin strategy for delivering the prescribed dose during the treatment, we evaluated the effect of different GTV-to-PTV margins for all targets. A GTV-to-PTV margin of 1 mm, as used in more than 85% of treated lesions, was able to ensure an adequate dose coverage for all treated lesions; when no additional margins were simulated, dose coverage remained significantly compromised in 18 lesions. Finally, we assessed the dosimetric effect of intrafraction target motion (Table 3). Based on ExacTrac image registrations, 10 isocenter positionings in 7 (22%) patients were out of tolerance, requiring corrections. For those, the maximum rotational and translational shifts were 0.8° and 0.7 mm, respectively, leading to a significant loss of GTV dose coverage in 21 (28%) of 74 lesions. With a GTV-to-PTV margin of 1 mm, as applied in our study, an adequate GTV dose coverage was maintained in all but 2 lesions.
Table 3.
Intrafraction translational and rotational shifts of treatment planning isocenter∗
| Timing | x (lateral) | y (vertical) | z (longitudinal) | 3D vector | Pitch (x) | Yaw (y) | Roll (z) |
|---|---|---|---|---|---|---|---|
| First mid treatment (<15') | –0.14 (0.29) | 0.18 (0.22) | –0.18 (0.28) | 0.32 (0.38) | –0.09 (0.28) | 0 (0.25) | 0.08 (0.33) |
| Second mid treatment (15-30') | –0.13 (0.37) | 0.22 (0.33) | –0.21 (0.4) | 0.41 (0.36) | –0.14 (0.30) | –0.05 (0.27) | 0.1 (0.33) |
| Shifts out of tolerance | |||||||
| First mid treatment | 3 of 31 (10%) | ||||||
| Second mid treatment | 7 of 38 (18%) |
Abbreviations: 3D = 3-dimensional; SD = standard deviation.
Based on 69 ExacTrac verifications. Tolerance values were 0.5 mm for translations and 0.5° for rotations.
Discussion
An essential prerequisite of the SIMT SRS technique is that multiple-target positioning is performed with a high degree of accuracy because small translational and rotational setup errors of the planning isocenter may produce significant loss of dose coverage of irradiated target volumes.14, 15, 16, 17 In our study, a significant loss of target coverage occurred in 14% of irradiated lesions, with the largest dosimetric changes seen for smaller volumes located far away from the plan isocenter. Analysis of dose variation showed that about 90% of the missing target coverage, as defined by V95 < 95%, was observed in lesions <0.4 cm3 that were located ≥3.9 cm distal to the treatment plan isocenter; however, dose coverage was maintained for all lesions after applying a GTV-to-PTV margin of 1 mm. Overall, our study indicates that residual setup rotational and translational isocenter errors during SIMT SRS have relatively little dosimetric effect, and small PTV margins are sufficient to assure adequate dose coverage of all lesions.
The finding that a small GTV-to-PTV margin of 1 mm may be applied during SIMT SRS without compromising target dose coverage is of paramount importance in clinical practice because larger GTV-to-PTV margins are expected to increase the risk of radiation-induced brain necrosis.21, 22, 23 Using V12Gy as a predictor of radionecrosis, Korytko et al21 observed a risk of 55.3% for V12Gy > 10 cm3 versus 22.5% for V12Gy < 10 cm3 in 198 intracranial tumors receiving gamma knife SRS. A significant correlation between V12Gy and the development of radionecrosis has been reported in other studies, showing a risk up to 69% for volumes larger than 10.8 cm3.22,23 A few examples of variations of target volumes using different margin strategies are shown in Table 4. For a spherical lesion of 1 cm (0.52 cm3), a margin expansion of 1, 2, and 3 mm results in an increase of the original volume of about 80%, 180%, and 330%, respectively, causing an expansion of perilesional normal brain irradiated at 8 to 16 Gy doses up to 4 to 6 times. Considering the benefit of keeping the V12Gy lower than 5 to 10 cc to reduce the risk of brain necrosis, the use of 1-mm margin may explain the relatively low risk of symptomatic radionecrosis after SIMT SRS observed in our study.
Table 4.
Simulation of target volume changes after applying different GTV-to PTV margins
| Diameter of spherical lesion | GTV-to-PTV margin 0 mm | GTV-to-PTV margin 1 mm | GTV-to-PTV margin 2 mm | GTV-to-PTV margin 3 mm |
|---|---|---|---|---|
| 0.5 cm | 0.07 cm3 | 0.18 cm3 | 0.38 cm3 | 0.7 cm3 |
| 1 cm | 0.52 cm3 | 0.9 cm3 | 1.43 cm3 | 2.21 cm3 |
| 1.5 cm | 1.76 cm3 | 2.57 cm3 | 3.59 cm3 | 4.85 cm3 |
| 2 cm | 4.18 cm3 | 5.57 cm3 | 7.23 cm3 | 9.2 cm3 |
Abbreviations: GTV = gross tumor volume; PTV = planning tumor volume.
A further essential characteristic of SIMT SRS is that accurate isocenter positioning is maintained during the treatment to ensure precise dose delivery to all targets. Analysis of intrafraction motion showed that isocenter deviations out of tolerance requiring correction occurred in about 15% of registrations, consistent with previous studies.24, 25, 26 For these patients, the effect of uncorrected rotational and translational shifts showed a significant loss of GTV dose coverage in about 28% of lesions, indicating that a continuous monitoring and correction of intrafraction motion is needed if small GTV-to-PTV margins are applied. In this regard, a robust clinical implementation of quality assurance measures concerning imaging, planning, and delivery to optimize the treatment is required during SIMT SRS. For example, postcontrast thin-slice (0.6-1 mm) gadolinium-enhanced T1-weighted axial MRI sequences acquired no more than 1 week from treatment and 6D intrafraction motion monitoring systems, as used in the current study. Other strategies to maximize treatment efficiency include distortion correction of MR imaging and the use of flattening filter photon beams that ensure a lower peripheral dose and shorten the treatment time.27
Together with the accuracy of target positioning, we evaluated the quality of our automated treatment planning software. Median conformity index and gradient index, which are commonly used to describe SRS quality planning, were 1.32 and 3.94, respectively, consistent with those reported in other published studies using either DCA or VMAT techniques.8, 9, 10, 11, 12, 13,28, 29, 30 In a comparative study of SIMT DCA and VMAT SRS plans generated by the commercially available automated planning software Multiple Brain Metastases (Brainlab) and HyperArc (Varian), respectively, Ruggeri et al29 observed comparable high plan quality in 20 patients with 2 to 10 BM, although the conformity index was slightly better for VMAT plans. In another study comparing SIMT DCA to VMAT SRS plans in 30 patients with 4 to 10 BM, Liu et al30 showed that the VMAT technique resulted in better conformity and V12Gy-8Gy volumes; in contrast, other studies suggest that DCA plans perform better than VMAT in terms of healthy brain sparing and treatment efficiency.13,28 Although such indexes remain of interest to assess the dosimetric quality of treatment plans, the correlation between planning quality and clinical outcomes remains to be clarified,31 and the superiority of one technique over another in terms of plan quality metrics is unsustained.
Because the ultimate validity of a procedure is measured in terms of clinical results, we have examined local control as significant outcome for assessing the accuracy of treatment delivery. Local control of 89% at 1 year is consistent with published results of single-target SRS3, 4, 5 and single-isocenter SRS32,33 in patients with up to 10 BM, confirming that SIMT SRS is a convenient treatment option that offers high local control and significant reduction of treatment delivery time compared with other techniques.34 The treatment was safe, with no grade 3 or 4 neurologic toxicity. For all lesions, the estimated risk of radionecrosis was 11% at 12 months, which is consistent with that reported by others.4,5,22,23,32,33 KPS scores improved or remained stable in the majority of patients with stable disease during the follow-up, without clinically evident neurocognitive impairment. Although our clinical findings are of some reassurance about the safety of SIMT SRS in patients with cumulative tumor volumes of less than 15 cm3, prospective studies with formal neuropsychological testing are needed to elucidate the effect of different factors on neurocognitive outcome, including number of lesions, total tumor volume, and radiation doses to normal brain volume and hippocampi.
In conclusion, our clinical experience indicates that SIMT DCA SRS offers high local control with an acceptable rate of toxicity in patients with multiple BM, representing a convenient approach associated with significantly reduced treatment time compared with single-target SRS techniques. With setup and intrafraction positioning and correction based on the ExacTrac X-Ray 6D imaging system, the effect of residual translational and rotational errors on GTV coverage is modest. The use of GTV-to-PTV margins and monitoring and correction of intrafraction motion are recommended in clinical practice to avoid significant loss in target coverage.
Footnotes
Sources of support: This research was not funded by external grants.
Disclosures: The authors declare no conflict of interest.
Research data are not available at this time.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.06.008.
Supplementary data
References
- 1.Kocher M., Soffietti R., Abacioglu U. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandhi R., Kondziolka D., Panczykowski D. Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg. 2012;117:237–245. doi: 10.3171/2012.4.JNS11870. [DOI] [PubMed] [Google Scholar]
- 4.Hunter G.K., Suh J.H., Reuther A.M. Treatment of five or more brain metastases with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:1394–1398. doi: 10.1016/j.ijrobp.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M., Serizawa T., Shuto T. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 6.Clark G.M., Popple R.A., Young P.E., Fiveash J.B. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys. 2010;76:296–302. doi: 10.1016/j.ijrobp.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Nath S.K., Lawson J.D., Simpson D.R. Single-isocenter frameless intensity-modulated stereotactic radiosurgery for simultaneous treatment of multiple brain metastases: Clinical experience. Int J Radiat Oncol Biol Phys. 2010;78:91–97. doi: 10.1016/j.ijrobp.2009.07.1726. [DOI] [PubMed] [Google Scholar]
- 8.Huang C., Ren L., Kirkpatrick J., Wang Z. SU-E-T-645: Treatment of multiple brain metastases using stereotactic radiosurgery with single-isocenter volumetric modulated arc therapy: Comparison with conventional dynamic conformal arc and static beam stereotactic radiosurgery. Med Phys. 2012;39:3854. doi: 10.1118/1.4735734. [DOI] [PubMed] [Google Scholar]
- 9.Lau S.K., Zakeri K., Zhao X. Single-isocenter frameless volumetric modulated arc radiosurgery for multiple intracranial metastases. Neurosurgery. 2015;77:233–240. doi: 10.1227/NEU.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limon D., McSherry F., Herndon J. Single fraction stereotactic radiosurgery for multiple brain metastases. Adv Radiat Oncol. 2017;2:555–563. doi: 10.1016/j.adro.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang J., Wernicke A.G., Pannullo S.C. Restricted single isocenter for multiple targets dynamic conformal arc (RSIMT DCA) technique for brain stereotactic radiosurgery (SRS) planning. J Radiosurg SBRT. 2018;5:145–156. [PMC free article] [PubMed] [Google Scholar]
- 12.Ruggieri R., Naccarato S., Mazzola R. Linac-based VMAT radiosurgery for multiple brain lesions: Comparison between a conventional multi-isocenter approach and a new dedicated mono-isocenter technique. Radiat Oncol. 2018;13:38. doi: 10.1186/s13014-018-0985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmaier J., Bodensohn R., Garny S. Single isocenter stereotactic radiosurgery for patients with multiple brain metastases: Dosimetric comparison of VMAT and a dedicated DCAT planning tool. Radiat Oncol. 2019;14:103. doi: 10.1186/s13014-019-1315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roper J., Chanyavanich V., Betzel G., Switchenko J., Dhabaan A. Single-isocenter multiple-target stereotactic radiosurgery: Risk of compromised coverage. Int J Radiat Oncol Biol Phys. 2015;93:540–546. doi: 10.1016/j.ijrobp.2015.07.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison J., Hood R., Yin F.F., Salama J.K., Kirkpatrick J., Adamson J. Is a single isocenter sufficient for volumetric modulated arc therapy radiosurgery when multiple intracranial metastases are spatially dispersed? Med Dosim. 2016;41:285–289. doi: 10.1016/j.meddos.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Stanhope C., Chang Z., Wang Z. Physics considerations for single-isocenter, volumetric modulated arc radiosurgery for treatment of multiple intracranial targets. Pract Radiat Oncol. 2016;6:207–213. doi: 10.1016/j.prro.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Ezzell G.A. The spatial accuracy of two frameless, linear accelerator-based systems for single-isocenter, multitarget cranial radiosurgery. J Appl Clin Med Phys. 2017;18:37–43. doi: 10.1002/acm2.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events (CTCAE), v4.03. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06 Available at: [Google Scholar]
- 19.Lin N.U., Lee E.Q., Aoyama H. Response Assessment in Neuro-Oncology (RANO) group. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 20.Gevaert T., Steenbeke F., Pellegri L. Evaluation of a dedicated brain metastases treatment planning optimization for radiosurgery: A new treatment paradigm? Radiat Oncol. 2016;11:13. doi: 10.1186/s13014-016-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korytko T., Radivoyevitch T., Colussi V. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419–424. doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 22.Blonigen B.J., Steinmetz R.D., Levin L., Lamba M.A., Warnick R.E., Breneman J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Minniti G., Clarke E., Lanzetta G. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;15:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishna N., Rosca F., Friesen S., Tezcanli E., Zygmanszki P., Hacker F. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol. 2010;95:109–115. doi: 10.1016/j.radonc.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Gevaert T., Verellen D., Tournel K. Setup accuracy of the Novalis ExacTrac 6DOF system for frameless radiosurgery. Int J Radiat Oncol Biol Phys. 2012;82:1627–1635. doi: 10.1016/j.ijrobp.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 26.Gevaert T., Boussaer M., Engels B. Evaluation of the clinical usefulness for using verification images during frameless radiosurgery. Radiother Oncol. 2013;108:114–117. doi: 10.1016/j.radonc.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Hartgerink D., Swinnen A., Roberge D. LINAC based stereotactic radiosurgery for multiple brain metastases: Guidance for clinical implementation. Acta Oncol. 2019;58:1275–1282. doi: 10.1080/0284186X.2019.1633016. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y., Chin K., Robbins J.R. Radiosurgery of multiple brain metastases with single-isocenter dynamic conformal arcs (SIDCA) Radiother Oncol. 2014;112:128–132. doi: 10.1016/j.radonc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Ruggieri R., Naccarato S., Mazzola R. Linac-based radiosurgery for multiple brain metastases: Comparison between two mono-isocenter techniques with multiple non-coplanar arcs. Radiother Oncol. 2019;132:70–78. doi: 10.1016/j.radonc.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Liu H., Thomas E.M., Li Interinstitutional plan quality assessment of 2 linac-based, single-isocenter, multiple metastasis radiosurgery techniques. Adv Radiat Oncol. 2020 doi: 10.1016/j.adro.2019.10.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torrens M., Chung C., Chung H.T. Standardization of terminology in stereotactic radiosurgery: Report from the Standardization Committee of the International Leksell Gamma Knife Society: Special topic. J Neurosurg. 2014;121:2–15. doi: 10.3171/2014.7.GKS141199. [DOI] [PubMed] [Google Scholar]
- 32.Minniti G., Capone L., Nardiello B. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neurooncol. 2020;148:47–55. doi: 10.1007/s11060-020-03442-7. [DOI] [PubMed] [Google Scholar]
- 33.Palmer J.D., Sebastian N.T., Chu J. Single-isocenter multitarget stereotactic radiosurgery is safe and effective in the treatment of multiple brain metastases. Adv Radiat Oncol. 2020;5:70–76. doi: 10.1016/j.adro.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas E.M., Popple R.A., Wu X. Comparison of plan quality and delivery time between volumetric arc therapy (RapidArc) and Gamma Knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014;75:409–417. doi: 10.1227/NEU.0000000000000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.