Abstract
A 62-year-old man presented with a rising serum concentration of prostate-specific antigen (PSA) to 53.3 ng/mL (to convert to μg/L, multiply by 1) and a PSA doubling time of 2.6 months. Computed tomography, fluorodeoxyglucose–positron emission tomography, and C-11 choline positron emission tomography demonstrated a parotid mass with innumerable lytic bone lesions and diffuse metastatic disease to the neck and mediastinal lymph nodes. Mediastinal lymph node biopsy revealed salivary ductal adenocarcinoma that produced PSA and demonstrated androgen receptor sensitivity. The patient had a prolonged clinical benefit to first- and second-line hormone therapy, and his PSA levels correlated with treatment response, development of hormone resistance, and progression. In summary, urologists, pathologists, and primary care providers should be aware that a rising PSA level in the setting of a head and neck mass in a patient without a history of prostate cancer does not constitute a diagnosis of metastatic prostate adenocarcinoma and that other primary tumors should be considered and a broader imaging and pathologic evaluation is indicated.
Abbreviations and Acronyms: ADT, androgen deprivation therapy; AR, androgen receptor; CAB, combined androgen blockade; CT, computed tomography; FDG-PET, fluorodeoxyglucose–positron emission tomography; LHRH, luteinizing hormone–releasing hormone; PSA, prostate-specific antigen
Prostate-specific antigen (PSA) is a proenzyme that is formed and released by the lining acini of the prostate into the duct system. It is released in an inactive form and then undergoes a proteolytic process to be active PSA.1, 2, 3 Prostate-specific antigen has been used as a serum marker to detect prostate cancer and to follow treatment response. In addition to prostate pathologies, many factors contribute to the serum concentration of PSA, including the patient’s age and race.4,5 Patients older than 60 years and African American men tend to have a faster rising rate than others, even without prostate cancer.
Moreover, a rising serum concentration of PSA has been reported in various tumors other than prostate cancer. Diamandis et al6 described rising PSA levels in 30% of patients with breast cancer, and interestingly, it was significantly associated with the presence of estrogen and progesterone receptors. In rare cases, PSA production can be seen in other tumor types, such as breast, colon, adrenal, and salivary cancers.7 The literature is limited on the incidence of PSA-producing salivary tumors; however, PSA expression was reported in 100% (n=56) of normal salivary gland tissue and 55% (n=6 of 11) of pleomorphic adenomas of the salivary gland.8,9 As well, androgen receptor (AR) expression has been reported in 70% to 100% of salivary ductal carcinoma and 21% of salivary adenocarcinoma.10,11
Awareness of the possibility of PSA secretion by nonprostate cancers is important for practicing urologists to avoid misdiagnosis and treatment delays, especially in patients without an identifiable prostatic primary tumor. Furthermore, on biopsy, metastases from salivary gland, breast, and prostate cancers demonstrate some pathologic similarities that make their differentiation from prostatic primary tumors challenging.12 This report describes a patient with widely metastatic parotid adenocarcinoma who presented with a rising PSA level and innumerable lytic bone lesions and nodal metastases. We discuss PSA as a potential marker of response to therapy in PSA-secreting salivary cancer and provide an update on the current use of hormone therapy for AR-positive salivary cancer.
Case Presentation
The case is one of a 62-year-old man with a complicated oncologic history. In 1999 he was diagnosed as having a left testis seminoma. He underwent resection with a retroperitoneal lymph node dissection followed by adjuvant radiation therapy. In 2001 he had a partial left nephrectomy for a cT1, grade 1 renal cell carcinoma. Then, in 2010, he was diagnosed as having a lymphoplasmacytic lymphoma, initially treated with rituximab for 4 cycles before starting cyclophosphamide and dexamethasone. He continued this therapy through April 2013.
In December 2012, the patient was found to have a rising PSA level of 14 ng/mL (was 5.7 ng/mL in October 2012) (to convert to μg/L, multiply by 1). Biopsy revealed high-grade prostatic intraepithelial neoplasia. He denied any lower urinary tract symptoms, and the patient was noted to have no nodularity of the prostate on examination.
In May 2013 he noted new left neck adenopathy. Repeated PSA measurement on May 28, 2013, was 53.3 ng/mL with a PSA doubling time of 2.6 months. May 30, 2013, computed tomography (CT) of the chest and abdomen demonstrated new right hilar and right paratracheal lymphadenopathy (4.5 cm), new pulmonary nodules, and progressive lytic lesions in the axial and proximal appendicular skeleton. Biopsy of a paratracheal lymph node on May 31, 2013, revealed poorly differentiated carcinoma that was strongly and diffusely immunoreactive with antibodies to cytokeratin 7 and negative for CD20, thyroid transcription factor-1, napsin, prostatic acid phosphatase, synaptophysin, and chromogranin. Prostate-specific antigen demonstrated focal/weak staining; electroretinographic findings were negative. Additional tissue samples were sent to CancerTYPE ID, and the expression profile predicted head and neck salivary gland adenocarcinoma with the highest probability score of 90% (Figure 1). Subsequently, additional immunohistochemical stains were performed and showed strong and diffuse reactivity to AR and MOC-31. The tumor was also focally positive to glypican-3 and Her2 in a membranous pattern (1+). In June 2013, fluorodeoxyglucose–positron emission tomography (FDG-PET) confirmed widespread metabolically active mixed lytic and sclerotic lesions throughout the visualized axial and appendicular skeleton consistent with metastatic disease. In addition, there was also a metabolically active left parotid mass with metabolically active left-sided cervical adenopathy and a large, approximately 4.8-cm metabolically active pretracheal/subcarinal lymph node in the chest. Neither C-11 choline PET nor FGD-PET demonstrated increased or suspicious uptake in the prostate (Figure 2).
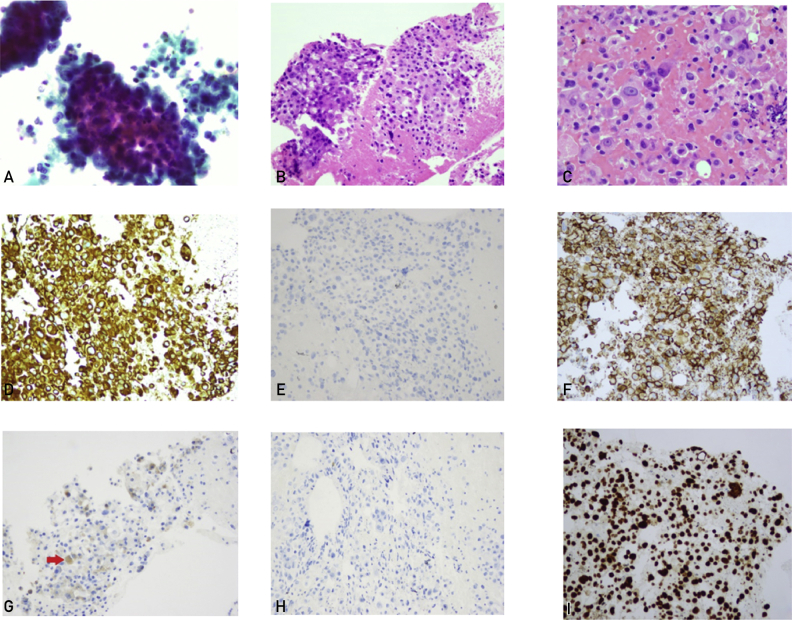
Figure 1.
Initial cytology findings of transbronchial needle aspiration, cell block, and immunohistochemical staining of the right station 4 lymph node. A, Hypercellular smears are showing 3-dimensional clusters of malignant cells with prominent nucleoli and increased nuclear to cytoplasmic ratios (ThinPrep, ×20). B and C, Cell block preparation demonstrating poorly differentiated malignant cells arranged in sheets (hematoxylin-eosin stain, ×20 and ×40). The tumor cells are immunoreactive for cytokeratin 7 (D), MOC-31 (F), and androgen receptor (I) and focally for prostate-specific antigen (G; red arrow) and are negative for cytokertin 20 (E) and prostatic acid phosphatase (H) (immunohistochemical staining, ×20).
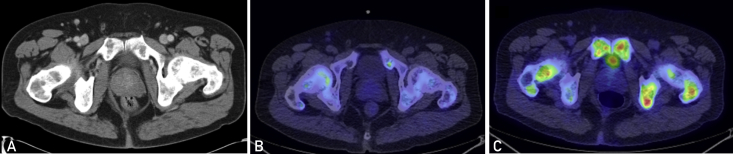
Figure 2.
Cross-sectional imaging from June 2013. A, Computed tomographic image of the abdomen and pelvis with contrast demonstrating slight enlargement of the prostate. B, C-11 choline positron emission tomographic (PET) image demonstrating no suspicious choline avidity in the prostate. C, Fluorodeoxyglucose (FDG)-PET image demonstrating no suspicious FDG uptake in the prostate.
In June 2013 the patient was initiated on a luteinizing hormone–releasing hormone (LRHR) agonist and bicalutamide. Clinically he noted treatment response with decreasing cervical lymphadenopathy. At the beginning of July 2013, a limited left neck dissection was performed. Pathologic findings from the neck dissection showed poorly differentiated adenocarcinoma, cytokeratin 7 and AR positive, PSA negative. Collectively, the final diagnosis was a primary (AR-positive) salivary gland adenocarcinoma or salivary duct carcinoma (Figure 3). A follow-up C-11 choline PET scan in July 2013 demonstrated a favorable response to therapy with decreasing activity in the bone disease and decreasing size and activity of pulmonary nodules and of the biopsy-proven nodal disease in the mediastinum (Figure 4). The PSA evaluation demonstrated a marked reduction in his PSA level from an initial value of 53.3 ng/mL down to 1.2 ng/mL. In August 2013 his PSA level continued to decrease to 0.81 ng/mL, and FDG-PET showed a significant interval response of metastatic salivary gland cancer with partial to complete response in all previously seen metastatic lesions.
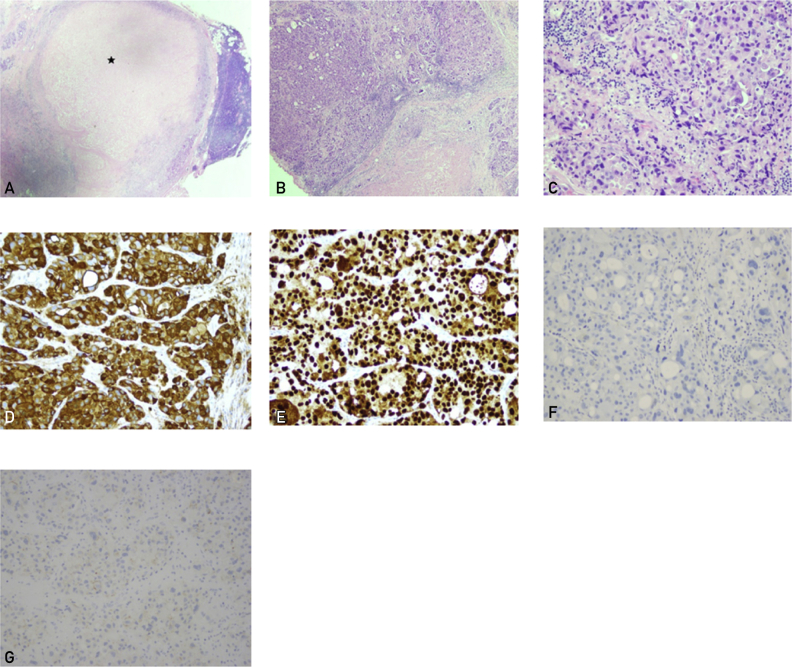
Figure 3.
Follow-up surgical excision of a level II cervical lymph node. A-C, Poorly differentiated tumor cells are almost replaced with necrosis (asterisk), forming sheets and clusters (hematoxylin-eosin stain, ×2, ×10, and ×20). The tumor cells are immunoreactive for cytokeratin 7 (D) and androgen receptor (E), and negative for prostatic-specific antigen (F) and HER2 (G) (immunohistochemical staining, ×20).
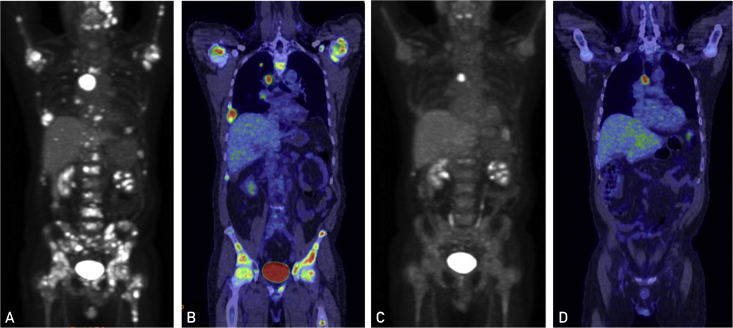
Figure 4.
Fluorodeoxyglucose (FDG) positron emission tomographic images showing widespread skeletal metastatic disease and FDG avid right pulmonary nodules before starting luteinizing hormone–releasing hormone (LHRH) agonist and bicalutamide (A and B). C and D, Significant interval response of metastatic androgen receptor–positive salivary cancer to LHRH agonist and bicalutamide with a partial to complete response in all previously seen metastatic lesions.
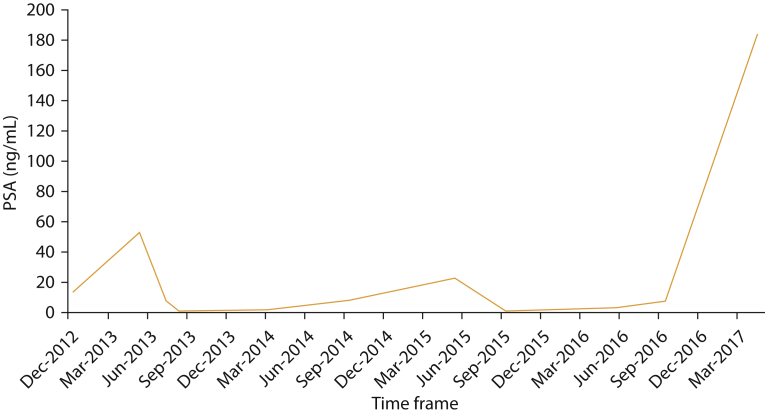
The patient continued first-line hormone therapy until September 2014, when he had significant radiographic progression and his PSA level was 8.1 ng/mL (duration of clinical response to initial hormone therapy was 15 months). He was subsequently treated with the mammalian target of rapamycin (mTOR) inhibitor everolimus based on next-generation sequencing of his tumor (with continuation of the LHRH agonist) without notable response radiographically or biochemically. In July 2015 he was started on second-line hormone therapy with abiraterone. An interim PSA level in September 2015 was suggestive of response with a value of 0.52 ng/mL. The response was confirmed in December 2015 with a FDG-PET demonstrating radiographic response and a PSA of 0.21 ng/mL. In April 2016, radiographic and biochemical progression was seen by FDG-PET, and his PSA level was 2.6 mg/mL (duration of response to second-line hormone therapy was 9 months). In May 2016 he was started on pembrolizumab and denosumab and had initial stabilization of his PSA (3.1 ng/mL in May 2016 and 2.8 ng/mL in June 2016) followed by progression in late August 2016, with a PSA of 9.7 ng/mL and radiographic progression on FDG-PET. September 2016 he started docetaxel and had a slight biochemical response with a PSA of 7.4 ng/mL in late September 2016 followed by a FDG-PET in November 2016 showing mixed response to therapy and a PSA of 7.5 ng/mL. In April 2017, repeated imaging with FDG-PET showed marked disease progression with new liver metastases. The PSA level at that time was 183 ng/mL. The patient died 1 month later. The PSA trend over time is shown in Figure 5.
Figure 5.
Prostate specific antigen (PSA) curve over time. To convert PSA values to μg/L, multiply by 1.
Discussion
Herein we report our experience with a case of a rising PSA level and innumerable metastases to bone, lymph nodes, and soft tissue of a PSA-producing salivary gland primary tumor. In this patient, the PSA level seemed to correlate with clinical and radiographic response to therapies, namely, first- and second-generation hormone therapies. With no standard first-line treatment for patients with metastatic salivary cancer, androgen deprivation therapy (ADT) has been studied and has documented efficacy. Fushimi et al13 investigated in a single-institution, open-label, single-arm, phase 2 trial the efficacy of combined androgen blockade (CAB) using leuprorelin and bicalutamide compared with conventional chemotherapy in the management of patients with AR-positive unresectable salivary cancer. Interestingly, the study showed that CAB is as effective as conventional chemotherapy with less toxicity. He reported median progression-free survival of 8.8 months and median overall survival of 30.5 months in CAB-treated patients.13 Androgen deprivation therapy is now recommended as part of mainstay treatment for metastatic AR-positive salivary cancers.14
Athough at first glance it may seem odd that a tumor with such rapid PSA production would stain only weakly for PSA on immunohistochemical analysis, this is not unexpected. Tremendous variability has been reported within AR-positive salivary gland tumors. Fan et al15 published a review of 13 AR-positive salivary gland tumors. Interestingly, only 2 patients’ tissue stained positive for PSA, and 7 patients had tissue stain positive for prostate acid phosphatase. Van Krieken9 actually described prostate marker immunoreactivity in salivary gland neoplasms as “a rare pitfall in immunohistochemistry.” Furthermore CancerTYPE ID reported with its highest predictive probability that the primary tumor is of salivary gland origin. CancerTYPE ID is an objective molecular test of 92 genes that is used to help identify the site of origin and the histologic subtype of tumors when it is in question.16
Boon et al17 reported on the use of first-line ADT in 35 patients with metastatic AR-positive salivary cancer and demonstrated that treatment with ADT showed better response, clinical benefit, and overall survival compared with patients treated with supportive care. The median progression-free survival for patients treated with ADT in their study was 4 months, with median duration of clinical benefit of 11 months. In addition, the median overall survival for ADT-treated patients was 17 months (range, 10-24 months) vs 5 months (range, 1-9 months) for patients treated with supportive treatment.17 Our patient had clinical benefit for 15 months with initial hormone therapy, and 9 months for second line. This is similar to the experience reported by Viscuse et al18 in which first-line treatment for AR-positive recurrent/metastatic salivary gland tumors treated with ADT vs chemotherapy were compared and found to be no different in overall survival.
Interestingly, for the present patient, the rise in serum PSA level preceding documentation of significant radiographic progression is similar to the biochemical recurrence seen with ovarian cancer (CA-125) and prostate adenocarcinoma.19 However, the present patient went on to have a prolonged clinical benefit to hormone therapy despite early biochemical progression, highlighting the importance of treating not just a blood test but the whole patient, incorporating biochemical, radiographic, and clinical data into treatment decisions.
The present case report illustrates similarities in the behavior of metastatic AR-positive salivary cancer to hormone-sensitive metastatic prostate cancers, including initial response to ADT followed by developing hormone refractory status. In summary, urologists, pathologists, and primary care providers should be aware that a rising PSA level in the setting of a head and neck mass in a patient without a history of prostate cancer does not constitute a diagnosis of metastatic prostate adenocarcinoma and that other primary tumors should be considered and a broader imaging and pathologic evaluation is indicated.
Acknowledgments
Drs Andrews and Ahmed contributed equally to this work.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Balk S.P., Ko Y.J., Bubley G.J. Biology of prostate-specific antigen. J Clin Oncol. 2003;21(2):383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- 2.Lilja H., Christensson A., Dahlen U. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37(9):1618–1625. [PubMed] [Google Scholar]
- 3.Mikolajczyk S.D., Marks L.S., Partin A.W., Rittenhouse H.G. Free prostate-specific antigen in serum is becoming more complex. Urology. 2002;59(6):797–802. doi: 10.1016/s0090-4295(01)01605-3. [DOI] [PubMed] [Google Scholar]
- 4.Oesterling J.E., Jacobsen S.J., Chute C.G. Serum prostate-specific antigen in a community-based population of healthy men: establishment of age-specific reference ranges. JAMA. 1993;270(7):860–864. [PubMed] [Google Scholar]
- 5.Morgan T.O., Jacobsen S.J., McCarthy W.F., Jacobson D.J., McLeod D.G., Moul J.W. Age-specific reference ranges for serum prostate-specific antigen in black men. N Engl J Med. 1996;335(5):304–310. doi: 10.1056/NEJM199608013350502. [DOI] [PubMed] [Google Scholar]
- 6.Diamandis E.P., Yu H., Sutherland D.J. Detection of prostate-specific antigen immunoreactivity in breast tumors. Breast Cancer Res Treat. 1994;32(3):301–310. doi: 10.1007/BF00666007. [DOI] [PubMed] [Google Scholar]
- 7.Levesque M., Hu H., D'Costa M., Diamandis E.P. Prostate-specific antigen expression by various tumors. J Clin Lab Anal. 1995;9(2):123–128. doi: 10.1002/jcla.1860090209. [DOI] [PubMed] [Google Scholar]
- 8.Tazawa K., Kurihara Y., Kamoshida S., Tsukada K., Tsutsumi Y. Localization of prostate-specific antigen-like immunoreactivity in human salivary gland and salivary gland tumors. Pathol Int. 1999;49(6):500–505. doi: 10.1046/j.1440-1827.1999.00900.x. [DOI] [PubMed] [Google Scholar]
- 9.van Krieken J.H. Prostate marker immunoreactivity in salivary gland neoplasms: a rare pitfall in immunohistochemistry. Am J Surg Pathol. 1993;17(4):410–414. doi: 10.1097/00000478-199304000-00012. [DOI] [PubMed] [Google Scholar]
- 10.D'Heygere E., Meulemans J., Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26(2):142–151. doi: 10.1097/MOO.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 11.Locati L.D., Perrone F., Losa M. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs) Oral Oncol. 2009;45(11):986–990. doi: 10.1016/j.oraloncology.2009.05.635. [DOI] [PubMed] [Google Scholar]
- 12.Barnes L., Eveson J.W., Reichart P., Sidransky D., editors. World Health Organization Classification of Tumors: Pathology and Genetics of Head and Neck Tumors. 3rd ed. IARC Press; Lyon, France: 2005. [Google Scholar]
- 13.Fushimi C., Tada Y., Takahashi H. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979–984. doi: 10.1093/annonc/mdx771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colevas A.D., Yom S.S., Pfister D.G. NCCN guidelines insights: head and neck cancers, version 1.2018. J National Compr Canc Netw. 2018;16(5):479–490. doi: 10.6004/jnccn.2018.0026. [DOI] [PubMed] [Google Scholar]
- 15.Fan C.Y., Wang J., Barnes E.L. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000;24(4):579–586. doi: 10.1097/00000478-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Bentley T.G., Schroeder B.E., Schnabel C.A. Cost effectiveness of a 92-gene assay for the diagnosis of metastatic cancer. J Med Econ. 2014;17(8):527–537. doi: 10.3111/13696998.2014.909817. [DOI] [PubMed] [Google Scholar]
- 17.Boon E., van Boxtel W., Buter J. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: a nationwide case series of 35 patients in The Netherlands. Head Neck. 2018;40(3):605–613. doi: 10.1002/hed.25035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viscuse P.V., Price K.A., Garcia J.J., Schembri-Wismayer D.J., Chintakuntlawar A.V. First line androgen deprivation therapy vs. chemotherapy for patients with androgen receptor positive recurrent or metastatic salivary gland carcinoma-a retrospective study. Front Oncol. 2019;9:701. doi: 10.3389/fonc.2019.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rustin G.J., Nelstrop A.E., Tuxen M.K., Lambert H.E. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Ann Oncol. 1996;7(4):361–364. doi: 10.1093/oxfordjournals.annonc.a010602. [DOI] [PubMed] [Google Scholar]