Abstract
Purpose
Recurrent intracranial metastases after whole-brain irradiation pose a clinical challenge owing to the escalating morbidity associated with their treatment. Although stereotactic radiosurgery is increasingly being used, there are still situations in which whole-brain reirradiation (ReRT) continues to be appropriate. Here, we report our experience using whole-brain pulsed reduced dose rate radiation therapy (PRDR), a method that delivers radiation at a slower rate of 0.067 Gy/min to potentially increase sublethal damage repair and decrease toxicity.
Methods and Materials
Patients undergoing whole-brain ReRT with PRDR from January 1, 2001 to March 2019 were analyzed. The median PRDR ReRT dose was 26 Gy in 2 Gy fractions, resulting in a median total whole-brain dose of 59.5 Gy. Cox regression analysis was used for multivariate analysis. The Kaplan-Meier method was used for overall survival, progression free survival, and to evaluate the ReRT score. Binary logistic regression was employed to evaluate variables associated with rapid death.
Results
Seventy-five patients were treated with whole-brain PRDR radiation therapy. The median age was 54 (range, 26-72), the median Karnofsky performance status (KPS) was 80, and 86.7% had recursive partitioning analysis scores of 2. Thirty-two patients had over 10 metastases and 11 had leptomeningeal disease. The median overall survival was 4.1 months (range, 0.29-59.5 months) with a 1 year overall survival of 10.4%. Age, KPS, dexamethasone usage, and intracranial disease volume were significantly correlated with overall survival on multivariate analysis. A KPS ≤70 was associated with rapid death after radiation. The prognostic value of the ReRT score was validated. The most common acute toxicities were fatigue (23.1%) and headache (16.9%).
Conclusions
In this large cohort of patients with advanced intracranial metastases, PRDR achieves acceptable survival and may decrease toxicity associated with ReRT. PRDR is an easily implemented technique and is a viable treatment option for ReRT of brain metastases.
Introduction
Brain metastases occur in approximately 170,000 patients with cancer per year with up to 25% of patients with melanoma, lung adenocarcinoma, and small cell lung cancer presenting with brain metastases.1,2 Although the indications for focal therapies with stereotactic radiosurgery are expanding with many centers treating up to 10 or even more lesions, whole-brain radiation therapy (WBRT) is still widely used. In a subset of patients receiving WBRT, subsequent progression in the brain will warrant retreatment where focal techniques may not be safe due to the size or extent of metastatic deposits. In these cases, whole-brain reirradiation (ReRT) represents the only feasible treatment, although its adoption has been limited owing to concerns for toxicity.
Several methods exist for limiting the toxicity associated with whole-brain ReRT. Based on previously published series, a common technique is decreasing the total radiation dose or fraction size.3, 4, 5, 6, 7, 8 Other techniques involve blocking the optic nerve and chiasm via a so-called peninsula block. Finally, although not expressly pursued in ReRT, hippocampal sparing WBRT may represent a technique that can decrease late effects in patients treated with ReRT.9,10
At the University of Wisconsin, we have attempted to decrease the late effects of whole-brain ReRT by using the technique of pulsed reduced dose-rate radiation therapy (PRDR), in which the rate of radiation therapy is decreased from standard rates of several gray per minute to 0.067 Gy per minute. Slowing the delivery of radiation may increase sublethal damage repair in normal tissues and has been associated with decreased release of transforming growth factor-ß.11,12 There may also be an increased therapeutic ratio through improved efficacy in tumor control as a result of low-dose hypersensitivity.13, 14, 15, 16 Here, we present a retrospective study of our outcomes in retreating patients with whole-brain PRDR radiation therapy.
Methods and Materials
Patient selection and characteristics
Patients with brain metastases treated with whole-brain ReRT at the University of Wisconsin between December 15, 1999, and November 29, 2017, were retrospectively identified. Inclusion criteria included age over 18 years and prior whole-brain radiation. This study was approved by the institutional review board. Two patients received prophylactic cranial irradiation while the remainder were treated to a median dose of 30 Gy during the initial treatment (range, 24-51.35 Gy). Patients who received whole-brain ReRT for primary brain tumors were excluded. Patients selected for ReRT were those for whom stereotactic radiosurgery was not considered to be an appropriate option. Rationale for retreatment has evolved with increasing use of stereotactic radiosurgery and decreasing utilization of whole-brain ReRT. Currently, we typically deliver whole-brain ReRT to patients with 10 or more metastases, leptomeningeal disease, or a recurrence in an area that is felt to be unsafe for further standard dose rate radiation (2 prior radiosurgeries or radiosurgery and fractionated stereotactic radiation therapy).
Radiation technique
Whole-brain ReRT was delivered using standard opposed lateral fields, similar to those used for the initial radiation therapy course. Patients were immobilized with a custom thermoplastic mask. Radiation was delivered at an effective dose rate of 0.067 Gy/min by delivering a pulse of 20 cGy at the standard dose rate every 3 minutes, as previously performed.17, 18, 19 There was no use of field in field or wedges to account for inhomogeneity of the plans. The median dose used was 26 Gy in 13 fractions (range, 24-30 Gy).
Statistical methods
Overall survival was measured from the start of the ReRT course until the date of death using the Kaplan-Meier method. A number of patient characteristics were extracted from chart review including age, primary tumor histology, Karnofsky performance status (KPS), dexamethasone dose, systemic disease status, size of largest brain metastases, number of brain metastases, and presence of leptomeningeal disease. Recursive partitioning analysis (RPA) scores were assigned as performed previously.20 The volume of intracranial metastatic disease was obtained by contouring all intracranial disease in MIM Maestro (MIM Software Inc, Cleveland, OH) for 55 patients with available magnetic resonance imaging (MRIs). Univariate and multivariate analysis were performed using a Cox regression analysis. Variables significant on univariate analysis were included in multivariate analysis. Binary logistic regression was used to evaluate variables associated with survival less than 2 months from initiation of radiation therapy.
Results
Seventy-five patients were identified who had been retreated with PRDR radiation therapy. Seventy-one of these 75 patients were also included in a prior study of 1361 patients treated for brain metastases from 1999 to 2014,21 suggesting a 5% rate of ReRT. The most common primary tumor site was breast in 27 patients (36.0%), followed by non-small cell lung cancer in 19 (25.3%), and small cell lung cancer in 13 (12.3%) (Table 1). The median age and KPS were 54 and 80, respectively (Table 1). A whole-brain RPA score of 2 was assigned to 86.7% of patients. Thirty-two patients (49.2% with available MRI) had more than 10 metastases (Table 1). Three patients had a single brain metastasis and were treated with WBRT owing to leptomeningeal disease, a large brain stem lesion, and small cell histology. A total of 11 patients had leptomeningeal disease. Forty patients had more than 10 metastases or leptomeningeal disease (53.3%), meeting our current requirement for ReRT. The median total tumor burden was 9.8 cm3. Systemic disease progression was present in 48.0% of patients. The primary tumor was controlled in 51.4% of patients. The median time from the previous treatment was 9.7 months. At consultation, 66.7% were symptomatic and receiving dexamethasone, with a 0median dexamethasone dose of 4 mg (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | |
|---|---|
| Median age | 54 y |
| Median time to recurrence | 9.7 mo |
| KPS | Number of patients (%) |
| ≤70 | 24 (32.0) |
| ≥80 | 51 (68.0) |
| Primary tumor | |
| NSCLC | 19 (25.3) |
| SCLC | 13 (12.3) |
| Breast | 27 (36.0) |
| Renal cell | 5 (6.7) |
| Melanoma | 4 (5.3) |
| Other | 7 (9.3) |
| RPA | |
| 1 | 4 (5.3) |
| 2 | 65 (86.7) |
| 3 | 6 (8.0) |
| Systemic disease status | |
| No evidence of disease | 6 (8.0) |
| Stable or responding | 20 (26.7) |
| Progressing | 36 (48.0) |
| Unknown | 12 (16.0) |
| Number of metastases | |
| 1-4 | 15 (23.1) |
| 5-9 | 18 (27.7) |
| ≥10 | 32 (49.2) |
| Leptomeningeal disease | 11 (14.7) |
| Size of metastases | |
| 0-1 cm | 16 (26.1) |
| 1-3 cm | 31 (59.4) |
| >3 cm | 10 (14.5) |
| Median total volume of disease | 9.8 cm3 |
Abbreviations: KPS = Karnofsky performance status; NSCLC = nonsmall cell lung cancer; RPA = recursive partitioning analysis; SCLC = small cell lung cancer.
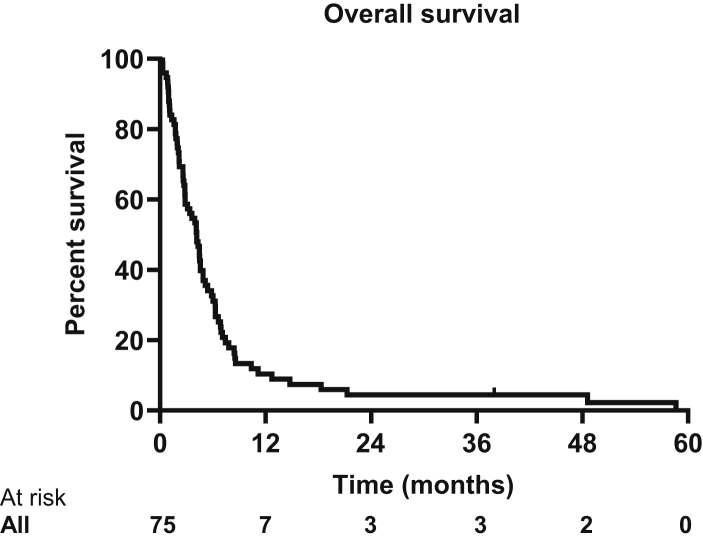
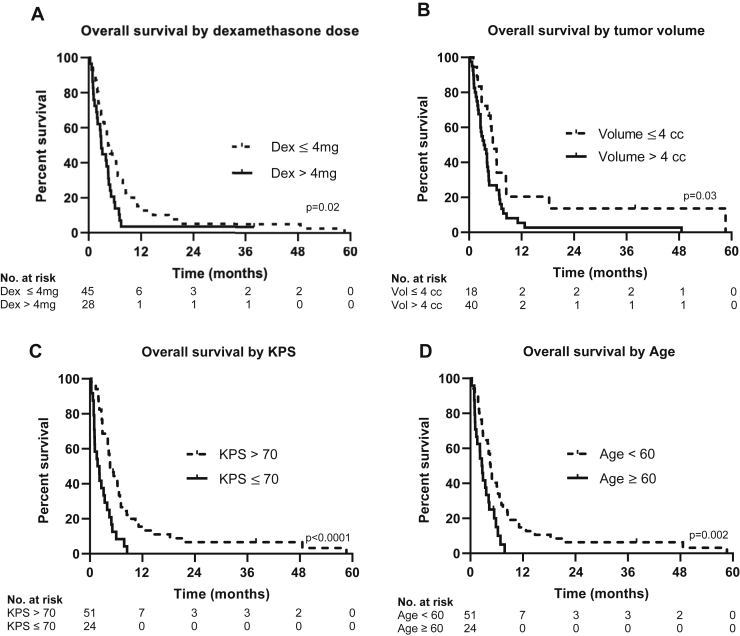
Overall survival for all patients was 4.1 months (95% confidence interval [CI], 3.16-5.19 months) (Fig. 1). The longest surviving patient lived 58.6 months. On univariate analysis, age, KPS ≤ 70, dexamethasone use of greater than 4 mg at initial consultation, and a volume of metastatic disease greater than 4 cm3 were all associated with worse overall survival (Table 2 and Fig. 2). These variables remained significant on multivariate analysis. Time from initial whole-brain radiation, progressive systemic disease, size of largest metastatic deposit, number of intracranial metastases, and leptomeningeal disease were not associated with worse survival in this cohort.
Figure 1.
Kaplan-Meier curve of overall survival with a median overall survival of 4.1 months (95% confidence interval [CI], 3.16-5.19 months).
Table 2.
Univariate analysis and multivariate analysis
| Univariate analysis | |||
|---|---|---|---|
| Variable | HR | 95% CI | P value |
| Dexamethasone >4 mg | 1.74 | 1.07-2.85 | .03 |
| KPS ≤70 | 2.78 | 1.65-4.66 | .0003 |
| Age (continuous) | 1.05 | 1.01-1.08 | .01 |
| ≤9.0 months from prior whole-brain radiation therapy | 1.40 | 0.87-2.26 | .17 |
| Volume >4 mL | 1.99 | 1.07-3.69 | .02 |
| Leptomeningeal disease | 1.83 | 0.93-3.59 | .08 |
| 10 or more brain metastases | 1.30 | 0.77-2.17 | .33 |
| Largest metastases ≥3 cm | 1.13 | 0.60-2.14 | .70 |
| Systemic disease progressing | 1.12 | 0.66-1.88 | .67 |
| Primary controlled | 0.84 | 0.52-1.35 | .47 |
| Free from extracranial disease | 0.56 | 0.26-1.23 | .15 |
| Small cell histology | 1.55 | 0.82-2.94 | .18 |
| Multivariate analysis | |||
|---|---|---|---|
| Variable | HR | 95% CI | P value |
| Dexamethasone >4 mg | 2.09 | 1.11-3.91 | .02 |
| KPS ≤70 | 2.10 | 1.14-3.87 | .02 |
| Age (continuous) | 1.05 | 1.01-1.08 | .01 |
| Volume >4 mL | 1.94 | 1.04-3.63 | .04 |
Abbreviations: CI = confidence interval; HR = hazard ratio; KPS = Karnofsky performance status.
Figure 2.
Overall survival stratified by (A) dexamethasone use, (B) volume of disease, (C) Karnofsky performance status (KPS), and (D) age.
Forty-eight patients did not have follow-up imaging, as initially we did not routinely obtain MRIs after WBRT, and patients who have clinical progression typically enroll in hospice with no further imaging. The median progression free survival for all patients in this study was 3.0 months. The overall response rate was 14.8% among all patients (repeat imaging was initially not routinely obtained) and 40.7% if only patients with repeat imaging are considered. On repeat imaging, 6 patients had a partial response, 5 patients had stable disease, and 16 patients had progressive disease.
Moderate rates of the expected side effects were observed. The most common acute side effect was fatigue (23.1%), followed by headache (16.9%) and weakness (7.7%) (Table 3). Seventeen patients (26.2%) were noted to have no side effects, while 4 patients had improvement in focal symptoms, such as leg weakness. One patient developed seizures, though whether this was caused by disease progression or as a result of ReRT is not known. Of the 49 patients with dexamethasone dosing available before and after radiation therapy, 17.5% had decreased dexamethasone use, 54.4% remained on the same dose, and 28.1% had increased dose at the completion of radiation therapy (Table 3).
Table 3.
Summary of acute toxicities
| Toxicity | Frequency (%) |
|---|---|
| None | 17/65 (26.2%) |
| Headache | 11/65 (16.9%) |
| Fatigue | 15/65 (23.1%) |
| Seizure | 1/65 (1.5%) |
| Focal symptoms improved | 4/65 (6.2%) |
| Skin | 4/65 (6.2%) |
| Weakness | 5/65 (7.7%) |
| Memory changes | 2/65 (3.1%) |
| Stopped radiation early | 8/75 (10.7%) |
| Dexamethasone decreased/same/increased | 10/31/16 |
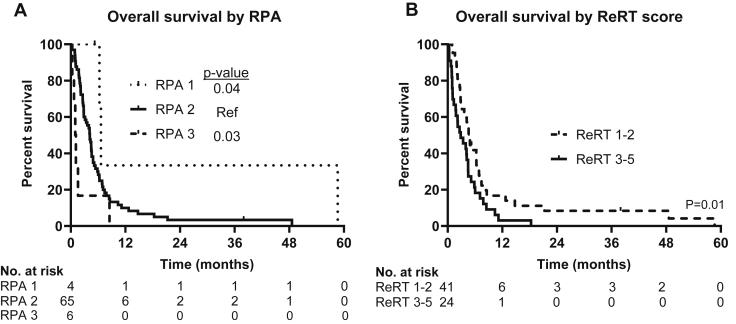
We validated the utility of previously published prognostic markers in our cohort. RPA appeared to be prognostic, with a statistically significant difference between RPA groups, although patient numbers were quite small in the RPA 1 and RPA 3 groupings (Fig. 3). We also assessed the utility of the previously published ReRT score, which incorporates KPS <80, interval to ReRT < 9 months, uncontrolled primary, small cell histology, and presence of extracranial metastases, with 1 point given for each variable present.8 ReRT score of 1 to 2 was associated with improved overall survival compared with a score of 3 to 4 with a median survival of 4.60 months (95% CI, 3.73-5.47 months) versus 2.86 months (95% CI, 0.86-4.86, P = .01), thus validating the original study (Fig. 3).
Figure 3.
Kaplan-Meier curves of overall survival stratified by (A) recursive partitioning analysis (RPA) and by (B) reirradiation (ReRT) scores.
One crucial aspect of patient selection for ReRT is to avoid treating patients who will die rapidly following the completion of radiation therapy. As a result, we endeavored to determine variables associated with demise within 1 and 2 months using binary logistic regression, evaluating all variables that were significant on univariate analysis. Using this technique, we found that KPS ≤70 was associated with increased risk of death within 2 months (hazard ratio, 6.67; P = .008). No other variables were found to be associated with rapid death.
Discussion
The decision of whether to pursue whole-brain ReRT requires an individualized approach with careful evaluation of the costs and the benefits of ReRT. Here, we evaluate the outcomes of patients treated with PRDR ReRT, a technique that could help limit toxicity and improve the quality of life of patients treated. The toxicity observed in this study was moderate and expected for WBRT, though due to the poor prognosis of this patient group, toxicity remains difficult to fully characterize.
The overall survival observed in this study was comparable to many previous studies in the retreatment setting (range, 2-6.9 months), which are reviewed in great detail in Logie et al.8 Our overall survival of 4.1 months was also in line with that expected for patients who underwent initial radiation with an RPA score of 2. Although patients in this study compared favorably with many prior studies in terms of KPS and RPA, they also had advanced intracranial disease. This has not been consistently reported across prior studies, but approximately 65% of patients here had more than 10 brain metastases or leptomeningeal disease. Thus, PRDR treatment appears to lead to comparable oncologic outcomes with the possibility of decreased toxicity due to dose rate and fractionation.
In terms of patient selection, validating the value of the ReRT score in this cohort suggests the validity of this tool in prognostication in this population.8 Certainly, the characteristics of this cohort are quite different from the ReRT cohort, as the ReRT score was built from 5 significant findings on univariate analysis of KPS <80, interval <9 months, uncontrolled primary, small cell histology, and presence of extracranial metastases. We found that only KPS was associated with worse survival in our cohort. The fact that the ReRT score remained significant in this cohort, where the individual variables were not significantly associated with survival, confirms the utility of this prognostic tool.
This study may further our understanding of the prognosis of these patients by identifying 2 novel prognostic variables. This appears to be the first study to find the volume of intracranial disease to be significantly associated with survival in the retreatment setting. We found that the overall intracranial disease burden is significantly correlated with overall survival in this cohort, while number of metastases and size of individual metastases are not. In particular, an overall disease burden of ≥4 cm3 is associated with worse overall survival. Interestingly, a similar finding was demonstrated in a cohort of patients undergoing stereotactic radiosurgery, suggesting the general importance of disease volume to prognosis.22 Additionally, we were able to use dexamethasone use as a surrogate for clinical symptoms instead of the previously used Radiation Therapy Oncology Group symptom score. Dexamethasone dose may be a more reliable prognostic variable due to its quantitative nature. The combination of volume of disease and dexamethasone dose provide additional, discrete prognostic information.
Perhaps the most important question is whether whole-brain ReRT has significant clinical benefit in general. Here, we found that 17.5% of patients had decreased steroid dose after radiation therapy. This is similar to our overall response rate and demonstrates an objective response in a subset of patients. Furthermore, we expect that this therapy delayed the progression of disease in additional patients. However, considering the results of the Quality of Life after Treatment for Brain Metastases study, where no benefit was seen in patients with nonsmall cell lung cancer who underwent whole-brain irradiation in the upfront setting, caution is needed whenever recommending WBRT and especially with ReRT.23 One situation where there appears to be very little benefit from whole-brain ReRT is when patients die rapidly after treatment. We found that KPS of ≤70 was prognostic in this regard, and the utility of whole-brain ReRT should be used with even more caution in this cohort.
In patients undergoing ReRT, selection of radiation technique and fraction size are critical. Certainly, hippocampal-sparing radiation can and should be considered in this cohort, as there is phase III randomized evidence showing this technique is associated with improved cognitive outcomes.10 There is less information in the context of ReRT. The potential advantages of PRDR include that it is a 3-dimensional technique that can easily be employed on a standard linear accelerator and has the potential to decrease toxicity to the whole-brain, though further work is needed to evaluate this assertion.
In summary, survival after whole-brain ReRT appears similar to initial whole-brain radiation. The data obtained here can be used to help inform patients of the likely benefit of this therapy. In particular, this data further suggest that patients with high ReRT scores or KPS ≤ 70 have relatively little to gain from this therapy and are likely better served by best supportive care. Avoiding the treatment of patients with extremely poor prognosis is crucial to the overall efficacy of this approach. PRDR radiation therapy requires no additional equipment and may tip the balance of cost versus benefit, though further work is needed to better assess the potential benefit of this technique.
Footnotes
Sources of support: No funding was received for this work.
Disclosures: We certify that we have no conflicts of interest.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Tabouret E., Chinot O., Metellus P., Tallet A., Viens P., Goncalves A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 2.Cagney D.N., Martin A.M., Catalano P.J. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 2017;19:1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scharp M., Hauswald H., Bischof M., Debus J., Combs S.E. Re-irradiation in the treatment of patients with cerebral metastases of solid tumors: Retrospective analysis. Radiat Oncol. 2014;9:4. doi: 10.1186/1748-717X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong W.W., Schild S.E., Sawyer T.E., Shaw E.G. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996;34:585–590. doi: 10.1016/0360-3016(95)02156-6. [DOI] [PubMed] [Google Scholar]
- 5.Son C.H., Jimenez R., Niemierko A., Loeffler J.S., Oh K.S., Shih H.A. Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:e167–e172. doi: 10.1016/j.ijrobp.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Ozgen Z., Atasoy B.M., Kefeli A.U., Seker A., Dane F., Abacioglu U. The benefit of whole brain reirradiation in patients with multiple brain metastases. Radiat Oncol. 2013;8:186. doi: 10.1186/1748-717X-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadikov E., Bezjak A., Yi Q.L. Value of whole brain re-irradiation for brain metastases—single centre experience. Clin Oncol. 2007;19:532–538. doi: 10.1016/j.clon.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Logie N., Jimenez R.B., Pulenzas N. Estimating prognosis at the time of repeat whole brain radiation therapy for multiple brain metastases: The reirradiation score. Adv Radiat Oncol. 2017;2:381–390. doi: 10.1016/j.adro.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gondi V., Tolakanahalli R., Mehta M.P. Hippocampal-sparing whole-brain radiotherapy: A "how-to" technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown P.D., Gondi V., Pugh S. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: Phase III trial nrg oncology cc001. J Clin Oncol. 2020;38:1019–1029. doi: 10.1200/JCO.19.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marples B., Collis S.J. Low-dose hyper-radiosensitivity: Past, present, and future. Int J Radiat Oncol Biol Phys. 2008;70:1310–1318. doi: 10.1016/j.ijrobp.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 12.Meyer J.E., Finnberg N.K., Chen L. Tissue tgf-beta expression following conventional radiotherapy and pulsed low-dose-rate radiation. Cell Cycle. 2017;16:1171–1174. doi: 10.1080/15384101.2017.1317418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin L.A., Smith C.E., Langston M.Y., Quashie D., Dillehay L.E. Response of glioblastoma cell lines to low dose rate irradiation. Int J Radiat Oncol Biol Phys. 1991;21:397–402. doi: 10.1016/0360-3016(91)90788-6. [DOI] [PubMed] [Google Scholar]
- 14.Short S.C., Kelly J., Mayes C.R., Woodcock M., Joiner M.C. Low-dose hypersensitivity after fractionated low-dose irradiation in vitro. Int J Radiat Biol. 2001;77:655–664. doi: 10.1080/09553000110041326. [DOI] [PubMed] [Google Scholar]
- 15.Tome W.A., Howard S.P. On the possible increase in local tumour control probability for gliomas exhibiting low dose hyper-radiosensitivity using a pulsed schedule. Br J Radiol. 2007;80:32–37. doi: 10.1259/bjr/15764945. [DOI] [PubMed] [Google Scholar]
- 16.Joiner M.C., Marples B., Lambin P., Short S.C., Turesson I. Low-dose hypersensitivity: Current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49:379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- 17.Burr A.R., Robins H.I., Bayliss R.A., Howard S.P. Pulsed reduced dose rate for reirradiation of recurrent breast cancer. Pract Radiat Oncol. 2020;10:e61–e70. doi: 10.1016/j.prro.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Witt J.S., Musunuru H.B., Bayliss R.A., Howard S.P. Large volume re-irradiation for recurrent meningioma with pulsed reduced dose rate radiotherapy. J Neurooncol. 2019;141:103–109. doi: 10.1007/s11060-018-03011-z. [DOI] [PubMed] [Google Scholar]
- 19.Adkison J.B., Tomé W., Seo S. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:835–841. doi: 10.1016/j.ijrobp.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 20.Gaspar L., Scott C., Rotman M. Recursive partitioning analysis (rpa) of prognostic factors in three radiation therapy oncology group (rtog) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 21.Brower J.V., Saha S., Rosenberg S.A., Hullett C.R., Robins H.I. Management of leptomeningeal metastases: Prognostic factors and associated outcomes. J Clin Neurosci. 2016;27:130–137. doi: 10.1016/j.jocn.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Baschnagel A.M., Meyer K.D., Chen P.Y. Tumor volume as a predictor of survival and local control in patients with brain metastases treated with gamma knife surgery. J Neurosurg. 2013;119:1139–1144. doi: 10.3171/2013.7.JNS13431. [DOI] [PubMed] [Google Scholar]
- 23.Mulvenna P., Nankivell M., Barton R. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (quartz): Results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]