Abstract
To clarify the effect of different respiratory sample types on SARS-CoV-2 detection, we collected throat swabs, nasal swabs and hock-a-loogie saliva or sputum, and compared their detection rates and viral loads. The detection rates of sputum (95.65%, 22/23) and hock-a-loogie saliva (88.09%, 37/42) were significantly higher than those in throat swabs (41.54%, 27/65) and nasal swabs (72.31%, 47/65) (P < 0.001). The Ct Values of sputum, hock-a-loogie saliva and nasal swabs were significantly higher than that in throat swabs, whereas no significant difference was observed between sputum and saliva samples. Hock-a-loogie saliva are reliable sample types that can be used to detect SARS-CoV-2, and worthy of clinical promotion.
Keywords: SARS-CoV-2, COVID-19, Hock-a-loogie saliva, RT-PCR
1. Introduction
During December 2019, an outbreak of unexplained cluster of pneumonia cases occurred in Wuhan, China [1], [2]. The outbreak was confirmed to be caused by a new coronavirus infection on January 10, 2020, which was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by the International Committee of Virus Taxonomy [3]. As of July 22, 2020, 216 countries have reported more than 14,500,000 confirmed cases and nearly 610,000 deaths [4].
The early identification and confirmation of SARS-CoV-2 is the key to the effective prevention and control of the epidemic [5]. At present, PCR-based nucleic acid detection is the most effective method to diagnose suspected patients. However, with an improved understanding of the disease, reports indicated that the laboratory test for SARS-CoV-2 had high false negatives, which has led to a large number of misdiagnosis, increasing the difficulty of epidemic prevention and control.
Several reasons account for false negatives. In addition to patient disease progression and reagent performance, the quality of sample collection is of great significance to the accuracy of test results. To understand the impact of sample type on the accuracy of SARS-CoV-19 test results, we collected different types of respiratory tract samples from confirmed patients and compared detection rates and viral loads.
2. Methods
2.1. Study design and participants
This was a prospective diagnostic validity study of patients with laboratory-confirmed Coronavirus Disease 2019 (COVID-19) admitted to the First Affiliated Hospital, School of Medicine, Zhejiang University from 19 Jan 2020 to 15 Feb 2020. The hospital is a large-scale general hospital with 3000 beds, which serves as a designated hospital for COVID-19 in the Zhejiang Province. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of the First Affiliated Hospital of Zhejiang University.
2.2. Sample collection
Before sampling, it was confirmed that the patient did not drink water, eat food, gargle or other similar behaviors within half an hour that might affect the sampling quality. The specific collection process is as follows: throat swabs were collected first, nasal swabs were collected after a 15 min’ interval, and after another 15-minutes the patient was instructed to wear a mask and deep cough 3–5 times before spitting hock-a-loogie saliva or sputum into a sterile container. Throat swabs and nasal swabs were collected by skilled medical personnel. A rayon throat swab (Copan Italia) was used to collect from the deep back of the throat, while nasal swabs were collected from the nasopharynx with a flocked mid-turbinate nasal swab (Copan Italia). Swabs were rotated and stayed for sufficient time to collect the fluid and epithelial cells. Immediately after collection, the swabs samples were placed in viral transport medium (Copan Italia) and all samples was sent to the laboratory for test within 1 h after sample collection.
2.3. Laboratory confirmation
Viral RNAs were extracted using the MagNA Pure LC 2.0 (Roche, Basel, Switzerland), and quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using a commercial kit specific for SARS-CoV-2 detection (BGI Genomics Co., Ltd., Shenzhen, China) approved by China Food and Drug Administration (CFDA). The detection limit of the ORFab1 RT-PCR assays was approximately 100 copies per mL. Specimens with Ct values ≤ 38.0 were considered positive, specimens with Ct values > 38.0 were repeated, specimens with repeated results of Ct values > 38 and specimens with undetectable Ct values were considered negative. Negative and positive controls were set for each test. In accordance with the guidelines of the Chinese Health Commission, all samples tested in this study were conducted in biosafety Level 2 laboratory (BSL-2).
2.4. Data collection
The clinical data collected included demographic data, medical comorbidities, date of symptom onset, symptoms and signs, progression and resolution of clinical illness. The severity of illness was evaluated according to the guidelines of the National Health Commission of the People’s Republic of China.
2.5. Statistical analysis
For most variables, descriptive statistics, such as the mean and standard deviation (SD; for data with normal distribution), median with interquartile range (IQR; for data with skewed distribution), and proportion (%), were calculated. T-test, Mann-Whitney U tests, Kruskal-Wallis tests, χ2 tests and Fisher exact test were used for comparisons when appropriate. The Kruskal-Wallis test was used to evaluate the duration of viral shedding among groups. Statistical analyses were performed using SPSS software, version 16.0 (SPSS). In all analyses, a P value < 0.05 was considered significant. All probabilities were 2-tailed.
3. Results
3.1. Patient description
The median age of the 65 cases of COVID-19 was 54 years (IQR 39.5–62). Hypertension was the most common underlying disease. Fever (84.6%), cough (53.8%) and expectoration (27.7%) were the most common clinical manifestations. Among these cases, 42 (64.6%) were severe. The time from illness onset to sample collection was 8 days (IQR, 6–11). Eight patients were admitted to ICU, and 2 of them were under mechanical ventilation. Demographic and clinical characteristics of all patients is shown in Table 1 .
Table 1.
Demographics and clinical characteristics of patients with SARS-CoV-2 infection.
| Variables | Total (N = 65) |
|---|---|
| Demographics | |
| Median age (median [IQR]) (yr) | 54 (39.5–62) |
| Male sex | 40 (61.5) |
| Current smoking | 7 (10.8) |
| Time from illness onset to sampling (median [IQR]) (days) | 8 (6–11) |
| Underlying disease | |
| Hypertension | 21 (32.3) |
| Chronic heart disease | 3 (4.6) |
| Chronic lung disease | 5 (7.7) |
| Chronic liver disease | 3 (4.6) |
| Symptoms | |
| Fever | 55 (84.6) |
| Cough | 35 (53.8) |
| Sputum | 18 (27.7) |
| Chest distress | 7 (10.8) |
| Dizziness | 4 (6.2) |
| Headache | 3 (4.6) |
| Diarrhea | 8 (12.3) |
| Myalgia | 12 (18.5) |
| Laboratory findings | |
| Leukocyte count (median [IQR]) (mm3) | 6.7 (4.8–10) |
| Lymphocyte count (median [IQR]) (mm3) | 0.9 (0.5–1.3) |
| Hemoglobin (median [IQR]) (g/L) | 141 (129–153) |
| Platelet count (median [IQR]) (mm3) | 187 (138–243) |
| Aspartate transaminase (median [IQR]) (UI/L) | 20 (17.5–30.3) |
| Creatinine (median [IQR]) (μmol/L) | 74 (61–88) |
| Creatine kinase isoenzyme (median [IQR]) (UI/L) | 20 (16.8–24) |
| Lactate dehydrogenase (median [IQR]) (μ/L) | 239.5 (194–320.8) |
| D-dimer (median [IQR]) (mg/L) | 310 (170–582) |
| C-reactive protein (median [IQR]) (mg/L) | 16.3 (6.2–49.2) |
| Disease severity | |
| Severe | 42 (64.6) |
| Oxygen supplement | 58 (89.2) |
| Invasive mechanical ventilation | 2 (3.1) |
| Intensive care unit admission | 10 (15.4) |
Bold texts indicate P < 0.05.
Abbreviation: IQR, interquartile range.
3.2. Comparison of detection rates of SARS-CoV-2 in different sample types
A total of 195 respiratory tract samples were collected from 65 patients, among which only 23 could produce sputum. In patients who had sputum collected, the detection rate in sputum (95.65%, 22/23) was significantly higher than those in throat swabs (34.78%, 8/23) and nasal swabs (65.22%, 15/23) (P < 0.001) (Table 2 ). Similarly, the detection rate in hock-a-loogie saliva was 88.09% (37/42), significantly higher than those in throat swabs (45.24%, 19/24) and nasal swabs (76.19%, 32/42) (P < 0.001). SARS-COV-2 detection rates were significantly higher in sputum and hock-a-loogie saliva than those in throat swabs and nasal swabs (P < 0.001). The detection rate of SARS-CoV-2 increased to 76.9% based on either positive throat swab or nasal swab.
Table 2.
Detection of SARS-CoV-2 in respiratory sites of COVID-19 cases.
| Groups | Sample types |
p values | |||
|---|---|---|---|---|---|
| Throat swabs (n/N, %) | Nasal swabs(n/N, %) | Sputum (n/N, %) | Saliva (n/N, %) | ||
| Sputum | 8/23 (34.78) | 15/23 (65.22) | 22/23 (95.65) | NA | <0.001 |
| Saliva | 19/42 (45.24) | 32/42 (76.19) | NA | 37/42 (88.09) | <0.001 |
| Total | 27/65 (41.54) | 47/65 (72.31) | 22/23 (95.65) | 37/42 (88.09) | <0.001 |
Bold texts indicate P < 0.05.
Abbreviation: NA, not applicable.
3.3. Comparison of SARS-COV-2 viral loads in different respiratory sample types
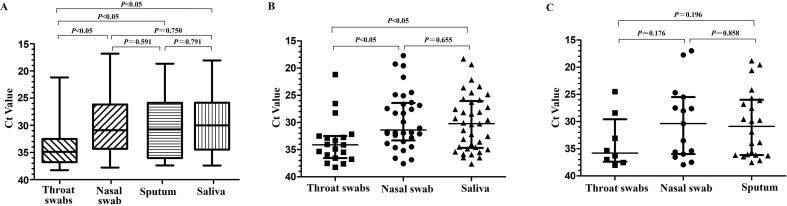
We found that the viral loads of sputum, hock-a-loogie saliva and nasal swabs were significantly higher than that of throat swabs (P < 0.05), while there was no significant difference between sputum and hock-a-loogie saliva (Fig. 1 A). In patients without sputum, the hock-a-loogie saliva and nasal swabs viral loads were significantly higher than that of throat swabs (P < 0.05), but there was no difference between the hock-a-loogie saliva and nasal swabs (Fig. 1B). In patients whose sputum were collected, there was no significant difference between sputum, nasal swabs and throat swabs (Fig. 1C).
Fig. 1.
SARS-CoV-2 viral load in different respiratory tract types (A) Comparison of Ct Value of different sample types in all patients. (B), Comparison of Ct Value of different sample types in patients without sputum. (C), Comparison of Ct Value of different sample types in patients with sputum.
4. Discussion
Rapid and accurate screening of suspected SARS-CoV-2 cases is of great significance for epidemic prevention and control [6], [7]. However, reports have shown false negative PCR testing in patients with SARS-CoV-2 infection, raising concerns of undetected spread [8]. Several factors may lead to false negatives by PCR. While the currently used clinical detection assays of SARS-CoV-2 has been approved by CFDA, the performance of these reagents has not been systematically evaluated, and the instability of the reagents may cause false negatives. Additionally, due to differences in tissue affinity, viral loads may differ between the different sample types. Therefore, it is of great significance to select appropriate sites to collect samples to improve detection rate and reduce false negatives.
In this study, we found that the detection rate of SARS-CoV-2 by RT-PCR was low in throat swabs, detected in less than half of confirmed patients. Lower detection rates in throat swabs is likely due to the ACE2 receptor being utilised by SARS-CoV-2 [9], [10]. The ACE2 receptor is also found in the closely related SARS virus, where the ACE2 expression level of pharyngeal squamous cells was shown to be significantly lower than those of lung and other lower respiratory tract tissues [11], directly leading to a lower amount of virus in the pharynx. In addition, throat swabs quality can be affected by eating, swallowing and other factors, further reducing the detection rate.
We found that spittle samples containing sputum had the highest detection rate compared to other respiratory samples, possibly because the sputum originates from the lower respiratory tract where the alveoli is shown to contain a high number of ACE2 receptors [11]. However, it is worth noting that, compared with other respiratory virus infections, one of the clinical symptoms of SARS-CoV-2 infection is cough without sputum [14], [15]. In this study, we also found that only 25% of patients with SARS-CoV-2 had sputum symptoms, and for the majority of patients, there was no sputum available for detection.
We found that the detection rate of nasal swabs was 72.31%, higher than that of throat swabs. However, the collection of nasal swabs requires a higher level of operators and cause patient discomfort, increasing patient resistance and affecting the sample quality [12], [13]. Whereas, sputum is a non-invasive specimen that has been used for the detection of respiratory viruses.
Our study has identified that the detection rate and viral loads in hock-a-loogie saliva samples were similar to those detected in sputum, despite the lack of sputum after deep cough. This may result from collection followed by deep cough, as a large number of viruses accumulated in the lower respiratory tract such as lungs and bronchi were expelled with high air pressure, making virus detection possible. Reports also indicated that saliva from deep cough may be mixed with unformed sputum with a high viral load [12].
In clinical practice, the use of hock-a-loogie saliva samples to detect SARS-CoV-2 has many advantages. Firstly, collecting hock-a-loogie saliva rather than throat swabs and nasal swabs avoids patient from discomfort. Secondly, the collection of hock-a-loogie saliva is suitable for patients for whom the collection of nasopharyngeal specimens is contraindicated, such as patients with severe bleeding tendency. Thirdly, patients can provide hock-a-loogie saliva specimens following simple instructions, whereas the collection of nasopharyngeal specimens must be performed by healthcare personnel [16]. Meanwhile, collection of throat swabs and nasal swabs increases risk of SARS-CoV-2 infection due to the proximity of face-to-face interactions with patients, while hock-a-loogie saliva collection does not. We recommend that patients wear masks for deep coughs, greatly reducing the risk of infection by sampling [12], [16].
There are several limitations to our study. Firstly, although the detection reagent we used in this study has passed CFDA certification, we have not evaluated its performance, and we do not know whether there is any difference in the detection sensitivity in different sample types. Secondly, the sample size of this study is small, so the influence of severity and progression of disease on detection rate cannot be evaluated. Thirdly, our study did not include severe unconscious patients who were unable to e spit. Finally, as this study only included hospitalized patients with more severe disease whose viral loads may be higher, further studies should be conducted on outpatients with milder symptoms.
In conclusion, this study showed that hock-a-loogie saliva sampling for SARS-CoV-2 has excellent sensitivity, and is highly accurate and reliable. Due to the higher convenience of sampling hock-a-loogie saliva than the throat swabs and nasal swabs, we strongly recommended detection in SARS-CoV-2 suspected patients using hock-a-loogie saliva.
Author contributions
Y. C was principal investigators, designed and supervised the study, and wrote the grant application (assisted by S. Z). S. Z, F. Y, J. F, X. W, L. Y, K. X, and J. S had roles in recruitment, data collection, and clinical management. S. Z, F. Y, Q. Z, B. L, G. X, X. Y, W. C, Q. W, D. Z, R. W, B. F, Y. D, L. H, Y. T and Z. D did clinical laboratory testing and analysis. S. Z, F. Y, J. F, X. W, and Y. drafted the Article. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
Acknowledgments
We acknowledge the contributions of other clinical and technical staff of the First Affiliated Hospital, College of Medicine, Zhejiang University; and Prof. Dhanasekaran Vijaykrishna from the Monash University for comments on the manuscript.
Financial support
This work was supported by the China National Mega-Projects for Infectious Diseases (grant number 2017ZX10103008 and 2018ZX10101001); and the National Natural Science Foundation of China (grant numbers 82072377 and 81702079).
Declaration of Competing Interest
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zhu N., Zhang D., Wang W., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q., Guan X., Wu P., et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya A.E., Susan C., et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. 2020;02(07) [Google Scholar]
- 4.National Health and Family Planning Commission of PRC. The national notifiable infectious disease report, [http://www.nhc.gov.cn/xcs/yqfkdt/202002/261f72a74be14c4db6e1b582133cf4 b7.shtml].
- 5.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Gayle A.A., Wilder-Smith A., et al. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel. Med. 2020 doi: 10.1093/jtm/taaa021. pii: taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D.K.W. Chu, Y. Pan, S.M.S. Cheng, et al., Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia, Clin Chem. 2020; pii: hvaa029. [DOI] [PMC free article] [PubMed]
- 8.Xie X., Zhong Z., Zhao W., et al. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020;200343 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Y., Shang J., Graham R., et al. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J. Virol. 2020 doi: 10.1128/JVI.00127-20. pii: JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamming I., Timens W., Bulthuis M.L., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004 Jun;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To K.K.W., Yip C.C.Y., Lai C.Y.W., et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin. Microbiol. Infect. 2019;25(3):372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.G., Yun S.G., Kim M.Y., et al. Comparison between Saliva and Nasopharyngeal Swab Specimens for Detection of Respiratory Viruses by Multiplex Reverse Transcription-PCR. J. Clin. Microbiol. 2016;55(1):226–233. doi: 10.1128/JCM.01704-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;pii: S0140–6736(20):30183–30185. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2019;2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.To K.K., Lu L., Yip C.C., et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg. Microbes Infect. 2017;6(6) doi: 10.1038/emi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]