Abstract

The degradation mechanism of benzo[a]pyrene (BaP) initiated by •OH and 1O2 in aqueous solution is investigated by density functional theory calculations. The main degradation products are BaP-1,6-quinone, BaP-3,6-quinone, BaP-4,6-quinone, and BaP-6,12-quinone. •OH and HO2 are the main intermediate radical species. At a low initial concentration of •OH, 1O2 could be a primary driver for BaP degradation. The degradation mechanism includes six consecutive elementary reactions: (1) 1O2 initiation forming BaP-6-OO. (2) 1,3 H-shift (H atom shifts to the OO group) that is promoted by H2O, forming BaP-6-OOH. (3) BaP-6-OOH decomposes into the •OH radical and BaP-6-O. (4) •OH addition to BaP-6-O forming BaP-6-O-1(3,4,12)-OH. (5) Extracting the H atom from the carbon with the OH group by 1O2. (6) Extracting the H atom from the OH group by HO2. At a high initial concentration of •OH, the •OH-initiated and 1O2-initiated degradation reactions of BaP are both feasible. The degradation mechanism includes six consecutive elementary reactions: (1) •OH initiation forming BaP-6-OH or 1O2 initiation forming BaP-6-OO. (2) 1O2 addition to BaP-6-OH forming BaP-6-OH-12(1,3,4)-OO or •OH addition to BaP-6-OO forming BaP-6-OO-12(1,3,4)-OH. (3) Extracting the H atom from the carbon with the OH group by 1O2, forming HO2. (4) 1,3 H-shift (H-shift from the carbon to the OO group), promoted by H2O. (5) The loss of the OH radical. (6) Abstracting the H atom from the OH group by HO2. In this paper, the formation of BaP-4,6-quinone via the BaP degradation is first reported. Water participates in the elementary reaction in which the H atom attached on the aromatic ring shifts to the OO group, serving as a bridge that stabilizes the transition state and transports the proton. A comprehensive investigation explains the degradation mechanism of BaP initiated by •OH and 1O2 in aqueous solution.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are main byproducts during the incomplete combustion process of fossil fuels, oil and fatty foods, automobile exhaust gas, and so on1−4 and can be oxidized to quinones, ring-retaining phenols, ring-opening carbonyls, and carboxylic acids in environmental systems.5,6 Because of long-range transport and resistance to degradation, PAHs are identified as persistent organic pollutants by the Stockholm convention, resulting in a degree of harm to human health and ecosystems.7−10
As a model compound for the study of PAHs, benzo[a]pyrene (BaP) composed of five fuse aromatic rings had attracted more attention. The World Health Organization ranks it as the first class of human carcinogens, and the European Union indicative limit value is set at 1 ng/m3 of BaP. Because of low volatility (PL = 7.9 × 10–6 Pa) and low solubility in aqueous solution, BaP is persistent in the environment and would be partitioned mostly into the particulate phase. BaP could also be deposited into surface water and soils.5
There are a few research reports on the removal of BaP from the environment. The main methods are chemical oxidation, microbial degradation, bioaccumulation, and photooxidation. Recently, some experiments have focused on the use of natural porphyrin rich in the environment to degrade BaP.11−15 It has been found that chlorophyll has a good degradation effect on BaP, and 98.5% of BaP in the chlorophyll solution is degraded after exposure to light for 4 days.11 A number of natural porphyrins such as chlorophyll a, sodium copper chlorophyllin, hematin, and so forth were used to degrade BaP. The transformation efficiency of BaP varied significantly with the stability of the porphyrin, and 97.75% of BaP in chlorophyll solution with lower stability was removed after exposure to light for 4 days.12 The major transformation products of BaP via porphyrins were identified as BaP-quinones, including BaP-1,6-dione, BaP-3,6-dione, and BaP-6,12-dione.11,12 Porphyrins have been proven to have excellent photosensitizing properties to generate singlet oxygen,16−19 and the degradation rate of BaP was positively correlated with the 1O2 concentrations generated by porphyrin photocatalysis; the higher the singlet oxygen content, the higher the BaP transformation.11,12•OH was also detected when BaP was degraded in the natural porphyrin aqueous solution.11,12 Both •OH and 1O2 were speculated to play a crucial role in the degradation of PAHs and other organic pollutants.20−25
In the process of the BaP degradation accelerated by porphyrins, •OH and 1O2 are detected and their role is emphasized in the experiments;11,12 however, the exact role of •OH and 1O2 is not clear, and the mechanism of the BaP transformation induced by •OH and 1O2 in the water still has not been elucidated. The present work aims to propose a comprehensive theoretical investigation on the degradation mechanism of BaP initiated by •OH and 1O2. Density functional theory (DFT) calculations are employed to figure out the degradation reaction routes and products.
2. Results and Discussion
2.1. Initial Reaction via •OH and 1O2
2.1.1. Initial Reaction via •OH
The favorable site of BaP attacked by the •OH radical is predicted by the Fukui function, in which it is generally believed that the larger the Fukui function value, the greater the corresponding reaction activity. The results shown in Figure 1b indicate that most of the potential sites for •OH attack are localized at C1, C3, C12 and especially at the C6 site. The average values of Fukui function distribution on the local molecular surface of BaP corresponding to each atom are listed in Table S1. It can be found that the reactivity index value of BaP’s C6 atom is twice that of its C1 atom. Consequently, it can be inferred that the favorable reactive site of BaP for •OH attack is the C6 atom. The prediction of reactive site C6 can also be confirmed by the Clar’s aromatic π-sextet approach.26 The following calculated potential energy surfaces (PESs) of the reaction between •OH and BaP also support this prediction.
Figure 1.

Numbering system and electronic structure plot of BaP. (a) General numbering system used for carbon atoms of BaP in the text. (b) Plot of the favorable site for BaP attacked by •OH radical (density = 0.01 a.u, isovalue = 0.003). (c) HOMO of BaP (isovalue = 0.1). The contribution values of carbon atoms to HOMO are labeled.
In this paper, addition and H-abstraction mechanisms are considered in the initial reaction of the BaP–OH system, and the calculated PESs are compared in Figure 2. Considering the rigid structure of the BaP molecule, the saturated bonding feature of each carbon atom, only the 12 C-atoms attached to the hydrogen atom are assigned as the possible sites for •OH attack. It can be found that the barriers and reaction energies of H-abstraction pathways for all 12 hydrogen atoms are higher than addition pathways, indicating that the H-abstraction mechanism is unfavorable both kinetically and thermodynamically. For the addition mechanism, the calculated barriers and reaction energies of the 12 different pathways show that (i) the calculated barriers are sensitive to the chemical environment of the carbon atoms, which vary between 4.4 and 9.8 kcal/mol; (ii) the calculated reaction energies indicate that these •OH addition reactions along all 12 pathways are exothermic and thus thermodynamically allowed; (iii) there are 1, 2, 2, 3, and 4 reaction sites on rings b, c, e, d, and a, respectively, ΔEr and ΔEa on these rings generally increase with the increasing numbers of reaction sites. For example, for ring b, there is only one reaction site (C6 atom) for the •OH addition; ΔEa (4.4 kcal/mol) and ΔEr (−26.3 kcal/mol) of •OH addition reaction on the C6 atom are lowest in comparison with the other addition sites. Therefore, the C6 pathway is the most favorable in both kinetics and thermodynamics, and the aromatic radical BaP-6-OH named P0 infra is the main intermediate.
Figure 2.
PESs for the OH-initiated reactions of BaP. (a–e) PES for the OH-initiated reactions on rings a, b, c, d, e. Abs and add denote the TSs of abstraction hydrogen and addition route, respectively.
2.1.2. Initial Reaction via 1O2
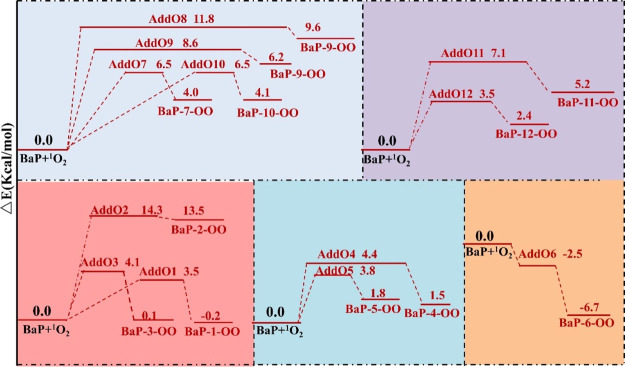
Considering the experiment results11,12 that the transformation of BaP is in direct proportion to the 1O2 concentration, the case of 1O2 addition to BaP is explored. For the addition mechanism shown in Figure 3, the calculated barriers and reaction energies for the 12 different pathways indicate that (i) the calculated barriers vary with different carbon atom sites from 14.3 to −2.5 kcal/mol. The highest and lowest barriers correspond to C2 and C6 pathways, respectively, which is same as •OH addition. The highest occupied molecular orbital (HOMO) of BaP shown in Figure 1c indicates that contribution of 12 carbon atoms to the pz orbital of HOMO varies greatly from 16.49% at the C6 site to 0.78% at the C2 site; when 1O2 and •OH approach BaP, PY in the lowest unoccupied molecular orbital (LUMO) of 1O2 or •OH will have the highest overlap with the pz orbital of C6, the lowest overlap with pz of C2. (ii) Except for the C6 pathway with ΔEr −6.7 kcal/mol, the 1O2-initiated reactions along the other pathways studied here are endothermic and unfavorable in thermodynamics; (iii) the most favorable pathway of 1O2 addition to BaP in both kinetics and thermodynamics is at the C6 position, so does the addition of •OH to BaP. The addition of 1O2 leads to a zwitter-ion product BaP-6-OO, which is highly reactive. Comparing ΔEr and ΔEb, •OH addition to BaP is more favorable than 1O2 addition in thermodynamics; 1O2 addition is more favorable than •OH addition in kinetics. Therefore, 1O2 and •OH addition to BaP on the C6 pathway is all feasible.
Figure 3.
PES for the 1O2-initiated reactions of BaP. AddOn (n = 1–12) denote the TSs of the addition route.
The rate constants for the primary reactions of 1O2 and •OH addition to BaP are studied using transition state theory (TST) at 298 K and 1 bar, shown in Table S3. The tunneling correction factor κ is fully considered by the asymmetric Eckart model, which can accurately analyze the analytic expression of projection probability p(E) as a function of energy. The rate constant k1 of the BaP-6-OO formation is calculated to be 1.70 × 10–5cm3·molecule–1·s–1, which is by 5 orders of magnitude higher than that of BaP-6-OH (k2 = 1.65 × 10–10 cm3·molecule–1·s–1); thus, 1O2 addition is more favorable than •OH addition in kinetics. The rate coefficient here for BaP + 1O2 is far more than the diffusion-control limit in the liquid phase. Whether the degradation of BaP is initiated by •OH addition or 1O2 will depend on their initial concentration in aqueous solution; 1O2 could be a primary driver for BaP degradation11,12 at a low OH concentration. Next, the fate of main intermediates BaP-6-OH(P0) and BaP-6-OO(PO1) is explored.
2.2. Subsequent Reactions of BaP-6-OH
2.2.1. Reaction of BaP-6-OH with 1O2
For the reactions of BaP-6-OH with 1O2, three routes are considered as shown in Figure 4: (i) direct α-H-abstractions to form BaP-6-phenol. (ii) The hydrogen extractions from OH group to form x,y-epoxide-BaP. (iii) O2 additions to form peroxy radicals. First, direct α-H-abstractions from rings by 1O2 are exothermic by 40.7 kcal/mol with barriers −14.6 kcal/mol, along with the formation of HOO• and BaP-6-phenol. Therefore, BaP-6-phenol is the product for direct α-H-abstractions route. The barrier to extracting hydrogen from OH groups with 1O2 to form an epoxide is 20 kcal/mol higher than the BaP-6-phenol route on PESs, which suggests that the epoxide route is impossible.
Figure 4.

PES of the reactions between 1O2 and BaP-6-OH. The relative Gibbs free energies (blue for C12 pathway, red for C1 pathway, green for C4 pathway, purple for C3 pathway) determined at the M06-2X/6-311G(d,p) level in aqueous media are also shown. For clarity, only the optimized structures of TSs and products involved in C12 pathway are provided.
The route of 1O2 addition to P0 is discussed in the following discussion. The electron spin density isosurface of P0 shown in Figure S4 figures out the addition sites of O2 to P0. The blue isosurface representing distribution of α electron clearly predicts that P0 has a larger spin density at C12, C1, C3, C4 positions, which are distributed over 0.258, 0.238, 0.232, 0.230 electrons, respectively; one might expect C12, C1, C3, C4 positions to be possible sites of electron transfer. The electrons distributed on C12, C1, C3, C4 atoms present in π(pz)-orbital are preferable to interact with the π* orbital of dioxygen to form covalent C–O bonds. Although the other two Cb1 and Cb2 sites have a relatively high spin density, 1O2 addition results in more bend of the rings, ΔEr and ΔEa are 2.1–4.0 and 2.8–4.4 kcal/mol higher than on the C12 site (shown in Table S5). In addition, the major transformation products of BaP via porphyrins were identified as BaP-1,6-dione, BaP-3,6-dione, and BaP-6,12-dione experimentally.11,12 Thus, the Cb1 and Cb2 sites are not considered infra. The subsequent O2 addition to P0 mainly proceeds in C12, C1, C3, C4, four different pathways, forming the peroxy radicals BaP-6-OH-OO(P1-n). For the sake of brevity, throughout this paper, n = 1, 3, 4, 12 are used to denote the C1, C3, C4, and C12 pathways, respectively. The formation of precomplexes of the 1O2 + P0 reaction is strongly exothermic by 32 kcal/mol because of 1O2 with high reactivity and energy. The barrier of O2 addition to P0 is 15.9 kcal/mol. The four pathways are highly competitive because of the little difference of 0.8 kcal/mol in ΔEa values. The formation of BaP-6-OH-OO has a little advantage in dynamics than direct α-H-abstraction to form BaP-6-phenol, and BaP-6-phenol is more favorable in thermodynamics. Thus, the reactions of BaP-6-OH with O2 can proceed with two routes: direct α-H-abstractions to form BaP-6-phenol and O2 additions to form peroxy radicals BaP-6-OH-OO(P1-n). The following investigations focus on the fate of peroxyl radicals BaP-6-OH-OO(P1-n).
2.2.2. Subsequent Reactions of BaP-6-OH-OO
For peroxyl radical P1-n, unimolecular decay and bimolecular reactions are explored. The RO2 + HOO route shown in Figure 5 is considered first, which can potentially form hydroperoxides and results in OH radical and oxygen recycling. P1-n react with HOO to form hydroperoxides P1-n-b with a ΔEr value of −30 kcal/mol and ΔEa value of 5 kcal/mol; thus, it is feasible in both kinetics and thermodynamics. However, the dissociation of P1-n-b into OH radical and epoxide P2-n is inhibited because of the higher barrier of 40 kcal/mol approximately. Thus, the RO2 + HOO route is not further discussed.
Figure 5.

PES of P1-n + P1-n route and P1-n + HO2 route. The relative Gibbs free energies (blue for C12 pathway, red for C1 pathway, green for C4 pathway, purple for C3 pathway) determined at the M06-2X/6-311G(d,p) level in aqueous media are also shown. For clarity, only the optimized structures of TSs and products involved in C12 pathway are provided.
The P1-n + P1-n route shown in Figure 5 emphatically focuses on the RO2 + RO2 → RO + RO + O2 channel, which can propagate reactive alkoxy radicals, making further transformations along it.27−29 The dimerization of P1-n forms RO–OO–OR tetroxide intermediates (IM1-n), which is expected to proceed without entrance barrier, and the details are provided in the Supporting Information. The dissociation of IM1-n through the O1–O2 bond cleavage needs to overcome a barrier height of 29 kcal/mol and is endothermic slightly. Therefore, the RO2 + RO2 route is infeasible.
The route in which P1-n reacts with 1O2 is explored and shown in the bottom of Figure 6, which includes four elementary reactions: extracting the H atom from the carbon with the OH group by 1O2, 1,3 H-shift (H-shift from the C12 site to the OO group), the loss of OH radical, and extracting the H atom from the OH group by HO2. 1O2 abstracts the H atom from the carbon with the OH group, forming P11-n and HO2. P11-n isomerize into P12-n via 1,3 H-shift, which can rapidly occur because of a low barrier height of 8.5–9.0 kcal/mol and is strongly exothermic by 50 kcal/mol. As the degradation experiment of BaP was conducted in aqueous solution,11,12 it is necessary to investigate whether water participates in the hydrogen transfer reaction. Considering the involvement of water, the 1,3 H-shift becomes a bimolecular reaction between P11-n and H2O. P11-n and H2O react via a six-membered ring transition state (TS-IM4-n), in which H2O is a bridge, donating the H atom to the O atom and accepting another H from the aromatic ring synchronously. In the process of this reaction, an O–H bond in the original water molecule is broken and a new one is formed. Hence, the water molecule in the products is not the original one. The involvement of H2O transports the proton and lowers the reaction barrier to 4.2–4.8 kcal/mol. A zwitter-ion P11-12 is formed through extracting the H atom from the carbon with the OH group by 1O2. The result of the nature bond orbital (NBO) analysis indicates that NPA charge on 1O, 1H, and 2H atoms of P11-12 are −0.67, 0.12, and 0.43, respectively (via. Table S7). Thus, acidic and alkali activated centers are formed on 2H and 1O atoms, which impels the proceeding of the 1,3 H-shift and reduces the activation energy of TS. P12-n decompose to P5-n through the loss of •OH, which proceeds with potential barrier of 18.5–19.8 kcal/mol and weak exothermicity; thus, the decomposition of P12-n is a rate-limiting step for this route. Finally, P5-n undergoes H-atom abstraction from the OH group, resulting in the formation of P6-n (BaP-6,12-quinone, BaP-1,6-quinone, BaP-3,6-quinone and BaP-4,6-quinone). The route in which the P1-n react with 1O2 forming P6-n is highly exothermic by 107–109 kcal/mol totally.
Figure 6.
PES of the P1-n + 1O2 route and P1-n unimolecular decay route. The relative Gibbs free energies (blue for C12 pathway, red for C1 pathway, green for C4 pathway, purple for C3 pathway) determined at the M06-2X/6-311G(d,p) level in aqueous media are also shown. For clarity, only the optimized structures of TSs and products involved in the C12 pathway are provided.
The route in which unimolecular decay of P1-n shown in the top of Figure 6 also generates P6-n involves four elementary steps. The first step is the formation of P1-n-a via 1,3 H-shift (H-shift from the C12 atom to the OO group); however, the barrier of direct 1,3 H-shift is up to 34 kcal/mol, which makes it difficult to occur. The involvement of H2O drastically lowers the reaction barrier to 19.1–20.3 kcal/mol, which enables the 1,3 H-shift. Water molecules act as a bridge, stabilizing the TS and transporting the proton. The unimolecular decay of P1-n becomes a bimolecular reaction between P1-n and H2O. The second step is rapid decomposition of P1-n-a, producing P4-n and •OH because of the lower barriers of 5 kcal/mol. The third and fourth steps result in the formation of P6-n via consecutive H abstraction.
For the formation of Bap-n,6-quinone, the route in which P1-n react with 1O2 is favorable than the unimolecular decay route of P1-n by comparing the PES of the first elementary reaction. The degradation mechanism of BaP into BaP-n-6-quinone can be interpreted as six consecutive elementary reactions: •OH addition forming BaP-6-OH, 1O2 addition forming BaP-6-OH-12(1,3,4)-OO peroxyl radical, extracting the H atom from the carbon with the OH group by 1O2, 1,3 H-shift (H-shift from the C12 atom to the OO group), the loss of OH radical, and abstracting the H atom from the OH group by HO2. There is competition in both kinetics and thermodynamics for C1, C3, C4, C12 pathways.
2.3. Subsequent Reactions of BaP-6-OO
As shown in Figures 7 and 8, the possible reactions are identified for BaP-6-OO(PO1). The first one is the unimolecular decomposition of PO1 via 1,3 H-shift (H-shift from the C6 atom to the OO group), which leads to an OH-loss. The calculated barriers are up to 41–42 kcal/mol via direct H atom shift; however, the water molecular acting as a bridge in a six-membered ring TS TS-PO2-n-1 drastically lowers the reaction barrier to 11–12 kcal/mol, producing the intermediate IMO1-n. Subsequent O–O bond cleavage of IMO1-n results in the formation of PO4-n and OH radical. The process is exothermic by 50.7–52.6 kcal/mol. Subsequently, PO4-n react with •OH, forming PO5-n, which is confirmed to be barrierless and exothermic by approximately 42 kcal/mol. The unimolecular decomposition IMO1-n and the formation of PO5-n are dominated by OH roaming mechanism.30,31 The reaction of 1O2 addition to PO4-n is confirmed to be energetically unfavorable. PO5-n undergo consecutive two-step abstraction H atom, yielding P6-n, which is also strongly exothermic by approximate 67 kcal/mol. The conversion mechanism of BaP-6-OO into P6-n via the unimolecular decomposition pathway includes five consecutive elementary reactions: (1) 1,3 H-shift (H-shift from the C6 atom to the OO group) that is promoted by H2O, forming BaP-6-OOH. (2) BaP-6-OOH decomposes into •OH radical and BaP-6-O. (3) •OH addition to BaP-6-O forming BaP-6-O-1(3,4,12)-OH. (4) Extracting the H atom from C1(3,4,12) with the OH group by 1O2. (5) Abstracting the H atom from the OH group by HO2.
Figure 7.
PES of the BaP-6-OO unimolecular decay route. The relative Gibbs free energies (blue for C12 pathway, red for C1 pathway, green for C4 pathway, purple for C3 pathway) determined at the M06-2X/6-311G(d,p) level in aqueous media are also shown. For clarity, only the optimized structures of TSs and products involved in C12 pathway are provided.
Figure 8.
PES of the BaP-6-OO + OH route. The relative Gibbs free energies (blue for C12 pathway, red for C1 pathway, green for C4 pathway, purple for C3 pathway) determined at the M06-2X/6-311G(d,p) level in aqueous media are also shown. For clarity, only the optimized structures of TSs and products involved in the C12 pathway are provided.
The bimolecular reaction of PO1 with •OH is shown in Figure 8. The spin density isosurface shown in Figure S4 clearly predicts C12, C1, C3, C4 positions to be •OH addition sites. PO1 is a diradical, which would be more reactive. The addition of •OH to PO1 occurs between two radicals, which is barrierless and strongly exothermic by 42.9–44.0 kcal/mol, resulting in the formation of PO3-n. For PO3-n, two possible routes are considered. The first route includes four elementary reactions: extracting the H atom from C1(3,4,12) with the OH group by 1O2, 1,3 H-shift (H-shift from the C6 atom to the OO group), the loss of OH radical, and abstracting the H-atom from the OH group by HO2. Because of low potential barriers and strong exothermicity shown in PESs, these elementary reactions are easy to proceed. Water molecules participate in 1,3 H-shift, transfer proton, and lower the potential barrier. These reactions ultimately result in the formation of P6-n. The second reaction route of PO3-n also can yield P6-n; the elementary reaction steps include direct H-shift or water-promoted H atom transfer, the loss of OH radical, H abstraction by 1O2, and H abstraction by HO2. This route is infeasible because of the high potential barrier for the initial elementary step of the H-shift. The conversion mechanism of BaP-6-OO into P6-n via the PO1 + •OH pathway includes five consecutive elementary reactions: •OH addition forming peroxyl radical, extracting H atom from C1(3,4,12) with the OH group by 1O2, 1,3 H-shift (H-shift from C6 atom to the OO group), the loss of •OH radical, and abstracting the H-atom from the OH group by HO2. For the conversion of BaP-6-OO into P6-n, the unimolecular decomposition of PO1 and the bimolecular reaction of PO1 with OH radical are all feasible; unimolecular decomposition of PO1 will be the primary route at a lower concentration of OH.
3. Conclusions
Experimentally, both •OH and 1O2 were speculated to play a crucial role in the degradation of BaP; the degradation rate was positively correlated with the 1O2 concentrations generated by porphyrin photocatalysis.11,12 Based on DFT-derived results, the degradation mechanism of BaP initiated by •OH and 1O2 in aqueous solution is summarized, shown in Figure 9.
-
(1)
1O2 and •OH addition to BaP are all feasible in aqueous solution.
-
(2)
The main degradation products of BaP are BaP-1,6-quinone, BaP-3,6-quinone, BaP-4,6-quinone, and BaP-6,12-quinone. At a low initial concentration of •OH, 1O2 could be a primary driver for BaP degradation. The mechanism includes six consecutive elementary reactions: (1) 1O2 initiation forming BaP-6-OO. (2) 1,3 H-shift (H-shift from the C6 atom to the OO group) that is promoted by H2O, forming BaP-6-OOH. (3) BaP-6-OOH decomposes into •OH radical and BaP-6-O. (4) •OH addition to BaP-6-O forming BaP-6-O-1(3,4,12)-OH. (5) Extracting the H atom from the carbon with the OH group by 1O2. (6) Extracting the H atom from the OH group by HO2. At a high initial concentration of •OH, the •OH-initiated and 1O2-initiated degradation reactions of BaP are both feasible. The mechanism includes six consecutive elementary reactions: •OH (1O2) initiation, 1O2 (•OH) addition forming the peroxyl radical, extracting the H atom from the carbon with the hydroxyl group by 1O2, 1,3 H-shift (H-shift from the C6 atom to the OO group), the loss of OH radical, and H abstraction by HO2. •OH and HO2 are the main intermediate radical species.
-
(3)
Water molecules play a positive catalytic role in the process of H shift. Water participates in the reactions, acting as a bridge that can stabilize the TS and transport the proton.
-
(4)
In this paper, the formation of BaP-4,6-quinone via the BaP degradation initiated by •OH and 1O2 is first reported.
Figure 9.
Degradation mechanism of BaP initiated by •OH and 1O2 in aqueous solution. For clarity, only the C12 pathway is provided.
4. Computational Details
All geometries discussed in this work are optimized at the level of M06-2X32/6-311G(d,p)33ithout any restriction on the symmetry. The M06-2× functional, a high-nonlocality functional with double the amount of nonlocal exchange (2×), has demonstrated a good performance for the thermochemistry, kinetics, and weak interactions for the main group elements, balancing accuracy and efficiency.34−36 The Gibbs free-energy (ΔG) and the corresponding enthalpy changes (ΔH) at different levels in Table S8 also prove that M06-2× is suitable. The local minimum points or first-order saddle points are identified by vibrational frequencies for all optimized species. An intrinsic reaction coordinate analysis for all TSs is performed. The solvent effect of aqueous media is estimated using the solvation model based on density.37 All calculations are performed with the Gaussian 09 Revision D.01 software suite.38 The Multiwfn39 program is used to analyze wave function to further assess the intrinsic characteristic of the key species studied here. All isosurface maps are rendered by the VMD 1.9.1 program40 based on the cube files generated by Multiwfn. Unless specified otherwise, we shall discuss only Gibbs free energies below. ΔEa and ΔEr are defined as the reaction barrier and the reaction energy respectively.
In the paper, the Fukui functions are calculated based on DFT calculations to predict the chemical reactivity of BaP associated with the •OH attack. Parr and Yang41 have demonstrated that the Fukui function describing the soft–soft interaction between the reactants can be defined by the following equation
| 1 |
where v(r) is the external potential. Because the equation has slope discontinuities, the left and right derivatives have different physical meanings, which is corresponding to a reactivity index for an electrophilic f– and a nucleophilic attack f+, respectively. Within a finite difference and frozen core approximation, these can be written as
| 2 |
| 3 |
By the same log, the reactivity index corresponding to a neutral (radical) attack can be written as
| 4 |
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (21373043).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01448.
Structure of BaP at the M062X/6-311 level; common reactions of the hydroxyl radicals; Fukui function distribution on the local molecular surface of BaP; NPA charge distribution on carbon atoms of BaP; calculated reaction energies, barrier heights, high-pressure-limit rate constants, and Boltzmann ratios; IRC pathways of BaP-6-OH and BaP-2-OH; basin analysis of C-O sigmatropic formation on the IRC pathway; PES of epoxide-BaP pathways; electron spin density isosurface and spin population results of free radicals; free energy change and reactive barrier of 1O2 addition; isomers’ configuration and relative Gibbs energy; energy profiles of the O2-O3 bond; scan results of O1-O2 and O3-O4 distance; number of oxygen atoms; enthalpy and Gibbs free energy change of BaP-OH and BaP-6-OH; and optimized xyz coordinates for most relevant structures (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Haritash A. K.; Kaushik C. P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- Pauzi Zakaria M.; Hideshige T.; Shinobu T.; Kei O.; Junya Y.; Eriko K.; Hidetoshi K. Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environ. Sci. Technol. 2002, 36, 1907–1918. 10.1021/es011278+. [DOI] [PubMed] [Google Scholar]
- Spencer Williams E.; Brian M.; Janet K. C. cancer risk from incidental ingestion exposures to PAHs associated with coal-tar-sealed pavement. Environ. Sci. Technol. 2013, 47, 1101–1109. 10.1021/es303371t. [DOI] [PubMed] [Google Scholar]
- Liu G.; Niu Z.; Van Niekerk D.; Xue J.; Zheng L. Polycyclic Aromatic Hydrocarbons (PAHs) from Coal Combustion: Emissions, Analysis, and Toxicology. Rev. Environ. Contam. Toxicol. 2008, 192, 1–28. 10.1007/978-0-387-71724-1_1. [DOI] [PubMed] [Google Scholar]
- Keyte I. J.; Harrison R. M.; Lammel G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons--a review. Chem. Soc. Rev. 2013, 42, 9333–9391. 10.1039/c3cs60147a. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Yeung L. W. Y.; Forbes M. W.; Mabury S.; Abbatt J. P. D. Epoxide formation from heterogeneous oxidation of benzo[a]pyrene with gas-phase ozone and indoor air. Environ.Sci.: Processes Impacts 2017, 19, 1292–1299. 10.1039/c7em00181a. [DOI] [PubMed] [Google Scholar]
- Izumi K.; Fukuyama T. Photochemical aerosol formation from aromatic hydrocarbons in the presence of NOx. Atmos. Environ., Part A 1990, 24, 1433–1441. 10.1016/0960-1686(90)90052-o. [DOI] [Google Scholar]
- Nakao S.; Qi L.; Clark C.; Sato K.; Tang P.; Cocker D.. Secondary Organic Aerosol Formation from m-Xylene Photooxidation: The Role of the Phenolic Product; Agu Fall Meeting, 2009.
- Borrás E.; Tortajada-Genaro L. A. Secondary organic aerosol formation from the photo-oxidation of benzene. Atmos. Environ. 2012, 47, 154–163. 10.1016/j.atmosenv.2011.11.020. [DOI] [Google Scholar]
- Kim K.-H.; Jahan S. A.; Kabir E.; Brown R. J. C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Luo L.; Lai X.; Chen B.; Lin L.; Fang L.; Tam N. F.; Luan T. Chlorophyll catalyse the photo-transformation of carcinogenic benzo[a]pyrene in water. Sci. Rep. 2015, 5, 12776–12782. 10.1038/srep12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L.; Xiao Z.; Chen B.; Cai F.; Fang L.; Lin L.; Luan T. Natural porphyrins accelerating the phototransformation of benzo[a]pyrene in water. Environ. Sci. Technol. 2018, 52, 3634–3641. 10.1021/acs.est.7b05854. [DOI] [PubMed] [Google Scholar]
- Zhong R.analyses of Pais metabolites using a peroxynitrite/Fe(III) porphyrin biomimetic system by HPLC. IEEE/ICME International Conference on Complex Medical Engineering, 2007.
- Luo Y.-j.; Ru-gang Z. Metabolism of benzo[a]pyrene in peroxynitrite/Fe(III) porphyrin system. J. Environ. Sci. 2007, 19, 385–386. 10.1016/s1001-0742(07)60063-1. [DOI] [PubMed] [Google Scholar]
- Romert L.; Dag J. Chlorophyllin is both a positive and negative modifier of mutagenicity. Mutagenesis 1992, 7, 349–355. 10.1093/mutage/7.5.349. [DOI] [PubMed] [Google Scholar]
- Kimel S.; Berns M. W. Singlet oxygen generation of porphyrins,chlorins, and phthalocyanines. Photochem. Photobiol. 1989, 50, 175–183. 10.1111/j.1751-1097.1989.tb04145.x. [DOI] [PubMed] [Google Scholar]
- Murtinho D.; Burrows H. D. Novel porphyrins and a chlorin as efficient singlet oxygen photosensitizers for photooxidation of naphthols or phenols to quinones. J. Chem. Soc., Perkin Trans. 2 2000, 65, 2441–2447. 10.1039/b006583h. [DOI] [Google Scholar]
- Morone M.; Pagani G. Enhancement of two-photon absorption cross-section and singlet-oxygen generation in porphyrins upon beta-functionalization with donor-acceptor substituents. Org. Lett. 2006, 8, 2719–2722. 10.1021/ol060742i. [DOI] [PubMed] [Google Scholar]
- Araki K.; Furuta H. Doubly N-Confused Porphyrins as Efficient Sensitizers for Singlet Oxygen Generation. Chem. Lett. 2003, 32, 244–245. 10.1246/cl.2003.244. [DOI] [Google Scholar]
- Fasnacht M. P.; Blough N. V. Mechanisms of the Aqueous Photodegradation of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 2003, 37, 5767–5772. 10.1021/es034389c. [DOI] [PubMed] [Google Scholar]
- Wang L.; Deng N. Photodegradation of aniline in aqueous suspensions of microalgae. J. Photochem. Photobiol., B 2007, 87, 49–57. 10.1016/j.jphotobiol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zuo Y. Photodegradation kinetics, products and mechanism of timolol under simulated sunlight. J. Hazard. Mater. 2013, 252–253, 220–226. 10.1016/j.jhazmat.2013.02.035. [DOI] [PubMed] [Google Scholar]
- Hirahara Y.; Nakamuro K. Aqueous photodegradation of fenthion by ultraviolet B irradiation: contribution of singlet oxygen in photodegradation and photochemical hydrolysis. Water Res. 2003, 37, 468–476. 10.1016/s0043-1354(02)00272-5. [DOI] [PubMed] [Google Scholar]
- Pajares A.; Bregliani M.; Garcia N. A.. Singlet oxygen-mediated photodegradation of water-contaminants. In Singlet Oxygen: Applications in Biosciences and Nanosciences, 1nd ed.; Santi N., Cristina F., Eds.; Royal Society of Chemistry and European Society for Photobiolog, 2016; pp 447–457. [Google Scholar]
- Zhang L. Q. Photodegradation Performance and Mechanisms of Carbamazepine and Its Impact Factors. Environ. Sci. 2012, 33, 4340–4345. [PubMed] [Google Scholar]
- Titaley I. A.; Walden D. M.; Dorn S. E.; Ogba O. M.; Massey Simonich S. L.; Cheong P. H.-Y.; Ha-Yeon C. P. Evaluating Computational and Structural Approaches to Predict Transformation Products of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 2019, 53, 1595–1607. 10.1021/acs.est.8b05198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S.; Reiman H.; Møller K. H.; Rissanen M. P.; Kjaergaard H. G.; Kurtén T. Computational Investigation of RO2 + HO2 and RO2 + RO2 Reactions of Monoterpene Derived First-Generation Peroxy Radicals Leading to Radical Recycling. J. Phys. Chem. A 2018, 122, 9542–9552. 10.1021/acs.jpca.8b09241. [DOI] [PubMed] [Google Scholar]
- Lee R.; Gryn’ova G.; Ingold K. U.; Coote M. L. Why are sec-alkylperoxyl bimolecular self-reactions orders of magnitude faster than the analogous reactions of tert-alkylperoxyls? The unanticipated role of CH hydrogen bond donation. Phys. Chem. Chem. Phys. 2016, 18, 23673–23679. 10.1039/c6cp04670c. [DOI] [PubMed] [Google Scholar]
- Ghigo G.; Mazzone A.; Porta C.; Fossati G.; Gritti D.; Mazzucchelli I.; Ricevuti G.; Fossati G.; Gritti D. Combustion and atmospheric oxidation of hydrocarbons: Theoretical study of the methyl peroxyl self-reaction. J. Chem. Phys. 2003, 118, 10575–10583. 10.1063/1.1574316. [DOI] [Google Scholar]
- Townsend D.; Lahankar S. A.; Lee S. K.; Chambreau S. D.; Suits A. G.; Zhang X.; Rheinecker J.; Harding L. B.; Boeman J. M. The Roaming Atom: Straying from the Reaction Path in Formaldehyde Decomposition. Sci 2004, 306, 1158–1161. 10.1126/science.1104386. [DOI] [PubMed] [Google Scholar]
- Suits A. G. Roaming Atoms and Radicals: A New Mechanism in Molecular Dissociation. Acc. Chem. Res. 2008, 41, 873–881. 10.1021/ar8000734. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Truhlar Donald G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor. Chem. Acc. 2008, 119, 525. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Krishnan R.; Binkley J. S.; Seeger R.; Pople J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. 10.1063/1.438955. [DOI] [Google Scholar]
- Li X.; Liao T.; Chung L. W. Computational Prediction of Excited-State Carbon Tunneling in the Two Steps of Triplet Zimmerman Di-π-Methane Rearrangement. J. Am. Chem. Soc. 2017, 139, 16438–16441. 10.1021/jacs.7b07539. [DOI] [PubMed] [Google Scholar]
- Mardirossian N.; Head-Gordon M. How accurate are the Minnesota density functionals for non-covalent interactions, isomerization energies, thermochemistry, and barrier heights involving molecules composed of main-group elements?. J. Chem. Theory Comput. 2016, 12, 4303–4325. 10.1021/acs.jctc.6b00637. [DOI] [PubMed] [Google Scholar]
- Walker M.; Dessent C. E. H. Performance of M06, M06-2X, and M06-HF Density Functionals for Conformationally Flexible Anionic Clusters: M06 Functionals Perform Better than B3LYP for a Model System with Dispersion and Ionic Hydrogen-Bonding Interactions. J. Phys. Chem. A 2013, 117, 12590–12600. 10.1021/jp408166m. [DOI] [PubMed] [Google Scholar]
- Marenich A. V.; Truhlar D. G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- Frisch M. J., Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Baken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09. Revision D.01; Gaussian Inc.: Wallingford, CT, 2009.
- Lu T.; Feiwu C. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.21992. [DOI] [PubMed] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: Visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Parr R. G.; Yang W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. 10.1021/ja00326a036. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.