Abstract
Purpose
The dosimetric parameters used clinically to reduce the likelihood of radiation pneumonitis (RP) for lung cancer radiation therapy have traditionally been V20Gy ≤ 30% to 35% and mean lung dose ≤ 20 to 23 Gy; however, these parameters are derived based on studies from photon therapy. The purpose of this study is to evaluate whether such dosimetric predictors for RP are applicable for locally advanced non-small cell lung cancer (LA-NSCLC) patients treated with proton therapy.
Methods and Materials
In the study, 160 (78 photon, 82 proton) patients with LA-NSCLC treated with chemoradiotherapy between 2011 and 2016 were retrospectively identified. Forty (20 photon, 20 proton) patients exhibited grade ≥2 RP after therapy. Dose volume histograms for the uninvolved lung were extracted for each patient. The percent lung volumes receiving above various dose levels were obtained in addition to V20Gy and Dmean. These dosimetric parameters and patient characteristics were evaluated with univariate and multivariate logistic regression tests. Receiver operating characteristic curves were generated to obtain the optimal dosimetric constraints through analyzing RP and non-RP sensitivity and specificity values.
Results
The multivariate analysis showed V40Gy and Dmean to be statistically significant for proton and photon patients, respectively. V35Gy to V50Gy were strongly correlated to V40Gy for proton patients. Based on the receiver operating characteristic curves, V35Gy to V50Gy had the highest area under the curve compared with other dose levels for proton patients. A potential dosimetric constraint for RP predictor in proton patients is V40Gy ≤ 23%.
Conclusions
In addition to V20Gy and Dmean, the lung volume receiving higher doses, such as V40Gy, may be used as an additional indicator for RP in LA-NSCLC patients treated with proton therapy.
Introduction
Lung cancer is the leading cause of cancer-related death, with more than 80% of patients presenting with non-small cell lung cancer (NSCLC). Of these patients, approximately one-third will be diagnosed with locally advanced (LA) disease, which is typically treated with concurrent chemoradiotherapy (CRT), and since 2018, followed by immunotherapy for a year. Radiation pneumonitis (RP) is one of the most common toxicities affecting lung cancer patients, with about 15% to 20% of patients developing RP,1 which is only going to get higher in the era of immunotherapy.2,3 Traditionally, patients with LA-NSCLC have been treated using photon-based external beam radiation therapy. However, proton therapy is becoming increasingly available in the United States and worldwide, with a growing number of proton centers opening. This provides an alternative option for treating LA-NSCLC.4 Compared with photon radiation therapy, proton radiation therapy provides superior sparing of low dose to the healthy lung, esophagus, and heart.4, 5, 6, 7, 8, 9 Sejpal et al found that proton therapy could result in less normal-tissue toxicity than photon radiation therapy when treating LA-NSCLC owing to significant sparing of organs at risk.7
Many studies have been performed to determine clinical and treatment characteristics that may influence the development of RP, such as pulmonary function before CRT,10 tumor location,11 concurrent chemotherapy,11 total dose,12 dose per fraction,13 and smoking status.12,14 Dosimetric constraints have also been investigated to evaluate likelihood of developing RP. The standard dosimetric parameters used clinically to reduce the likelihood of RP have traditionally been V20Gy (percent volume receiving greater than or equal to 20 Gy) ≤ 30% to 35%13,15, 16, 17 and mean lung dose ≤ 0 to 23 Gy.13,16, 17, 18, 19 Jin et al studied patients with NSCLC who were treated with definitive CRT and patients treated with RT without chemotherapy and found that meeting constraints of the combination of V20 < 25%, V25 < 20%, V35 < 15%, and V50 < 10% results in a 2% chance of RP incidence regardless of treatment regimen.14
However, most studies investigating dose constraints to reduce lung toxicities are based on data from photon therapy. There are preliminary studies that investigated RP prediction in proton cohorts.20 Owing to the inherent dose distribution differences in photon and proton lung treatment, the dose predictors derived to reduce the risk of RP in photon therapy patients may no longer hold true for proton therapy patients. Our study identified cohorts of LA-NSCLC patients treated with either photon and proton therapy and studied the differences in the correlation of the dosimetric parameters with the incidences of RP for proton therapy.
Methods and Materials
Proton dose prescription
All proton treatments were prescribed in Gy (relative biologic effectiveness [RBE]) in which a constant of RBE of 1.1 was used. For simplicity, proton Gy (RBE) will be represented as Gy in this report.
Patient selection
The institutional review board approved this study. Between 2011 and 2016, 160 patients (78 photon, 82 proton) with LA-NSCLC were treated with definitive intent with either concurrent or sequential CRT. No patients in the study received adjuvant immunotherapy. To be eligible for this study, the patient had to complete therapy to a prescription of at least 60 Gy. The exclusion criteria included patients with prior thoracic treatments or patients treated with combination therapy (photon and proton in the same course of treatment). The patients’ characteristics, including modality, sex, age, stage of cancer, tumor location, chemotherapy regimen, chemotherapy type, and smoking status were collected. The grade of RP after treatment was graded using Common Terminology Criteria for Adverse Events version 4.0.21 In this cohort, 40 patients (20 photon, 20 proton) exhibited grade ≥2 RP (required medical intervention) after photon or proton therapy. Five out of the 82 proton patients were treated with pencil beam scanning, and the rest were treated with double scattering technique. Twenty-six out of the 78 photon patients were treated with 3-dimensional CRT, and the rest of the photon patients were treated with intensity modulated radiation therapy (IMRT) or volumetric arc therapy. Table 1 shows a summary of the patient cohort characteristics for RP versus non-RP patients. The dose volume histograms for the uninvolved lung region, defined as lungs minus internal gross tumor volume (IGTV), were generated from the patient treatment plans. In addition to the traditional lung dosimetric parameters V20Gy and mean lung dose, the percent lung volume receiving above various dose levels from V5Gy to V70Gy in increments of 5 Gy were obtained from the dose-volume histograms. Table 2 shows patient characteristics and for proton versus photon patients to help understand the baseline characteristic differences between the 2 cohorts.
Table 1.
Patient characteristics
| Pneumonitis (grade ≥2) | Nonpneumonitis | P value | |
|---|---|---|---|
| Modality | |||
| Proton | 20 | 62 | |
| Photon | 20 | 58 | .855 |
| Sex | |||
| Male | 21 | 52 | |
| Female | 19 | 68 | .313 |
| Age (y) | |||
| Mean | 68.75 | 65.94 | |
| SD | 9.77 | 11.11 | .231 |
| Stage | |||
| IIA | 0 | 2 | .909 |
| IIB | 0 | 1 | |
| IIIA | 23 | 72 | |
| IIIB | 17 | 44 | |
| IV | 0 | 1 | |
| Tumor location | |||
| RUL | 15 | 41 | .002 |
| RML | 8 | 4 | |
| RLL | 8 | 15 | |
| LUL | 5 | 40 | |
| LLL | 3 | 7 | |
| Hilum | 1 | 13 | |
| PTV volume | |||
| Proton (Mean ± SD) | 546.64 ± 265.54 | 465.79 ± 279.70 | .180 |
| Photon (mean ± SD) | 496.06 ± 317.61 | 618.45 ± 413.96 | |
| Chemo | |||
| Concurrent | 37 | 113 | |
| Sequential | 3 | 7 | 1.000 |
| Chemo type∗ | |||
| Carboplatin/taxol | 22 | 64 | .944 |
| Cisplatin/etoposide | 9 | 30 | |
| Other | 8 | 26 | |
| Smoking status | |||
| Never | 9 | 10 | .006 |
| Former | 29 | 83 | |
| Current | 2 | 27 |
Abbreviations: LLL = left lower lobe; LUL = left upper lobe; PTV = planning target volume; RLL = right lower lobe; RML = right middle lobe; RP = radiation pneumonitis; RUL = right upper lobe; SD = standard deviation.
All RP patients were ≥2 grade.
Missing 1 pneumonitis patient.
Table 2.
Patient characteristics for proton versus photon patients
| Proton | Photon | P value | |
|---|---|---|---|
| Modality | |||
| Non-RP | 62 | 58 | .855 |
| RP | 20 | 20 | |
| Sex | |||
| Male | 41 | 30 | .142 |
| Female | 41 | 48 | |
| Age (y) | |||
| Mean | 69.18 | 64.54 | .003 |
| SD | 9.78 | 10.76 | |
| Stage | |||
| IIA | 2 | 0 | .020 |
| IIB | 1 | 0 | |
| IIIA | 55 | 40 | |
| IIIB | 24 | 37 | |
| IV | 0 | 1 | |
| Tumor location | |||
| RUL | 26 | 30 | .563 |
| RML | 5 | 7 | |
| RLL | 11 | 12 | |
| LUL | 27 | 18 | |
| LLL | 4 | 6 | |
| Hilum | 9 | 5 | |
| PTV Volume | |||
| Mean | 488.9 | 587.07 | .200 |
| SD | 275.75 | 395.15 | |
| Chemo | |||
| Concurrent | 79 | 72 | .319 |
| Sequential | 3 | 6 | |
| Chemo type∗ | |||
| Carboplatin/taxol | 58 | 34 | <.001 |
| Cisplatin/etoposide | 20 | 19 | |
| Other | 4 | 24 | |
| Smoking status | |||
| Never | 6 | 13 | .175 |
| Former | 60 | 53 | |
| Current | 16 | 12 |
Abbreviations: LLL = left lower lobe; LUL = left upper lobe; PTV = planning target volume; RLL = right lower lobe; RML = right middle lobe; RP = radiation pneumonitis; RUL = right upper lobe; SD = standard deviation.
All RP patients were ≥2 grade.
Missing 1 photon patient.
Statistical analysis
All statistical tests were performed using IBM SPSS Statistics v21.0. software. The level of statistical significance was chosen to be P < .05.
Patient characteristics were tested for statistical significance using a univariate logistic regression analysis. Univariate analysis was used to study the association between the dosimetric parameters of V5Gy to V70Gy and Dmean to the risk of RP. Both the photon and proton cohorts were examined and the values were compared with the clinical dose constraint levels of V20Gy and Dmean.
A multivariate logistic regression analysis was also performed for all dosimetric parameters that were found to be statistically significant at P < .05 in the univariate tests for each of the photon and proton patient cohorts. The patient characteristics that were found to be statistically significant in the univariate analysis based on the RP and non-RP patient cohorts were also tested. A likelihood ratio test was adopted for a stepwise forward variable selection. Odds ratio (OR) with 95% confidence interval (CI) was estimated using the model. All hypothesis-testing were 2-sided at a significance level of 5%. Spearman nonparametric correlation coefficients were computed to assess the correlation between each pair of dose volume parameters.
Receiver operating characteristic analysis
Receiver operating characteristic (ROC) curves were generated for various dose levels for the photon and proton groups, and the area under the curve (AUC) values were calculated. The volumetric constraint for the optimal dosimetric parameters were obtained through analyzing RP and non-RP sensitivity and specificity values.
Results
Table 3 shows the univariate logistic regression results for the proton therapy patients. All dose volume parameters except V70Gy, including Dmean, were shown to be statistically significant. Table 4 shows the univariate logistic regression results for the photon therapy patients. In this test, V5Gy to V45Gy and Dmean were shown to be statistically significant. Based on the patient characteristics, tumor location and smoking status were found to be statistically significant with a P value of 0.002 and 0.006, respectively. All P values calculated for the patient characteristics based on RP and non-RP cohorts can be found in Table 1, and those based on proton and photon cohorts can be found in Table 2.
Table 3.
Univariate analysis for proton therapy patient patients with RP and those without in each dose level (in increments of 5 Gy)
| Dose (Gy) | RP (n = 20) |
No RP (n = 62) |
P value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| V5 | 40.24 (7.47) | 33.45 (9.30) | .004∗ |
| V10 | 37.14 (6.42) | 30.59 (8.48) | .002∗ |
| V15 | 34.78 (5.59) | 28.41 (7.92) | .002∗ |
| V20 | 32.57 (4.70) | 26.44 (7.57) | .001∗ |
| V25 | 30.32 (4.26) | 24.59 (7.18) | .001∗ |
| V30 | 28.33 (4.40) | 22.54 (6.79) | .001∗ |
| V35 | 26.34 (4.54) | 20.02 (6.46) | <.001∗ |
| V40 | 24.45 (4.45) | 18.11 (5.99) | <.001∗ |
| V45 | 22.48 (4.42) | 16.37 (5.89) | <.001∗ |
| V50 | 20.51 (4.41) | 14.71 (5.62) | <.001∗ |
| V55 | 18.36 (4.40) | 12.84 (5.59) | <.001∗ |
| V60 | 15.43 (5.13) | 10.92 (4.98) | .001∗ |
| V65 | 10.69 (4.64) | 7.68 (4.00) | .007∗ |
| V70 | 1.70 (2.12) | 1.38 (1.59) | .463 |
| Dmean | 18.36 (2.94) | 14.39 (4.16) | <.001∗ |
Abbreviations: RP = radiation pneumonitis; SD = standard deviation.
All RP patients were ≥2 grade.
P < .05.
Table 4.
Univariate analysis for photon therapy patients with RP and those without in each dose level (in increments of 5 Gy)
| Dose (Gy) | RP (n = 20) |
No RP (n = 59) |
P value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| V5 | 53.93 (8.81) | 46.38 (12.24) | .013∗ |
| V10 | 41.98 (7.91) | 35.92 (10.21) | .018∗ |
| V15 | 35.42 (5.71) | 30.05 (8.79) | .013∗ |
| V20 | 31.28 (3.99) | 26.23 (7.83) | .008∗ |
| V25 | 28.31 (3.47) | 23.16 (7.93) | .007∗ |
| V30 | 25.56 (3.42) | 20.55 (7.85) | .008∗ |
| V35 | 22.84 (3.69) | 18.02 (7.65) | .009∗ |
| V40 | 19.90 (4.18) | 15.39 (7.37) | .012∗ |
| V45 | 16.61 (4.39) | 13.01 (6.93) | .033∗ |
| V50 | 13.72 (4.47) | 10.92 (6.52) | .077 |
| V55 | 11.07 (4.43) | 8.91 (6.04) | .142 |
| V60 | 8.35 (4.30) | 6.87 (5.45) | .269 |
| V65 | 5.15 (3.63) | 4.48 (4.33) | .528 |
| V70 | 1.62 (2.70) | 1.55 (3.18) | .930 |
| Dmean | 18.15 (2.08) | 14.91 (4.67) | .004∗ |
Abbreviations: RP = radiation pneumonitis; SD = standard deviation.
All RP patients were ≥2 grade.
P < .05.
For the proton patient cohort, V40Gy (P <.001, OR [95% CI] = 1.309 [1.130-1.516]) and smoking history (P = .036) were found statistically significant in the multivariate analysis. Based on the Spearman nonparametric correlation coefficients, V40Gy was strongly correlated with dose volume metrics from V35Gy to V50Gy. The correlation coefficients for all dose levels from V35Gy to V50Gy compared with V40Gy were r ≥ 0.975, and all P values were <.001.
For the photon patient cohort, Dmean (P = .005, OR [95% CI] = 1.288 [1.072-1.549]) was found statistically significant in the multivariate analysis. Spearman nonparametric correlation coefficients were computed. Dmean v. V20Gy to V40Gy were r ≥ 0.95 and all P values were <.001.
ROC analysis
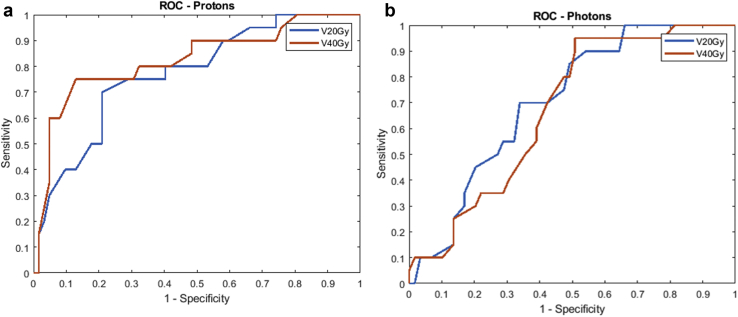
Figure 1a shows the ROC curves for V20Gy and V40Gy proton patients. V35Gy to V50Gy resulted in higher AUC values all being ≥0.80 compared with the other dose levels, as shown in Table 5. Figure 1b shows the ROC curves for photon patients to validate the already-shown clinically relevant dose volume of V20Gy, which had the highest AUC value of 0.707. The AUC values for the photon therapy patients are shown in Table 5.
Figure 1.
Receiver operating characteristics curves (ROC) for V20Gy and V40Gy for (a) proton patients and (b) photon patients. V35Gy to V50Gy resulted in the highest area under the curve values of ≥0.80 compared with the other dose levels for proton patients. V20Gy resulted in the highest area under the curve value of 0.707 compared with the other dose levels for photon patients.
Table 5.
AUC values for various dose levels for proton therapy patients and photon patients
| Proton patients |
Photon patients |
||
|---|---|---|---|
| Dose level | AUC | Dose level | AUC |
| V10Gy | 0.736 | V10Gy | 0.677 |
| V20Gy | 0.771 | V20Gy | 0.707 |
| V35Gy | 0.821 | V35Gy | 0.694 |
| V40Gy | 0.824 | V40Gy | 0.674 |
| V50Gy | 0.805 | V50Gy | 0.624 |
| V50Gy | 0.785 | V50Gy | 0.596 |
Abbreviation: AUC = area under the curve.
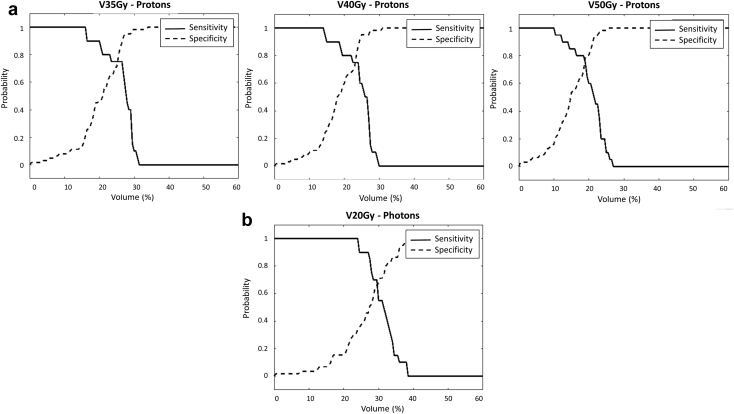
Figure 2a shows the specificity and sensitivity versus volume percentage curves for proton therapy patients (for V35Gy, V40Gy, and V50Gy), and Figure 2b shows photon therapy patients (for V20Gy). These plots were used to determine the optimal volume constraint for the dose predictors. Based on the intersection of the specificity and sensitivity curves shown in Figure 2a, the optimal volume constraint of V35Gy is ≤25%, V40Gy ≤23% and V50Gy ≤19% for proton therapy. The constraint of V20Gy ≤30% is confirmed for photon patients, as shown in Figure 2b.
Figure 2.
Specificity and sensitivity versus volume percentage for V35Gy, V40Gy, and V50Gy for (a) proton therapy patients and V20Gy for (b) photon therapy patients. The point of intersection between the solid and dashed line indicate at what volume percentage receiving 20 Gy, 35Gy, 40 Gy, and 50 Gy bests predicts radiation pneumonitis.
Discussion
As development of additional radiation treatment techniques become more available, such as proton radiation therapy, determination of new dose predictors needs to be established owing to the inherent physical difference between photon radiation compared with proton radiation. Mean lung dose and V20Gy have been shown to be the best predictors for lung toxicities when treating lung patients with concurrent CRT. These constraints are also the current standard dose predictors used when treating lung cancer with proton therapy, due to the lack of data suggesting otherwise. This study aimed to investigate whether the current standard dose predictors used in photon therapy hold true for proton therapy.
Results in Table 5 show that V35Gy to V50Gy had the highest AUC compared with other dose levels, indicating that a higher dose level may be a better dose predictor for proton therapy patients, compared with V20Gy used for photon patients. Table 5 also showed that V20Gy had the highest AUC for photon therapy patients, which is the clinically used dose predictor for RP currently. Based on the multivariate analysis, V40Gy was found to be statistically significant, while V35Gy to V50Gy yielded strong correlation with V40Gy based on the Spearman nonparametric correlation test. This indicates that in general V35Gy, V40Gy, and V50Gy can all be valid dose predictors for RP for patients treated with proton therapy, in addition to the current clinical standard V20Gy and mean lung dose constraints. This is further supported by the higher AUC values of V35Gy to V50Gy compared with other dose-volume parameters in the ROC curves for proton patients. All dose levels, except for V70Gy, were found the be statistically significant based on the univariate analysis for proton therapy patients, which may indicate that lower dose levels may be used as predictors for RP, although higher dose levels, such as V40Gy, may be stronger predictors. Dmean was found to be statistically significant for photon patients based on the multivariate analysis, which is consistent with current clinical standards. Figure 2a indicates that possible additional dosimetric constraints could be V35Gy ≤25%, V40Gy ≤23%, or V50Gy ≤19% for proton therapy patients, whereas the V20Gy ≤30% was confirmed for photon therapy patients, as shown in Figure 2b.
From Table 5, it is observed that absolute AUC values are generally higher for proton therapy than photon therapy. It implies that proton dose (RBE) has a stronger effect on RP than photon dose at the same level. This was indirectly supported by the slightly elevated ORs at the corresponding V40Gy (the best predictor) for proton therapy (OR: 1.309) and Dmean (the best predictor) for photon therapy (OR: 1.208).
Tucker et al investigated the use of effective dose for predicting RP for proton patients compared with using mean lung dose. They found that in proton therapy, delivering higher doses to smaller lung volumes resulted in increased RP risk.20 Vogelius et al found that the low dose bath from photon therapy may be more relevant for RP risk compared with proton therapy when radiation is combined with chemotherapy.22 Our study showed using different dose volume constraints, such as V35Gy, V40Gy, or V50Gy, for proton therapy patients could cause fewer lung toxicities. Using specialized dose predictors for proton therapy that were determined based on a proton patient cohort, compared with those typically used for photon therapy patients, could further benefit patients getting treated with proton therapy.
In this study, total lung minus IGTV was used as the healthy lung volume. One standard volume definition is used in our clinic to denote healthy lung, regardless of fractionation scheme. Because RTOG 0618, RTOG 0813, RTOG 0915, and RTOG 1106 all suggest the definition of healthy lung volume to be total lung minus GTV, rather than total lung minus CTV, which is what RTOG 0617 suggests, our clinic chose the former as the definition.23, 24, 25, 26, 27 Additionally, acquiring a 4-dimensional CT is part of our standard of practice for locally advanced disease, so we also incorporate motion into our volume definitions. This study did not investigate the effect of different definitions of healthy lung. As the CTV includes microscopic disease and usually encompasses larger volumes than GTV, the healthy lung volume defined by lung minus IGTV is expected to show more lung dose than defined by lung minus CTV, thus it is more conservative in obtaining a clinical treatment plan. It would be interesting to investigate the causal differences in pneumonitis outcome in the future.
It is important to note that this is an initial retrospective study, and further studies should be done before clinically accepting these new possible constraints for proton therapy patients. These results are dependent on a single institution, and planning strategies used at other institutions could result in different outcomes. However, Shusharina et al 2018 present similar DVH values for proton patients for high dose volumes (V40 and V60) when treating the lung, illustrating that it is the nature of proton therapy plans to have a higher percent volume receiving these higher doses (compared with photon therapy).28
In this study the majority (52 patients) of the photon patients were treated with IMRT or volumetric arc therapy, and 26 patients were treated with either 2 field or 4 field 3D CRT. Although quantitative analyses of normal tissue effects in the clinic lung dose constraints were derived based on 3-dimensional planning, they still hold true for IMRT patients.29 At our institution, our typical beam selection for proton planning is to use a posterior and posterior oblique beam for patients with lung cancer. When choosing beams for proton therapy, we aim to reduce the uncertainty in the beam path and limit the amount of lung tissue being traversed. The healthy lung tissue along the proton beam paths have a concentrated region of higher dose compared with the low dose spread from photon treatments. It is our intention in this study to investigate dosimetric parameters on volumes with higher dose to observe and regulate the incidence of radiation pneumonitis in proton radiation therapy.
In regard to the proton therapy delivery technique, intensity modulated proton therapy is recognized in general for being better at sparing organs at risk at the proximal end of the tumor, compared with double scattering. Although in this study the number of patients treated with intensity modulated proton therapy is too small to warrant a statistical analysis, it is interesting to note that all 5 patients treated with PBS were non-RP patients, which could suggest that PBS technique may have better sparing of the lung. Future studies with PBS lung patients should be conducted to investigate the lung sparing effects compared with double scattering.
Jin et al 2009 provided constraints for higher dose volumes (V35 <15% and V50 <10%). However, these constraints were derived from only a photon patient population.14 Although such criteria appear to be consistent with our photon non-RP cohort, the mean percent volume in our proton population (Table 2) for the non-RP population are higher than those specified (V35Gy = 20.02 and V50Gy = 14.71), further illustrating the guidelines currently available may not apply to the proton therapy population. Also, it should be emphasized that the suggestion for using higher dose constraints for proton patients should be in addition to, and not instead of, the currently used clinical dose constraints of V20Gy and Dmean.
Conclusions
While mean lung dose and V20Gy are strong predictors for RP in photon therapy, the volume receiving a higher dose, such as V35Gy, V40Gy, or V50Gy, may be a better indicator for RP for LA-NSCLC patients treated with proton therapy. This new dosimetric criteria could potentially help with the RP control in LA-NSCLC treated with proton therapy.
Footnotes
Sources of support: none. Research data are not available at this time.
Disclosures: Dr Dong has personal fees from Varian Medical Systems, outside the scope of the manuscript. The other authors report no conflicts.
References
- 1.Simone C.B., 2nd Thoracic radiation normal tissue injury. Semin Radiat Onco. 2017;27:370–377. doi: 10.1016/j.semradonc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Jain V., Berman A.T. Radiation pneumonitis: Old problem, new tricks. Cancers (Basel) 2018;10:222. doi: 10.3390/cancers10070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino M., Giobbie-Hurder A., Hatabu H., Ramaiya H.N., Hodi F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2016;2:1607–1616. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 4.Chang J.Y., Jabbour S.L., De Ruysscher D. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;95:505–516. doi: 10.1016/j.ijrobp.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Jy, Verman V., Li Ming. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: Final results of a phase 2 study. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang J.Y., Zhang X., Xiaochun W. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 7.Sejpal S., Komaki R., Tsao A. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004–3013. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen Q.N., Ngoc B.L., Ritsuko K. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol. 2015;115:367–372. doi: 10.1016/j.radonc.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang J.Y., Risuko K., Lu C. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117:4707–4713. doi: 10.1002/cncr.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robnett T.J., Machtay M., Vines E.F. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. doi: 10.1016/s0360-3016(00)00648-9. [DOI] [PubMed] [Google Scholar]
- 11.Yamada M., Kudoh S., Kirata S. Risk factors of pneumonitis following chemoradiotherapy for lung cancer. Eur J Cancer. 1998;34:71–75. doi: 10.1016/s0959-8049(97)00377-8. [DOI] [PubMed] [Google Scholar]
- 12.Roach M., 3rd, Gandara D.R., Yuo H.S. Radiation pneumonitis following combined modality therapy for lung cancer: Analysis of prognostic factors. J Clin Oncol. 1995;13:2606–2612. doi: 10.1200/JCO.1995.13.10.2606. [DOI] [PubMed] [Google Scholar]
- 13.Hernando M.L., Marks L.B., Bentel G.C. Radiation-induced pulmonary toxicity: A dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 14.Jin H., Tucker S.L., Liu H.H. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol. 2009;91:427–432. doi: 10.1016/j.radonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham Mv, Purdy J.A., Emami B. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 16.Yorke E.D., Jackson A., Rosenzweig K.E. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54:329–339. doi: 10.1016/s0360-3016(02)02929-2. [DOI] [PubMed] [Google Scholar]
- 17.Lind P.A., Marks L.B., Hollis D. Receiver operating characteristic curves to assess predictors of radiation-induced symptomatic lung injury. Int J Radiat Oncol Biol Phys. 2002;54:340–347. doi: 10.1016/s0360-3016(02)02932-2. [DOI] [PubMed] [Google Scholar]
- 18.Kwa S.L., Theuws J.C., Wagenaar A. Evaluation of two dose-volume histogram reduction models for the prediction of radiation pneumonitis. Radiother Oncol. 1998;48:61–69. doi: 10.1016/s0167-8140(98)00020-6. [DOI] [PubMed] [Google Scholar]
- 19.Kwa S.L., Lebseque J.V., Theuws J. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. doi: 10.1016/s0360-3016(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 20.Tucker S.L., Xu T., Paganetti H. Validation of effective dose as a better predictor of radiation pneumonitis risk than mean lung dose: Secondary analysis of a randomized trial. Int J Radiat Oncol Biol Phys. 2019;103:403–410. doi: 10.1016/j.ijrobp.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, National Institutes of Health and National Cancer Institute. US Department of Health and Human Services; Washington, DC: 2009. [Google Scholar]
- 22.Vogelius I.R., Westerly D.C., Aznar M.C. Estimated radiation pneumonitis risk after photon versus proton therapy alone or combined with chemotherapy for lung cancer. Acta Oncol. 2011;50:772–776. doi: 10.3109/0284186X.2011.582519. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman R.D., Paulus R., Pass H.I. Stereotactic body radiation therapy for operable early-stage lung cancer: Findings from the NRG oncology RTOG 0618 trial. JAMA Oncol. 2018;4:1263–1266. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bezjak A., Paulus R., Gaspar L.E. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non–small-cell lung cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol. 2019;37:1316–1325. doi: 10.1200/JCO.18.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Videtic G.M., Hu C., Singh A. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer: NRG Oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys. 2015;93:757–764. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong F.M., Ten Haken R.K., Schipper M. Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non-Small-Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2017;3:1358–1365. doi: 10.1001/jamaoncol.2017.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shusharina N., Liao Z., Mohan R. Differences in lung injury after IMRT or proton therapy assessed by (18)FDG PET imaging. Radiother Oncol. 2018;128:147–153. doi: 10.1016/j.radonc.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Bentzen S.M., Constine L.S., Deasy J.O. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): An introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–S9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]