Abstract

Copper and palladium/copper nanoparticles supported on reduced graphene oxide catalysts were synthesized and evaluated for the selective NO reduction by CO. The catalysts were characterized by XRD, nitrogen adsorption–desorption, TGA, XPS, TPR, in situ XRD, STEM, and HRTEM. The STEM and HRTEM results showed high metal oxide dispersions on the rGO. XPS results showed the presence of Cu and Pd oxide species. The reduction of copper supported on the rGO occurred in two steps for CuOx/rGOc, while that for CuOx-PdOy/rGOc occurred in one step for temperatures lower than 350 °C. Noteworthy is that the in situ XRD results showed that the rGO structure was not affected after reduction at 350 °C. The in situ XRD of reduction revealed the appearance of new phases for copper during the reduction. The catalysts were evaluated in NO reduction by CO. The tests showed that the reduced catalysts presented high performance with NO conversions and N2 selectivity above 85% at 350 °C.
1. Introduction
The abatement of NOx, nitric oxide (NO) and nitrogen dioxide (NO2), is of paramount importance since these gases are pollutants that generate various undesirable effects, such as photochemical smog, acid rain, and aggravation of the destruction of the ozone layer.1 NOx also causes various health problems and can lead to death in cases of severe intoxication.2 NO and carbon monoxide (CO) gases, emitted by different sources, must be eliminated, and the principal problem is the catalyst for total NOx abatement.3
Catalysts based on copper have been used for the reduction of NO by CO.4 Copper supported on AlPO4,4 Al2O3,5 CeO2,5 zeolites,6 and carbon nanotubes7 was investigated for this reaction. Bimetallic transition-metal oxides (Cu/Fe) supported on commercial and synthesized carbon nanotubes and γ-Al2O3 were also investigated recently. Dasireddy et al.7 reported that Cu/Fe supported on synthesized carbon nanotubes presented high catalytic performance due to the strong interaction of Cu and Fe with the CNT and the acid and base surface sites on the carbon nanotubes. Hu et al.5 studied the NO + CO reaction over CuO/CeO2 and CuO/γ-Al2O3 at low temperatures and showed that NO conversions are related to the loading of CuO and of the support at temperatures lower than 200 °C. At 300 °C, the NO is completely converted to N2.
As reported in the literature, the Cu/Fe bimetallic catalyst presented high activity, and although Pd is more expensive, it facilitates the CO adsorption for the reaction.
Ultimately, graphene in catalysis has aroused great interest.8 Graphene is a two-dimensional material, one of the allotropes of carbon, and has a hexagonal structure of carbon atoms. Graphene has unique and remarkable properties, so its use has been extensively investigated for catalysis, including electrocatalysis, photocatalysis, and conventional heterogeneous catalysis.9 The graphene can be used like an active phase or as a support for metals and metal oxides.10−15
Reduced graphene oxide has a high specific surface area, which is highly desirable in catalysis, and acts as a two-dimensional support for metallic nanoparticles with high dispersion.16 In addition, it has a high adsorption capacity, defects in its structure that can generate new surface functionalities and increase the interactions with the metal nanoparticles,8,17,18 and excellent mechanical properties that allow high stability and durability, increasing the lifetime of the catalyst.9 Reduced graphene oxide (rGO) can be obtained from graphite at a relatively low cost.19,20
Several catalysts based on graphene have been developed and applied in numerous catalytic reactions, such as Fischer–Tropsch synthesis,21,22 selective hydrogenation,23,24 and NOx abatement.25,26 Nanocomposite TiO2/graphene was used for photocatalytic NOx oxidation under UV and visible light irradiation. Cerium and manganese oxides were supported on a TiO2–graphene nanocomposite that showed higher activity and N2 selectivity for low-temperature selective catalytic reduction of NOx with NH3.26 The reduced graphene oxide is an excellent support for nanocomposites since the high specific area allows distribution of the nanoparticles evenly. Furthermore, the structural defects of the rGO may contribute to higher dispersion of the nanoparticle composite.
Therefore, we selected the CuOx/rGOc catalyst for the NO + CO reaction and also verified if by addition of PdO to the CuOx/rGOc catalyst changes the performance of the reaction.
There are a few works using reduced graphene oxide as a support for the NO + CO reaction. In the present work, we present the synthesis and characterization of rGO, impregnated first with copper and impregnated palladium oxide on the CuOx/rGOc catalyst for the selective reduction of NO by CO. We studied the influence of temperature and the promoting effect of palladium on both unreduced and reduced catalysts for the NO + CO reaction tests. Therefore, we can compare the performance of CuOx/rGOc and Cu/rGOr and the promoting effect of PdO on the NO + CO reaction for the rGO.
2. Results and Discussion
2.1. Characterizations
Figure 1 shows XRD patterns of rGO, CuOx/rGOc and CuOx-PdOy/rGOc. In this diffractogram, the broad and low intensity diffraction peak located at 2θ = 25.6° was attributed to the rGO (002) crystalline plane, corresponding to an interlayer distance of 0.347 nm. The diffraction peak at 40.1° for CuOx-PdOy/rGOc belongs to Pd (111).27 The peak at 43.1° showed a diffraction angle, which coincides with the Cu (111) and rGO (100) planes.28,29 No peaks were identified for copper oxide in the diffractograms. However, comparing the XRD patterns of pure rGO (Figure 1a) and the catalysts (Figure 1b,c), we can observe that the diffraction peak at 25.6° was more intense, suggesting that bonding between the rGO layers were reestablished after the impregnation process.30
Figure 1.
XRD patterns of (a) rGO, (b) CuOx/rGOc, and (c) CuOx-PdOy/rGOc samples.
The results of the textural properties (specific area, pore volume, and average pore size) of the graphite, rGO, and catalysts are presented in Table 1. The rGO presented a high specific area (439 m2/g). Graphite showed a low specific area and pore volume. Defects are observed in the regular hexagonal structure of the rGO sheet after the oxidation process of graphite and exfoliation and reduction of graphite oxide.31 There was also a significant reduction of the specific surface area, pore volume, and average pore size after the incorporation of the metals. We did Raman spectroscopy for the original graphite and the reduced graphene oxide, as shown in Figure S1. We can observe the bands D and G and the ID/IG, indicating that reduced graphene oxide presents a high ratio and thus defects as expected.32
Table 1. Textural Properties of the rGO and Catalystsa.
| sample | SBET (m2/g) | Vp (cm3/g) | Rm (Å) |
|---|---|---|---|
| graphite | 12 | 0.1 | 132.6 |
| rGO | 439 | 1.3 | 88.4 |
| CuOx/rGOc | 183 | 0.1 | 66.5 |
| CuOx-PdOy/rGOc | 188 | 0.1 | 69.7 |
SBET, specific surface area; Vp, BJH desorption pore specific volume; Rm, BJH desorption average pore radius.
Figure 2 shows N2 adsorption–desorption isotherms and pore size distributions of rGO and the catalysts. The isotherms and pore size distribution indicate the presence of mesopores and macropores (Figure 2a).33 The hysteresis corresponded to the porous structure of a slit type of the rGO structure with distinct carbon layers and defects in the hexagonal structure (Figure 2b) due to the thermal exfoliation and reduction processes.
Figure 2.
(a) Nitrogen adsorption and desorption isotherms and (b) pore size distribution for rGO, CuOx/rGOc, and CuOx-PdOy/rGOc.
The synthesis method used in this work for obtaining the reduced graphene oxide consists of four steps: oxidation of graphite, purification, drying and expansion, and simultaneous reduction of graphite oxide. The thermal method employed removed considerably the oxygenated groups from the structure, forming the reduced graphene oxide, as can be seen in the FTIR analysis of graphite, graphene oxide (GO), and rGO (Figure 3).
Figure 3.

FTIR analysis of graphite, graphene oxide (GO), and rGO.
As shown, the graphite oxide exhibited the presence of different compounds, indicating the effective functionalization, indicating different oxide and carbonyl groups besides the formation of O–H and C–C and C–O–C groups.34 After reduction, the reduced graphene oxide showed residual functionalized compounds.
TGA results display the mass loss of rGO, CuOx/rGOc, and CuOx-PdOy/rGOc under a N2 atmosphere, as shown in Figure 4. Below 100 °C, all the samples showed a mass loss of water. The total mass losses under a N2 atmosphere were 58, 46.5, and 49.5% for rGO, CuOx/rGOc, and CuOx-PdOy/rGOc, respectively. This can be attributed to the decomposition of oxygen complexes on the surface rather than the decomposition of carbon.
Figure 4.
Mass loss profile of rGO, CuOx/rGOc, and CuOx-PdOy/rGOc under a N2 and air atmosphere.
TGA results with air showed the decomposition of pure rGO from 450 °C until completing at 600 °C. However, with the addition of the metals, the decomposition began earlier at lower temperature. It started at 300 °C and ended almost at 450 °C, with remaining masses of 18.7 and 11.6% for CuOx/rGOc and CuOx-PdOy/rGOc, respectively. The process of reduction of graphene oxide affects the structure, resulting in oxygenated groups and structural defects, and when copper is incorporated in rGO, the decomposition of oxygenated groups and the carbon surface is favored. Therefore, the presence of copper accelerated the decomposition of rGO.30
STEM and HRTEM micrographs and the size distribution of nanoparticles on rGO are shown in Figure 5. The micrographs showed that the metal nanoparticles supported on the rGO are well dispersed. The average sizes of the nanoparticles were 3.8 nm for the CuOx-PdOy/rGOc. The spacing lattice fringes of the nanoparticles were 0.20 and 0.21 nm, attributed to the Cu (111) and CuPd (110) planes, respectively.35 However, the high-resolution image suggests the formation a CuPd nanocomposite, which corresponds to the bcc structure of the CuPd nanocluster. The catalysts were prepared at 300 °C under nitrogen flow, and the reduced graphene oxide has functionalized groups that favored the formation of CuPd nanocomposites during the reduction process of rGO. Therefore, rGO can act as a reducing agent for metal oxides when subjected to high temperatures.
Figure 5.

Micrographs STEM of (a) CuOx/rGOc and (b) CuOx-PdOy/rGOc, (c) size distribution of CuOx/rGOc nanoparticles and (d) CuOx-PdOy/rGOc nanoparticles, and HRTEM of (e) CuOx/rGOc and (f) CuOx-PdOy/rGOc.
The XPS spectra for the CuOx/rGOc and CuOx-PdOy/rGOc catalysts are shown in Figures 6 and 7, respectively. The surface chemical composition of the catalysts was evaluated. The XPS spectrum of CuOx/rGOc presented signals of Cu 2p, C 1s, and O 1s, and that of CuOx-PdOy/rGOc presented signals of Pd 3d, Cu 2p, C 1s, and O 1s. Table 2 shows the atomic percentage of the elements present at the surface. The main element in reduced graphene oxide is carbon as a regular hexagonal network. Oxygen was also identified due to the oxygen groups present at the surface of rGO.
Figure 6.

(a) Pattern and (b–d) high-resolution XPS spectra of (b) C 1s, (c) O 1s, and (d) Cu 2p for the CuOx/rGOc catalyst.
Figure 7.

(a) Pattern and (b–e) high-resolution XPS spectra of (b) C 1s, (c) O 1s, (d) Cu 2p, and (e) Pd 3d for the CuOx-PdOy/rGOc catalyst.
Table 2. Atomic Percentages Calculated from the XPS Spectra of the Catalysts.
| sample | element | peak (eV) | atomic (%) |
|---|---|---|---|
| CuOx/rGOc | C 1s | 284.9 | 80.9 |
| O 1s | 532.0 | 17.3 | |
| Cu 2p | 934.0 | 1.8 | |
| CuOx-PdOy/rGOc | C 1s | 284.9 | 79.9 |
| O 1s | 532.0 | 17.9 | |
| Cu 2p | 934.0 | 1.7 | |
| Pd 3d | 336.7 | 0.5 |
The spectrum of C 1s presents four deconvolution peaks. The main peak at 284.6 eV was attributed to the presence of C=C/C–C in the aromatic rings of the rGO structure.36 The other peaks at 286.1, 288.1, and 291.0 eV were associated to the C–O, and COOH groups, and the satellite peak (π → π*), typical for aromatic carbon structure, respectively.37
The spectrum of Cu 2p showed four deconvolution peaks related to Cu 2p1/2 and Cu 2p3/2. The binding energies of Cu the 2p3/2 peak at 934.2 eV and of the Cu 2p1/2 peak at 954.0 eV are assigned to CuO.38 The binding energies for Cu species are close to the values presented by Biesinger et al.,39 and thus, since the samples were not reduced, the oxidation states of the copper species at the surface are CuO and Cu2O. In fact, the peaks at 932.4 and 951.9 eV are probably associated to Cu2O. The peaks of Pd 3d5/2 at 335.4, 336.1, 337.2, and 338.2 eV are attributed to Pd0, PdOx<1, PdO, and PdO2, respectively. The peaks of Pd 3d3/2 at 340.5, 341.4, 342.5, and 343.8 eV are assigned to Pd0, PdOx<1, PdO, and PdO2, respectively.40,41 The reduction of PdO at the surface to metallic Pd0 particles are probably formed by the X-ray beam spectrum during the XPS experiments. The O 1s spectrum showed the presence of C–O and COOH groups, since the reduced graphene oxide presented these groups in its structure, as shown in the FTIR experiments (Figure 3).
Table 3 shows the atomic percentage of the copper and palladium species present on the surface of the catalysts. The percentages of the copper and palladium species were calculated through the Cu 2p3/2 and Pd 3d5/2 regions, respectively. The calculated Cu2O/CuO ratios were 0.76 and 0.86 for CuOx/rGOc and CuOx-PdOy/rGOc, respectively. Therefore, Cu2O and CuO species coexisted at the surface of the catalysts, with CuO being the major content. Moreover, metallic palladium and oxide were also observed on the CuOx-PdOy/rGOc catalyst.
Table 3. Atomic Percentages of the Copper and Palladium Species Calculated from the XPS Spectra of the Catalysts.
| sample | species | peak (eV) | atomic (%) |
|---|---|---|---|
| CuOx/rGOc | Cu2O | 932.4 | 43.2 |
| CuO | 934.2 | 56.8 | |
| CuOx-PdOy/rGOc | Cu2O | 932.4 | 46.3 |
| CuO | 934.2 | 53.7 | |
| CuOx-PdOy/rGOc | Pd0 | 335.4 | 14.1 |
| PdOx < 1 | 336.1 | 32.2 | |
| PdO | 337.2 | 30.8 | |
| PdO2 | 338.2 | 22.9 |
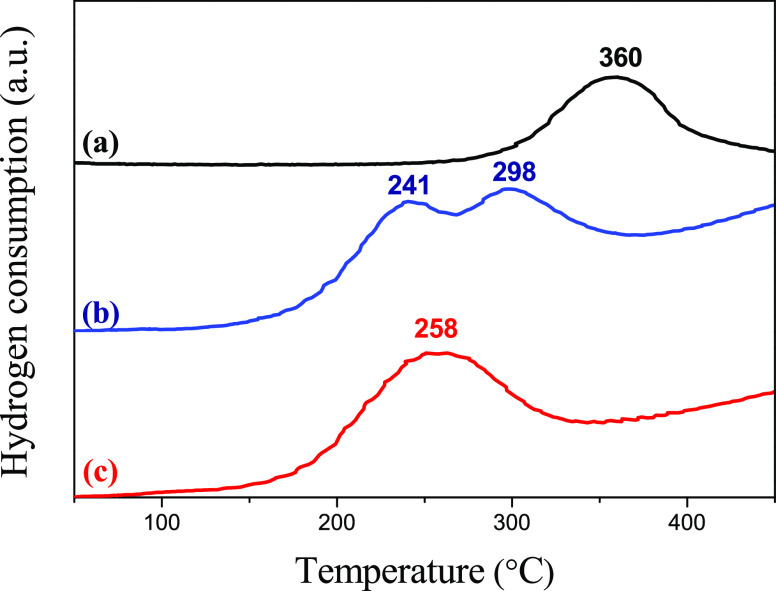
Figure 8 shows the TPR profiles of the hydrogen consumption (H2) of CuO (used as reference), CuOx/rGOc, and CuOx-PdOy/rGOc. The reduction peaks for the catalysts appeared at temperatures lower than for the pure material, which suggests that copper is well dispersed on rGO.5 For the CuOx/rGOc catalyst, copper reduction occurred in two steps with two maximum peaks at 241 and 298 °C. In fact, XPS results showed CuO and Cu2O species at the surface of the rGO catalyst. Therefore, the first peak was associated with the reduction of Cu2+ to Cu+ and the second was associated with the reduction of Cu+ to Cu0. Noteworthy is that for CuOx-PdOy/rGOc, copper reduction occurred in one step, displaying only one peak at 258 °C. Interestingly, we observed a consumption of hydrogen at room temperature for CuOx-PdOy/rGOc, which can be assigned to the reduction of PdO to metallic Pd0 (not shown). It is well known that the reduction of PdO normally takes place at room temperature.42−44 The metallic Pd particles at the surface of rGO may interact with Cu species on the rGO support and catalyzes the reduction of Cu species by shifting the Cu2+ toward lower temperatures from 298 to 258 °C. Therefore, the presence of palladium favored the direct reduction of Cu2+ to Cu0.
Figure 8.
H2 consumption profiles during the TPR of (a) CuO, (b) CuOx/rGOc, and (c) CuOx-PdOy/rGOc.
Figure 9 displays the in situ XRD measurements, which were employed to evaluate the changes in the crystalline structure of copper in the CuOx/rGOc catalyst during the reduction process under H2 flow. Figure 9a shows the diffraction patterns of CuOx/rGOc at room temperature without H2 flow, and Figure 9b–d shows those at different reduction temperatures (250, 300, and 350 °C) under H2 flow. Indeed, with increasing temperature, the structure of copper presented well-defined crystalline planes. The diffraction patterns at 250, 300, and 350 °C displayed four peaks at 25.4, 43.0, 50.0, and 73.5°, relative to the planes of rGO (002), Cu (111), Cu (200), and Cu (220), respectively.29 Furthermore, the Cu (111) peak became more intense and narrow, indicating very crystalline copper nanoparticles. The sizes of copper nanoparticles measured by the Scherrer45 equation were 5.1, 6.0, and 6.0 nm at 250, 300, and 350 °C, respectively. To confirm this, Table S1 presents the data on the full-width at half-maximum (FWHM) and the peak intensity. The data indicates that the Cu (111) peak became more intense and narrow. Comparing the data between 300 and 350 ° C, there was no significant change with the same particle size.
Figure 9.
Diffraction patterns of CuOx/rGOc at (a) room temperature without H2 and at three different temperatures of (b) 250 ° C, (c) 300 ° C, and (d) 350 ° C under H2 flow.
Noteworthy is that the rGO structure was not affected after reduction at 350 °C, as indicated at 2θ = 25.4°, in accordance with the TGA results. SEM images in Figure 10 show the EDS maps of elements, and well-distributed particles and TEM results confirm the presence of nanoparticles on Cu and CuPd supported on rGO samples. The in situ XRD and TPR results showed that using a temperature of 350 ° C under H2 flow made possible obtaining the reduced copper on the surface of rGO.
Figure 10.
(a) SEM, (b–d) EDS maps of elements (b) C, (c) Cu, and (d) Pd, and (e) EDS analysis of Cu and Pd of the CuOx-PdOy/rGOc catalyst.
2.2. Catalytic Test
2.2.1. NO + CO Tests
The performances of the calcined (CuOx/rGOc and CuOx-PdOy/rGOc) and reduced (Cu/rGOr and Cu-Pd/rGOr) catalysts were evaluated in the selective reduction reaction of NO using CO as a reducing agent at different temperatures (150, 250, and 350 °C), as shown in Figure 11. The catalytic tests were performed to analyze the effect of temperature, copper reduction, and palladium addition on the NO and CO conversion and N2 selectivity of the catalysts. The stability test of the catalysts during the NO + CO reaction at T = 350 °C is shown in Figure S2.
Figure 11.

NO and CO conversion and N2 selectivity for unreduced and reduced catalysts: black bar, CuOx/rGOc; red bar, CuOx-PdOy/rGOc; blue bar, Cu/rGOr; green bar, Cu-Pd/rGOr.
The CuOx/rGOc sample presented low NO conversion (12%) at 250 °C, indicating only NO decomposition at this temperature; however, the NO + CO reaction occurred at a higher temperature (350 °C), showing conversions of 24% NO and 10% CO. Palladium was added in small amounts to the copper catalyst supported on rGO in order to evaluate the promoting effect of palladium on the CuOx/rGOc catalyst. Noteworthy is that for the CuOx-PdOy/rGOc sample, the reaction of NO + CO occurred even at a low temperature of 150 ° C, with conversions of 11% NO and 19% CO. The NO conversions of CuOx-PdOy/rGOc were 37 and 61% for 250 and 350 °C, respectively. Therefore, the presence of palladium improved significantly the catalytic activity of the unreduced bimetallic catalyst. However, both unreduced catalysts showed increasing NO and CO conversions by raising the temperature and the highest N2 selectivity was found at 350 °C.
As shown in Figure S2, the Cu/rGOr catalyst was deactivated initially, but NO conversion was constant after some time, increasing with time-on-stream (TOS). On the other hand, the Cu-Pd/rGOr catalyst was very stable in 1 h. Therefore, under such conditions, the catalysts are stable.
The reduced catalysts were studied after treatment in H2/He flow at 350 °C. In fact, the reduction improved the NO and CO conversions markedly. The bimetallic Cu-Pd/rGOr catalyst presented the highest NO conversion (92%) and N2 selectivity (100%) at 350 °C. The reduced Cu/rGOr sample showed 88% NO conversion and 95% N2 selectivity at 350 °C. The NO and CO conversions were significantly higher for both reduced Cu/rGOr and Cu-Pd/rGOr catalysts compared to the unreduced samples. In fact, TPR results confirm that the presence of palladium favored the direct reduction of Cu2+ species and metallic Pd and so is the catalytic activity of the CuOx-PdOy/rGOc catalyst, which we attributed to the presence of Cu ion species and PdOx species at the surface, as observed by XPS, enhancing the reaction cycle of NO + CO. Moreover, the TPR profile confirm that there is interaction between Pd and Cu particles since the maximum peak was shifted to a lower temperature, about 250 °C, which suggests interaction. The XPS indicated the presence of copper oxide in the calcined catalyst under N2 flow. After reduction, these oxides are reduced to metallic particles, according to the TPR results.
The reaction of NO + CO results in N2, N2O, and CO2 formation, according to Scheme 1:
Scheme 1. Reaction of NO + CO.

According to Roy et al.,3 the NO molecule must be dissociated when adsorbed to form N2. Therefore, the reduction of NO by CO occurs on specific sites since CO must be adsorbed and NO be chemisorbed dissociatively. Xu et al.46 studied the co-adsorption of NO and CO on Pd (100) and Pd supported on silica and concluded that the structure equilibrium and coverage for the adsorption of the mixture CO/NO depend strongly on the temperature and crystal orientation.
The performances of various catalysts based on copper and copper/palladium for the NO and CO reaction on different supports were compared with the present catalyst supported on rGO, as shown in Table 4. Flores-Sanchez et al.47 investigated different catalysts based on NiO, CuO, and ZnO for the selective NO reduction by CO. The CuO catalyst exhibited the highest conversion of NO at 275 °C and 94% selectivity to N2. Hu et al.5 investigated the influence of the supports for different copper oxide contents at low temperatures and showed that the reduced oxide species dispersed on the support surface enhanced the activity of the NO reduction by CO. Kacimi et al.4 observed a relationship between the quantity of copper(II) species with a dispersed surface and its ease of reduction at low temperature and the NO reduction activity of the catalyst, like what was done in this work. The copper(I) and (II) species on the rGO surface are easily reduced at low temperatures under H2 flow, indicating that the greater reducibility of copper supported in rGO compared to CuO and other supports favors the NO + CO reaction. Muñoz et al.48 attributed the coexistence of Cu+ and Cu2+ to the formation of N2O at low temperature; however, at temperatures above 250 °C, CO can reduce copper to Cu0 favoring the formation of N2. Fernández-Garcia et al.49 indicated the formation of a CuPd alloy in ceria, which is thermodynamically more stable than when the two metals are separate. The presence of copper improved the rate of CO oxidation on Pd surfaces and dissociation of N–O.49
Table 4. Comparative Catalytic Performance of Different Catalysts for Selective Reduction of NO by CO.
| catalysts | temperature (°C) | XNO (%) | SN2 (%) | reaction condition | reference |
|---|---|---|---|---|---|
| CuOx/rGOc | 350 | 23.8 | 100 | 1 vol % CO + 0.5 vol % NO in He | this work |
| Cu/rGOr | 350 | 87.6 | 95.2 | 1 vol % CO + 0.5 vol % NO in He | this work |
| CuOx-PdOy/rGOc | 350 | 61.1 | 95.1 | 1 vol % CO + 0.5 vol % NO in He | this work |
| Cu-Pd/rGOr | 350 | 91.7 | 100 | 1 vol % CO + 0.5 vol % NO in He | this work |
| CuO | 275 | 100 | 94 | 3.3 vol % CO + 0.7 vol % NO in He | [47] |
| 5CuAIPO4 | 400 | 48 | 100 | 1.5 vol % CO + 0.2 vol % NO + 0.65 vol % O2 in He | [4] |
| 5CuAIPO4 reduced | 250 | 50 | 100 | 1.5 vol % CO + 0.2 vol % NO + 0.65 vol % O2 in He | [4] |
| Cu–Al hydrotalcite | 360 | 100 | 100 | 1 vol % CO + 1 vol % NO in He | [48] |
| 11.1CuO/γ-Al2O3 | 200 | <60 | 0 | 10 vol % CO + 5 vol % NO in He | [5] |
| 4.9CuO/CeO2 | 200 | <98 | 100 | 10 vol % CO + 5 vol % NO in He | [5] |
| 9.3CuO/CeO2 | 300 | 100 | 100 | 10 vol % CO + 5 vol % NO in He | [5] |
| Pd–Cu/Al2O3 | 200 | <30 | 1 vol % CO + 0.45 vol % O2 + 0.1 vol % NO in N2 | [49] | |
| Pd–Cu/CeO–Al2O3 | 200 | 100 | 1 vol % CO + 0.45 vol % O2 + 0.1 vol % NO in N2 | [49] |
Cheng et al.50 prepared copper catalysts supported on Fe2O3, CeO2, Fe2O3, and CeO2 mixed metal oxide supports for the NO + CO reaction. Mixed oxide catalysts performed better than catalysts of only one type of oxide. The high fraction of oxygen vacancies in the catalyst may facilitate the redox cycle of oxygen during the NO + CO reaction. Bai et al.51 prepared a catalyst of CuO supported on CeO2-Al2O3. The mechanism of the NO + CO reaction on the Cu/CeAl catalyst was proposed. At low temperatures, CO reacts with the adsorbed NO, favoring the formation of N2O. By increasing the temperature, the formation of N2 was favored. The authors attributed the improvement of NO decomposition to the synergistic effect of Ce3+ + Cu2+ ↔ Ce4+ + Cu+.
The reduction of NO by CO is a structure-sensitive reaction since the Pd particle sizes and the morphology may influence the NO dissociation and there is a NO and O2 competition established for the reductant agent.52 STEM micrographs revealed small nanoparticles sizes (3.8 nm) in CuOx-PdOy/rGOc, which explains its higher activity compared to CuOx/rGOc.
Indeed, the activity of copper oxide catalysts involves the ions Cu2+/Cu+, which also have a strong relationship with the supports;5 however, the greater the reducibility of copper oxide species dispersed on the support without interaction with the support, the higher is the activity at lower temperatures. However, at higher temperatures, the activity of copper oxide catalysts involves the Cu ions, which also strongly influence the reaction. In fact, the TPR results (Figure 8) confirm that copper species is reduced at temperatures below 200 °C under H2 flow on both CuOx/rGOc and CuOx-PdOy/rGOc samples. The reduction of CuOx/rGOc occurred in two steps: Cu2+ to Cu+ and Cu+ to Cu0. However, the reduction of CuOx-PdOy/rGOc occurred in one step. Thus, copper is easier reduced when supported on rGO rather than on oxide supports. When increasing the temperature under hydrogen flow during in situ XRD (Figure 9), new peaks related to metallic copper planes (Cu (111), Cu (200), and Cu (220)) appeared. The adsorption of NO is an essential step for the formation of the desired product since NO is adsorbed and CO removes the oxygen from NO to form N2. The new planes of metallic copper after reduction may favor the adsorption of NO on the surface of the catalyst.
The XPS results (Figures 6 and 7) showed Cu2+ and Cu+ species at the surface of CuOx/rGOc and CuOx-PdOy/rGOc. Cu2+ and Cu+ species at the surface. When comparing the reduced and unreduced samples, we observed that the reduced samples presented higher conversions at temperatures lower than 250 °C. It evidences that Cu ion species and Pd on the surface enhance the activity, in agreement with the reported results in the literature.53−55
The influence of the reducibility seems to influence the palladium that subtly changed NO and CO conversions on the reduced catalyst, which can be attributed to Pd0 particles or PdOx species distributed on Cuδ+ ions, as observed by XPS, or dispersed on the rGO support, modifying the sites and the adsorption of NO and CO molecules.56
NO reduction by CO is a specific reaction in which CO is molecularly adsorbed on metallic sites and NO is associatively adsorbed on CuO, in accordance with Cheng et al.,50 and not dissociatively.3 Thus, CO can act by removing the oxygen atoms left by the dissociation of NO–CuO sites, allowing continuous N2 formation. In fact, CO can act in two ways: as a reducing agent in the catalytic reduction of NO and in the reduction of the metal oxide.6
Therefore, these results suggest that the CO molecules are chemically adsorbed on metallic surface Pd0 and simultaneously reduce the oxide PdO at the surface. On the other hand, the Cuδ+ ions at the surface can also oxidize NO molecules with the formation of N2O and transforming them into higher-oxidation state Cu2+, while the CO adsorbed on Pd0 reduces the Cu2+ to lower-oxidation state Cu+, releasing CO2 and Pd0, in agreement with refs (5) and (57).
Noteworthy is that the reduced graphene oxide (rGO) structure was not affected after reduction at 350 °C and that the CuOx/rGOc catalyst favors significantly the formation of N2 and bimetallic CuOx-PdOy/rGOc favors mostly the formation of N2O and less N2.
Shelef and Kummer58 reported for Al2O3 supports that the reduction of NO by CO occurred in two steps: partial reduction of NO to N2O and then subsequent reduction of N2O to N2. These steps correspond to the oxidation of the reduced site. The higher the reducibility of copper oxide species, the better is the cycling of the re-oxidation since NO is adsorbed on Cu2+ and CO on Cu+ sites.59,60
These surface phenomena suggest a different sequence of steps for the NO + CO reaction in which Cu and Pd sites act in different pathways for the mono- and bimetallic catalysts during the NO+CO reaction, either for the unreduced and reduced catalysts. In fact, CO and NO reduce and oxidize Cuδ+, releasing N2 and N2O and CO2 molecules, hence regenerating Cu ions and Pd0 at the surface atoms for new CO adsorption. These mechanisms are described in the following reaction (Scheme 2) for the mono- and bimetallic CuOx/rGOc and the CuOx-PdOy/rGOc catalysts.
Scheme 2. Reaction of NO + CO on the CuOx/rGOc and the CuOx-PdOy/rGOc Catalysts.
According to Cheng et al.,50 NO can also be adsorbed on CuO, increasing the active sites and the reaction rate of the reduced samples. This is also supported by Hu et al.’s5 work that showed the presence of Cu2p3/2 for different supports. For the Cu2+ on Al2O3, the valence of the dispersed copper oxide did not change during the reaction. However, the copper oxide species on CeO2 could decrease the amount of Cu2+ cations during the reaction. Thus, the authors assumed that the reduction of NO by CO might involve cycling of Cu2+/Cu+ at lower temperatures as shown in our proposed schemes.
In this study, the influence of the support is not significant and the presence of Cu ions and of Pd in the metallic form or as a PdOx species play an important role in the reduction of NO by CO either on the reduced or unreduced form. However, the reduced form facilitates the reduction of Pd and the adsorption of CO, while NO can be adsorbed on Cu ions and provide the oxidation-reduction step.
3. Conclusions
Defects were observed in the regular hexagonal structure of the rGO sheet after the oxidation process of graphite and exfoliation and reduction of graphite oxide. Copper and palladium were homogeneously distributed on the rGO surface. The addition of palladium on the catalyst resulted in a high dispersion of nanoparticles with an average size of 3.8 nm. XPS results showed Cu2O/CuO ratios of 0.76 and 0.86 for CuOx/rGOc and CuOx-PdOy/rGOc, respectively, and that Cu2O and CuO species coexisted at the surface of the catalysts, with CuO being the major content. The in situ XRD results showed that the rGO structure was not affected after reduction at 350 °C and revealed also new crystalline metallic copper planes. The bimetallic Cu-Pd/rGOr catalyst presented 92% NO conversion and 100% N2 selectivity at 350 °C. The reduced Cu/rGOr sample presented 88% NO conversion and 95% N2 selectivity at 350 °C. The NO and CO conversions were significantly higher for both reduced catalysts than for the unreduced samples.
4. Experimental Section
4.1. Materials
Graphite powder was supplied by Nacional de Grafite Ltda (Brazil). Potassium permanganate (KMnO4) and palladium nitrate (Pd(NO3)2) solution were purchased from Sigma-Aldrich. Concentrated sulfuric acid (H2SO4), hydrochloric acid (HCl), cupric nitrate trihydrate (Cu(NO3)2·3H2O), and hydrogen peroxide (30 wt %) were purchased from VETEC Quimica Fina Ltda. All chemicals were used as received without any further purification.
4.2. Preparation of Reduced Graphene Oxide (rGO)
Graphite oxide (GO) was prepared by the modified Hummer’s method61 with the oxidation time set at 2 h. First, 12 g of graphite was added to 280 mL of H2SO4 under stirring in an ice bath. KMnO4 (36 g) was added slowly under stirring, while the temperature was kept at lower than 20 °C and then the mixture was 4heated up to 40 °C for 2 h. Then, 600 mL of distilled water was added slowly, and the solution was heated to 95 °C under stirring for 15 min. Finally, 2000 mL of distilled water and 60 mL of hydrogen peroxide were added and the solution was purified by centrifugation in water and diluted HCl (10 wt %) and then dried in air at 60 °C for 24 h. The final GO was treated by thermal expansion and reduction under air flow at 30 °C/min up to 300 °C and then under He flow at 10 °C/min between 300 and 500 °C to obtain the reduced graphene oxide (rGO).
4.3. Preparation of CuOx/rGOc and CuOx-PdOx/rGOc Catalysts
The catalysts were prepared by an incipient wetness impregnation method. A solution of Cu(NO3)2·3H2O was prepared and added slowly to the rGO. After impregnation, the sample was dried at 110 °C overnight followed by calcination at 300 °C in nitrogen for 1 h. The catalyst calcined was designated CuOx/rGOc (x = 0–2). Subsequently, palladium was impregnated on the CuOx/rGOc catalyst using Pd(NO3)2 as the precursor salt, dried, and calcined similarly. The nominal loading of copper and palladium was 5 and 1 wt %, respectively. The bimetallic catalyst was designated CuOx-PdOy/rGOc (y = 0–2).
4.4. Characterizations
X-ray powder diffraction (XRD) analysis was carried out in a Rigaku Miniflex diffractometer operated at 20 kV and 15 mA using Cu Kα radiation (λ = 1.5406 Å). Diffraction data were recorded in the 2θ range from 5 to 90° with a step size of 0.05°/s. Crystal phases were identified using the JCPDS database. Thermogravimetric analysis (TGA) was carried out on Hitachi equipment (model STA7300). TGA curves were obtained under nitrogen and air gas flow (80 mL/min) at a heating rate of 10 °C/min from 30 to 1000 °C. Textural properties were obtained by N2 adsorption/desorption at −196 °C using a Micromeritics ASAP2010 instrument. Before each measurement, the samples were degassed at 200 °C overnight under vacuum in order to remove adsorbed species. Fourier-transform infrared spectroscopy (FTIR) analyses were performed on a Perkin Elmer spectrometer (model Spectrum 100), equipped with a DTGS (triglycine sulfate) detector. The spectra were obtained in the range between 4000 and 600 cm–1 in the transmittance mode with a number of scans of 32 and a resolution of 4 cm–1. Raman analyses were performed at room temperature using a He/Ne laser (λ = 632.84 nm) in the 1000–1800 cm–1 wavelength range to identify the formation of D and G bands.
High-resolution transmission electron microscopy (HRTEM) and scanning transmission electron microscopy (STEM) analyses were performed on a JEOL 2100F at an accelerating voltage of 200 kV. Scanning electron microscopy (SEM) analyses were performed using a field emission scanning electron microscope (FEG-SEM) from FEI Co. (model Quanta 400) with an acceleration voltage of 30 kV coupled with X-ray-dispersive energy detectors (EDS). The surface chemical state of the atoms and their relative abundance were evaluated by X-ray photoelectron spectroscopy (XPS) using an Escalab 250Xi Thermo Scientific spectrometer with a monochromatic Al Kα (1486.6 eV) X-ray source. The XPS spectra were acquired in constant analyzer energy mode (CAE) with a pass energy of 100 eV for survey and 25 eV for high resolution.
Temperature-programmed reduction (H2-TPR) was performed with a mixture of 1.53 vol % H2/Ar (60 mL/min) and a heating rate of 10 °C/min from 30 to 450 °C. Prior to TPR analysis, the sample (150 mg) was pretreated under flowing pure He (60 mL) at 300 °C for 1 h.
In situ XRD analyses were performed on a Rigaku DMAX 2200 diffractometer equipped with an XRK 900 (Anton Paar) reaction chamber. The catalyst was subjected to a flow rate of 50 mL/min with a mixture of 1.53 vol % H2/He, and XRD patterns were obtained at three different temperatures (250, 300, and 350 °C). The XRD patterns were obtained in the 2θ range from 5 to 90° with a step size of 0.05°/s.
4.5. Catalytic Test
The catalysts were tested for the NO reduction with CO as the reducing agent. The tests were performed with unreduced and reduced catalysts. Catalysts were pretreated in situ with flowing He (45 mL/min) at 350 °C for 30 min and reduced with 10 vol % H2/He flow (50 mL/min) at 350 °C for 20 min. The catalyst reduced was designated Cu/rGOr and Cu-Pd/rGOr.
The feed composition consisted of 0.5 vol % NO, 1 vol % CO, and balance in He. The total gas flow rate was 37.5 mL/min and 100 mg of catalyst. The GHSV was 9000/h. The tests were performed at different temperatures (150, 250, and 350 °C) and at atmospheric pressure in a fixed bed quartz microreactor. The effluent gases were analyzed on line using a gas chromatograph (Shimadzu GC-17A) equipped with a thermal conductivity detector (TCD) and a Carboxen1010 column. Helium was employed as a carrier gas.
The NO (XNO) and CO conversion (XCO) and N2 selectivity (SN2) were calculated using eqs 1, 2, and 3, respectively.
| 1 |
where ANO(input) and ANO(output) are the areas of NO peaks before the reaction and during the reaction from the chromatograms, respectively.
| 2 |
ACO(input) and ACO(output) are the areas of CO peaks before the reaction and during the reaction from the chromatograms, respectively.
| 3 |
where AN2 and AN2O are the corrected areas with the response factors of N2 and N2O, respectively.62
Acknowledgments
The authors acknowledge FAPERJ for scholarships; Maria Luiza Rocco (IQ/UFRJ), Cilene Labre Alves da Silva and André Luiz Pinto (LABNANO/CBPF) for the HRTEM/STEM analysis and Nucleus of Catalysis (NUCAT/UFRJ).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02417.
Raman spectroscopy for graphite and reduced graphene oxide (Figure S1); stability test of the catalysts (Figure S2); and data on the full-width at half-maximum (FWHM) and the peak intensity of in situ XRD (Table S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Skalska K.; Miller J. S.; Ledakowicz S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. 10.1016/j.scitotenv.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Weinberger B.; Laskin D. L.; Heck D. E.; Laskin J. D. The Toxicology of Inhaled Nitric Oxide. Toxicol. Sci. 2001, 59, 5–16. 10.1093/toxsci/59.1.5. [DOI] [PubMed] [Google Scholar]
- Roy S.; Hegde M. S.; Madras G. Catalysis for NOx abatement. Appl. Energy 2009, 86, 2283–2297. 10.1016/j.apenergy.2009.03.022. [DOI] [Google Scholar]
- Kacimi M.; Ziyad M.; Liotta L. F. Cu on amorphous AlPO4: Preparation, characterization and catalytic activity in NO reduction by CO in presence of oxygen. Catal. Today 2015, 241, 151–158. 10.1016/j.cattod.2014.04.003. [DOI] [Google Scholar]
- Hu Y.; Dong L.; Shen M.; Liu D.; Wang J.; Ding W.; Chen Y. Influence of supports on the activities of copper oxide species in the low-temperature NO + CO reaction. Appl. Catal., B 2001, 31, 61–69. 10.1016/S0926-3373(00)00269-1. [DOI] [Google Scholar]
- Panayotov D.; Dimitrov L.; Khristova M.; Petrov L.; Mehandjiev D. Reduction of nitric oxide with carbon monoxide on the surface of copper-containing catalysts based on aluminophosphates, silicoaluminosulphates and ZSM-5 zeolite. Appl. Catal., B 1995, 6, 61–78. 10.1016/0926-3373(95)00002-X. [DOI] [Google Scholar]
- Dasireddy V. D. B. C.; Likozar B. Selective catalytic reduction of NOx by CO over bimetallic transition metals supported by multi-walled carbon nanotubes (MWCNT). Chem. Eng. J. 2017, 326, 886–900. 10.1016/j.cej.2017.06.019. [DOI] [Google Scholar]
- Huang C.; Li C.; Shi G. Graphene based catalysts. Energy Environ. Sci. 2012, 5, 8848. 10.1039/c2ee22238h. [DOI] [Google Scholar]
- Deng D.; Novoselov K. S.; Fu Q.; Zheng N.; Tian Z.; Bao X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. 10.1038/nnano.2015.340. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Zhao Q.; Li Y.; Peng W.; Zhang G.; Zhang F.; Fan X. Gold nanoparticles supported on layered TiO2-RGO hybrid as an enhanced and recyclable catalyst for microwave-assisted hydration reaction. RSC Adv. 2016, 6, 76151–76157. 10.1039/C6RA08021A. [DOI] [Google Scholar]
- Nongbe M. C.; Ekou T.; Ekou L.; Yao K. B.; Le Grognec E.; Felpin F.-X. Biodiesel production from palm oil using sulfonated graphene catalyst. Renewable Energy 2017, 106, 135–141. 10.1016/j.renene.2017.01.024. [DOI] [Google Scholar]
- Roy B.; Ghosh S.; Ghosh P.; Basu B. Graphene oxide (GO) or reduced graphene oxide (rGO): Efficient catalysts for one-pot metal-free synthesis of quinoxalines from 2-nitroaniline. Tetrahedron Lett. 2015, 56, 6762–6767. 10.1016/j.tetlet.2015.10.065. [DOI] [Google Scholar]
- Khalili D. Graphene oxide: A reusable and metal-free carbocatalyst for the one-pot synthesis of 2-amino-3-cyanopyridines in water. Tetrahedron Lett. 2016, 57, 1721–1723. 10.1016/j.tetlet.2016.03.020. [DOI] [Google Scholar]
- Rana S.; Maddila S.; Yalagala K.; Jonnalagadda S. B. Organo functionalized graphene with Pd nanoparticles and its excellent catalytic activity for Suzuki coupling reaction. Appl. Catal., A 2015, 505, 539–547. 10.1016/j.apcata.2015.07.018. [DOI] [Google Scholar]
- Dey A.; Athar J.; Varma P.; Prasant H.; Sikder A. K.; Chattopadhyay S. Graphene-iron oxide nanocomposite (GINC): An efficient catalyst for ammonium perchlorate (AP) decomposition and burn rate enhancer for AP based composite propellant. RSC Adv. 2015, 5, 1950–1960. 10.1039/C4RA10812D. [DOI] [Google Scholar]
- Hu M.; Yao Z.; Wang X. Graphene-Based Nanomaterials for Catalysis. Ind. Eng. Chem. Res. 2017, 56, 3477–3502. 10.1021/acs.iecr.6b05048. [DOI] [Google Scholar]
- Julkapli N. M.; Bagheri S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy 2015, 40, 948–979. 10.1016/j.ijhydene.2014.10.129. [DOI] [Google Scholar]
- Machado B. F.; Serp P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. 10.1039/C1CY00361E. [DOI] [Google Scholar]
- Edwards R. S.; Coleman K. S. Graphene synthesis: relationship to applications. Nanoscale 2013, 5, 38–51. 10.1039/C2NR32629A. [DOI] [PubMed] [Google Scholar]
- Bai H.; Li C.; Shi G. Functional composite materials based on chemically converted graphene. Adv. Mater. 2011, 23, 1089–1115. 10.1002/adma.201003753. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Zhu Q.; Gao Y.; Zhai P.; Ma D. Iron oxide nanoparticles supported on pyrolytic graphene oxide as model catalysts for Fischer Tropsch synthesis. Appl. Catal., A 2013, 456, 233–239. 10.1016/j.apcata.2013.03.006. [DOI] [Google Scholar]
- Karimi S.; Tavasoli A.; Mortazavi Y.; Karimi A. Cobalt supported on Graphene - A promising novel Fischer-Tropsch synthesis catalyst. Appl. Catal., A 2015, 499, 188–196. 10.1016/j.apcata.2015.04.024. [DOI] [Google Scholar]
- Rana S.; Jonnalagadda S. B. A facile synthesis of Cu-Ni bimetallic nanoparticle supported organo functionalized graphene oxide as a catalyst for selective hydrogenation of p-nitrophenol and cinnamaldehyde. RSC Adv. 2017, 7, 2869–2879. 10.1039/C6RA26443C. [DOI] [Google Scholar]
- Yan H.; Cheng H.; Yi H.; Lin Y.; Yao T.; Wang C.; Li J.; Wei S.; Lu J. Single-Atom Pd1/Graphene Catalyst Achieved by Atomic Layer Deposition: Remarkable Performance in Selective Hydrogenation of 1,3-Butadiene. J. Am. Chem. Soc. 2015, 137, 10484–10487. 10.1021/jacs.5b06485. [DOI] [PubMed] [Google Scholar]
- Trapalis A.; Todorova N.; Giannakopoulou T.; Boukos N.; Speliotis T.; Dimotikali D.; Yu J. TiO2/graphene composite photocatalysts for NOx removal: A comparison of surfactant-stabilized graphene and reduced graphene oxide. Appl. Catal., B 2016, 180, 637–647. 10.1016/j.apcatb.2015.07.009. [DOI] [Google Scholar]
- Lu X.; Song C.; Jia S.; Tong Z.; Tang X.; Teng Y. Low-temperature selective catalytic reduction of NOX with NH3 over cerium and manganese oxides supported on TiO2-graphene. Chem. Eng. J. 2015, 260, 776–784. 10.1016/j.cej.2014.09.058. [DOI] [Google Scholar]
- Wei Z.; Pan R.; Hou Y.; Yang Y.; Liu Y. Graphene-supported Pd catalyst for highly selective hydrogenation of resorcinol to 1, 3-cyclohexanedione through giant π-conjugate interactions. Sci. Rep. 2015, 5, 15664. 10.1038/srep15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Cao H.; Shao J.; Li G.; Qu M.; Yin G. Co3O4@graphene composites as anode materials for high-performance lithium ion batteries. Inorg. Chem. 2011, 50, 1628–1632. 10.1021/ic1023086. [DOI] [PubMed] [Google Scholar]
- Elzey S.; Baltrusaitis J.; Bian S.; Grassian V. H. Formation of paratacamite nanomaterials via the conversion of aged and oxidized copper nanoparticles in hydrochloric acidic media. J. Mater. Chem. 2011, 21, 3162–3169. 10.1039/c0jm03705b. [DOI] [Google Scholar]
- Zhao Y.; Song X.; Song Q.; Yin Z. A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. CrystEngComm 2012, 14, 6710–6719. 10.1039/c2ce25509j. [DOI] [Google Scholar]
- Lin L.-C.; Grossman J. C. Atomistic understandings of reduced graphene oxide as an ultrathin-film nanoporous membrane for separations. Nat. Commun. 2015, 6, 8335. 10.1038/ncomms9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumbilavil S.; Sankar P.; Rose T. P.; Philip R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400–700 nm region. Appl. Phys. Lett. 2015, 107, 051104 10.1063/1.4928124. [DOI] [Google Scholar]
- Stankovich S.; Dikin D. A.; Piner R. D.; Kohlhaas K. A.; Kleinhammes A.; Jia Y.; Wu Y.; Nguyen S. B. T.; Ruoff R. S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. 10.1016/j.carbon.2007.02.034. [DOI] [Google Scholar]
- Ţucureanu V.; Matei A.; Avram A. M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. 10.1080/10408347.2016.1157013. [DOI] [PubMed] [Google Scholar]
- Cheng G.; Walker A. R. H. Transmission electron microscopy characterization of colloidal copper nanoparticles and their chemical reactivity. Anal. Bioanal. Chem. 2010, 396, 1057–1069. 10.1007/s00216-009-3203-0. [DOI] [PubMed] [Google Scholar]
- Xu C.; Shi X.; Ji A.; Shi L.; Zhou C.; Cui Y. Fabrication and characteristics of reduced graphene oxide produced with different green reductants. PLoS One 2015, 10, e0144842 10.1371/journal.pone.0144842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M.-Y.; Teng C.-C.; Hsiao M.-C.; Liu P.-I.; Chuang W.-P.; Ma C.-C. M.; Hsieh C.-K.; Tsai M.-C.; Tsai C.-H. Platinum nanoparticles/graphene composite catalyst as a novel composite counter electrode for high performance dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 12880. 10.1039/c1jm11850a. [DOI] [Google Scholar]
- Lv J.; Zhao M.; Shang F.; Song Y.; Wang F.; Zhou Z.; Zi Z.; Wei Y.; Chen X.; He G.; Zhang M.; Song X.; Sun Z. Effect of solution concentration on surface morphology, chemical composition and photoresponse of CuO/Cu2O composite thin films grown by hydrothermal synthesis. J. Mater. Sci.: Mater. Electron. 2014, 25, 4877–4882. [Google Scholar]
- Biesinger M. C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. 10.1002/sia.6239. [DOI] [Google Scholar]
- Rahul R.; Singh R. K.; Bera B.; Devivaraprasad R.; Neergat M. The role of surface oxygenated-species and adsorbed hydrogen in the oxygen reduction reaction (ORR) mechanism and product selectivity on Pd-based catalysts in acid media. Phys. Chem. Chem. Phys. 2015, 17, 15146–15155. 10.1039/C5CP00692A. [DOI] [PubMed] [Google Scholar]
- Holade Y.; Canaff C.; Poulin S.; Napporn T. W.; Servat K.; Kokoh K. B. High impact of the reducing agent on palladium nanomaterials: new insights from X-ray photoelectron spectroscopy and oxygen reduction reaction. RSC Adv. 2016, 6, 12627–12637. 10.1039/C5RA24829A. [DOI] [Google Scholar]
- de Correa C. M.; Castrillón F. C. Supported bimetallic Pd–Co catalysts: characterization and catalytic activity. J. Mol. Catal. A: Chem. 2005, 228, 267–273. 10.1016/j.molcata.2004.09.033. [DOI] [Google Scholar]
- Kumar N.; Smith M. L.; Spivey J. J. Characterization and testing of silica-supported cobalt–palladium catalysts for conversion of syngas to oxygenates. J. Catal. 2012, 289, 218–226. 10.1016/j.jcat.2012.02.011. [DOI] [Google Scholar]
- Stonkus V.; Edolfa K.; Leite L.; Sobczak J. W.; Plyasova L.; Petrova P. Palladium-promoted Co–SiO2 catalysts for 1,4-butanediol cyclization. Appl. Catal., A 2009, 362, 147–154. 10.1016/j.apcata.2009.04.033. [DOI] [Google Scholar]
- Patterson A. L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. 10.1103/PhysRev.56.978. [DOI] [Google Scholar]
- Xu X.; Chen P.; Goodman D. W. A Comparative Study of the Coadsorption of CO and NO on Pd(100), Pd(III), and Silica-Supported Palladium Particles with Infrared Reflection-Absorption Spectroscopy. J. Phys. Chem. 1994, 98, 9242–9246. 10.1021/j100088a025. [DOI] [Google Scholar]
- Flores-Sanchez L. A.; Quintana-Melgoza J. M.; Olivas A.; Avalos-Borja M. Reduction of nitric oxide by carbon monoxide over NiO , CuO , and ZnO catalysts. React. Kinet., Mech. Catal. 2015, 114, 597–609. 10.1007/s11144-014-0820-1. [DOI] [Google Scholar]
- Muñoz V.; Zotin F. M. Z.; Palacio L. A. Copper-aluminum hydrotalcite type precursors for NOx abatement. Catal. Today 2015, 250, 173–179. 10.1016/j.cattod.2014.06.004. [DOI] [Google Scholar]
- Fernández-García M.; Martínez-Arias A.; Belver C.; Anderson J. A.; Conesa J. C.; Soria J. Behavior of palladium-copper catalysts for CO and NO elimination. J. Catal. 2000, 190, 387–395. 10.1006/jcat.1999.2751. [DOI] [Google Scholar]
- Cheng X.; Zhang X.; Su D.; Wang Z.; Chang J.; Ma C. NO reduction by CO over copper catalyst supported on mixed CeO2 and Fe2O3: Catalyst design and activity test. Appl. Catal., B 2018, 239, 485–501. 10.1016/j.apcatb.2018.08.054. [DOI] [Google Scholar]
- Bai Y.; Bian X.; Wu W. Catalytic properties of CuO/CeO2-Al2O3 catalysts for low concentration NO reduction with CO. Appl. Surf. Sci. 2019, 463, 435–444. 10.1016/j.apsusc.2018.08.229. [DOI] [Google Scholar]
- Iglesias-Juez A.; Hungría A. B.; Martínez-Arias A.; Anderson J. A.; Fernández-García M. Pd-based (Ce,Zr)Ox-supported catalysts: Promoting effect of base metals (Cr, Cu, Ni) in CO and NO elimination. Catal. Today 2009, 143, 195–202. 10.1016/j.cattod.2008.12.013. [DOI] [Google Scholar]
- Peter S. D.; Garbowski E.; Perrichon V.; Pommier B.; Primet M. Activity enhancement of mixed lanthanum-copper-iron-perovskites in the CO+NO reaction. Appl. Catal., A 2001, 205, 147–158. 10.1016/S0926-860X(00)00551-2. [DOI] [Google Scholar]
- Liu L.; Liu B.; Dong L.; Zhu J.; Wan H.; Sun K.; Zhao B.; Zhu H.; Dong L.; Chen Y. In situ FT-infrared investigation of CO or/and NO interaction with CuO/Ce0.67Zr0.33O2 catalysts. Appl. Catal., B 2009, 90, 578–586. 10.1016/j.apcatb.2009.04.019. [DOI] [Google Scholar]
- Yao X.; Yu Q.; Ji Z.; Lv Y.; Cao Y.; Tang C.; Gao F.; Dong L.; Chen Y. A comparative study of different doped metal cations on the reduction, adsorption and activity of CuO/Ce0.67M0.33O2 (M=Zr4+, Sn4+, Ti4+) catalysts for NO+CO reaction. Appl. Catal., B 2013, 130-131, 293–304. 10.1016/j.apcatb.2012.11.020. [DOI] [Google Scholar]
- Schmal M.; Baldanza M. A. S.; Vannice M. A. Pd-xMo/Al2O3 catalysts for NO reduction by CO. J. Catal. 1999, 185, 138–151. 10.1006/jcat.1999.2465. [DOI] [Google Scholar]
- Sárkány J.; D’ltri J. L.; Sachtler W. M. H. Redox chemistry in excessively ion-exchanged Cu/Na-ZSM-5. Catal. Lett. 1992, 16, 241. 10.1007/BF00764336. [DOI] [Google Scholar]
- Shelef M.; Kummer J. T. The behavior of nitric oxide in heterogeneous catalytic reactions. Chem. Eng. Prog. Symp. Ser. 1971, 67, 74. [Google Scholar]
- Fu Y.; Tian Y.; Lin P. A low-temperature IR spectroscopic study of selective adsorption of NO and CO on CuO/γ-Al2O3. J. Catal. 1991, 132, 85–91. 10.1016/0021-9517(91)90249-4. [DOI] [Google Scholar]
- Lokhov Y. A.; Davydov A. A. Study of the state of transition-metal cations on the surface of catalysts by the method of IR spectroscopy of adsorbed test molecules (CO, NO). Kinet. Catal. 1980, 21, 1523. [Google Scholar]
- Chen J.; Yao B.; Li C.; Shi G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. 10.1016/j.carbon.2013.07.055. [DOI] [Google Scholar]
- Dietz W. A. Response factors for gas chromatographic analyses. J. Chromatogr. Sci. 1967, 68–71. 10.1093/chromsci/5.2.68. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.