Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has placed unprecedented burden on the delivery of intensive care services worldwide.
Research Question
What is the global point estimate of deaths and risk factors for patients who are admitted to ICUs with severe COVID-19?
Study Design and Methods
In this systematic review and meta-analysis Medline, Embase, and the Cochrane library were searched up to August 1, 2020. Pooled prevalence of participant characteristics, clinical features, and outcome data was calculated with the use of random effects models. Subgroup analyses were based on geographic distribution, study type, quality assessment, sample size, end date, and patient disposition. Studies that reported in-hospital mortality rate of adult patients (age >18 years) with confirmed COVID-19 admitted to an ICU met study eligibility criteria. Critical evaluation was performed with the Newcastle Ottawa Scale for nonrandomized studies.
Results
Forty-five studies with 16,561 patients from 17 countries across four continents were included. Patients with COVID-19 who were admitted to ICUs had a mean age of 62.6 years (95% CI, 60.4-64.7). Common comorbidities included hypertension (49.5%; 95% CI, 44.9-54.0) and diabetes mellitus (26.6%; 95% CI, 22.7-30.8). More than three-quarters of cases experienced the development of ARDS (76.1%; 95% CI, 65.7-85.2). Invasive mechanical ventilation was required in 67.7% (95% CI, 59.1-75.7) of case, vasopressor support in 65.9% (95% CI, 52.4-78.4) of cases, renal replacement therapy in 16.9% (95% CI, 12.1-22.2) of cases, and extracorporeal membrane oxygenation in 6.4% (95% CI, 4.1-9.1) of cases. The duration of ICU and hospital admission was 10.8 days (95% CI, 9.3-18.4) and 19.1 days (95% CI, 16.3-21.9), respectively, with in-hospital mortality rate of 28.1% (95% CI, 23.4-33.0; I2 = 96%). No significant subgroup effect was observed.
Interpretation
Critically ill patients with COVID-19 who are admitted to the ICU require substantial organ support and prolonged ICU and hospital level care. The pooled estimate of global death from severe COVID-19 is <1 in 3.
Key Words: coronavirus, critical illness, intensive care, respiratory medicine, SARS-CoV-2
Since emerging in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has placed an unprecedented burden on ICUs around the world. SARS-CoV-2 is a highly transmissible upper respiratory tract virus that causes coronavirus disease 2019 (COVID-19). A striking feature of COVID-19 is rapidly progressive respiratory failure, which develops in approximately 5% of infected adults.1
At the time of writing (August 28, 2020), there have been >24 million confirmed cases of COVID-19 and more than three-quarters of a million deaths worldwide.2 In early case series, mortality rates for critically ill patients with COVID-19 were between 40% and 61%, despite advanced ICU supports.3, 4, 5 This mortality rate is substantially greater than in previous viral pneumonitis pandemics, such as the 2009 H1N1 influenza pandemic with morality rates between 10% and 30%.6 , 7 Usual provision of ICU level support has also been strained during the current pandemic by the natural history of severe COVID-19 with reports of protracted ICU lengths of stay.8
Although COVID-19 is a global pandemic, the burden of disease has not been homogenous, and a number of regions that experienced earlier, rapid community spread reported strained or resource limited health care systems, which may have contributed to the high mortality rates.3 , 4 , 9 More recent ICU series from regions with lesser COVID-19 population prevalence have reported lower ICU mortality rates of approximately 15%.10 Although there is a need to measure the international burden of critical illness,11 there is limited understanding of the global impact and outcomes of COVID-19 infection requiring ICU admission.
The objective of this systematic review and meta-analysis was to provide a contemporary and global assessment of the point estimate of death and risk factors for severe disease in patients admitted to an ICU with COVID-19.
Methods
Search Strategy and Selection Criteria
This review was performed in accordance with the Meta-analysis of Observational studies in Epidemiology12 and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis13 reporting guidelines (e-Appendix 1).
Three electronic databases (MEDLINE, EMBASE, and the Cochrane Library) were searched from inception to August 1, 2020. Key search terms included “coronavirus,” “COVID-19,” “SARS-CoV-2” or “severe acute respiratory syndrome,” “intensive care,” “critically ill,” “critical care,” “severe.” Exact terms used are presented in e-Appendix 2. In addition, reference lists of relevant studies and review articles were searched manually for potentially eligible studies not captured in the primary search. Corresponding authors were contacted for additional data necessary for a meta-analysis. Two reviewers (E. T., J. S.) independently screened titles and abstracts of all identified studies for eligibility. Any discrepancies were resolved by consensus after discussion with a third reviewer (M. P. P.).
The inclusion criteria for studies were (1) design that included randomized controlled trials, nonrandomized controlled trials (case control or controlled cohort), observational studies and case series, (2) study population that included adult patients (≥18 years old) admitted to an ICU or high dependency unit, which included studies that compared ICU and non-ICU cohorts, (3) disease that confirmed COVID-19 or SARS-CoV-2, and (4) outcome that reported in-hospital mortality rates. Exclusion criteria were (1) review articles, opinion articles, case reports, (2) studies that did not define COVID-19 severity or did not include baseline physiologic data, (3) retracted studies, (4) studies that reported probable COVID-19 only, (5) duplicate patient data (from the same source and capture period) with preference given to sample size and quality for inclusion (e-Appendix 3), and (6) studies published in a language other than English.
Data Analysis
Two authors (E. T., J. S.) independently extracted predefined data. Data estimated values of the mean and SD were derived by formulas designed by Wan et al.14 Extracted information included study characteristics (author, geographic location, design, sample size, and start and end date), participant characteristics (age, sex, smoking status, BMI, comorbidities), presenting symptoms, admission pathologic data, and pulmonary radiologic findings (radiographic and CT imaging, organ system dysfunction, complications, severity of illness scores, treatment and ICU supports, and outcome data (ICU and hospital length of stay and in-hospital mortality rate).
The methodologic quality of included studies was assessed according to the modified versions of the Newcastle-Ottawa scale or the Quality and Synthesis of Case Series and Case Reports Protocol, as appropriate.15, 16, 17 Two reviewers independently assessed each study. Any discrepancies were discussed and resolved by consensus.
The criterion to undertake modelling in the outcome of interest was a minimum of three studies reporting relevant data. The random-effects model (DerSimonian and Laird method) was applied to estimate the pooled prevalence and 95% CIs.18 To account for extreme prevalence data, prevalence estimates were transformed with the use of the double arcsine method then back-transformed for ease of interpretation.19 Publication bias was assessed with the use of the funnel plot with asymmetry ascribed to an LFX index greater than ±1. Statistically significant heterogeneity was assessed as a Cochrane’s Q test (P < .10) and I2 > 75%. Heterogeneity was further explored through subgroup analyses with the use of the following categoric study characteristics: (1) geographic distribution (North America vs Asia vs Europe vs Middle East), (2) quality assessment - risk of bias (high vs low), (3) sample size (>150 vs ≤150 patients), (4) center type (multicenter vs single center), (5) study type (case series vs retrospective cohort vs case control vs prospective cohort vs prospective cross-sectional vs chart review and national audit), (6) study end date (before April 2020 vs after April), and (7) patient disposition-proportion censored at study end date (<20% in-hospital at time of publication vs ≥20% in-hospital). Statistical analyses for pooled prevalence were performed with MetaXL (version 5.3; EpiGear International Pty Ltd). For categoric variables (mortality rate by sex), pooled ORs and associated 95% CIs were calculated with the use of a Mantel-Haenszel model; for continuous variables (length of stay), mean differences and associated 95% CIs were calculated with an inverse-variance with the use of Review Manager (RevMan; version 5.3; The Cochrane Collaboration).
Results
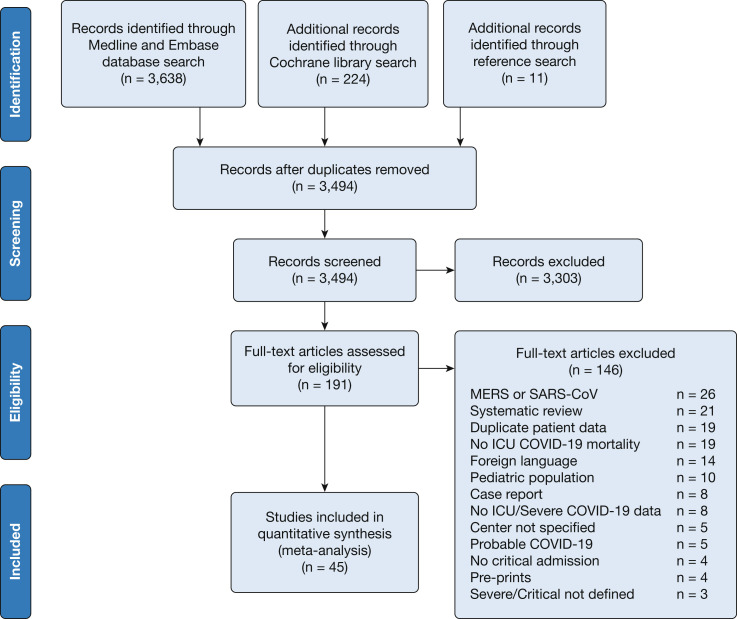
A total of 3,873 articles were retrieved with the use of the search strategy. After screening was performed by abstract and title, 191 articles were selected for full-text assessment. Forty-five studies met the inclusion criteria and were included in this review and meta-analysis (Fig 1 ).
Figure 1.
Preferred reporting items for systematic reviews and meta-analysis study flow diagram. COVID-19 = coronavirus disease 2019; MERS = Middle East respiratory syndrome; SARS-CoV = severe acute respiratory syndrome coronavirus.
The 45 studies included 16,561 patients from 17 countries from December 29, 2019, to July 30, 2020. There were 16 studies from Europe (n = 13,485),20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 15 studies from China (n = 1,385),37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 11 studies from North America (n = 1,469),52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 and three studies from Middle East (n = 222).63, 64, 65 There were 18 case series,22 , 24 , 25 , 29 , 31, 32, 33 , 36 , 38 , 41 , 48, 49, 50 , 52 , 55 , 60 , 62 , 65 nine retrospective cohort studies,21 , 23 , 35 , 40 , 45 , 53 , 59 , 61 , 63 nine case-control studies,30 , 37 , 39 , 42, 43, 44 , 46 , 51 , 58 five prospective cohort studies,20 , 26 , 34 , 54 , 56 two chart reviews,57 , 64 one cross-sectional study,47 and one national audit.27 The main characteristics of the included studies are shown in Table 1 and e-Appendix 4. Sixty variables were analyzed for this meta-analysis (Table 2 ).
Table 1.
Characteristics of Included Studies
| Study | Country | Study Design | Sample Size | Dates | Quality |
|---|---|---|---|---|---|
| Almazeedi et al63 (2020) | Kuwait | Retrospective cohort | 42 | 24/02-20/4/2020 | Good |
| Amit et al64 (2020) | Israel | Chart review | 156 | 05/03-27/04/2020 | Good |
| Arentz et al52 (2020) | United States | Case series | 21 | 20/02-05/03/2020 | Fair |
| Auld et al53 (2020) | United States | Retrospective cohort | 217 | 06/03-17/04/2020 | Poor |
| Barrasa et al20 (2020) | Spain | Prospective cohort | 48 | 04/03-24/03/2020 | Poor |
| Bhatla et al54 (2020) | United States | Prospective cohort | 79 | 06/03-19/05/2020 | Good |
| Bhatraju et al55 (2020) | United States | Case series | 24 | 24/02-09/03/2020 | Fair |
| Borobia et al21 (2020) | Spain | Retrospective cohort | 75 | 25/02-19/04/2020 | Good |
| Cardoso et al22 (2020) | Portugal | Case series | 20 | 10/03/2020 | Poor |
| Chen et al37 (2020) | China | Retrospective case control subject | 51 | 22/01-25/03/2020 | Poor |
| Cui et al38 (2020) | China | Case series | 81 | 30/01-22/03/2020 | Poor |
| Cummings et al56 (2020) | United States | Prospective cohort | 257 | 02/03-01/04/2020 | Good |
| Ferguson et al57 (2020) | United States | Chart review | 21 | 13/03-11/04/2020 | Poor |
| Grasselli et al23 (2020) | Italy | Retrospective cohort | 1715 | 20/02-22/04/2020 | Poor |
| Halasz et al24 (2020) | Italy | Case series | 242 | 02-04/2020 | Fair |
| Halvatsiotis et al25 (2020) | Greece | Case series | 90 | 10/03-13/04/2020 | Fair |
| Helms et al26 (2020) | France | Prospective cohort | 150 | 03/03-31/03/2020 | Good |
| Hur et al58 (2020) | United States | Retrospective case control subject | 138 | 01/03-08/04/2020 | Good |
| ICNARC et al27 (2020) | England | National clinical audit | 10624 | 01/03-30/07/2020 | … |
| Khamis et al65 (2020) | Oman | Case series | 24 | 24/02-24/04/2020 | Poor |
| Klok et al28,29 (2020) | Netherlands | Case series | 184 | 07/03-05/04/2020 | Poor |
| Li J et al39 (2020) | China | Retrospective case control subject | 74 | 25/01-26/02/2020 | Poor |
| Ling et al40 (2020) | China | Retrospective cohort | 8 | 22/01-11/02/2020 | Poor |
| Llitjos et al30 (2020) | France | Retrospective case control subject | 26 | 19/03-11/04/2020 | Poor |
| Longchamp et al31 (2020) | Switzerland | Case series | 25 | 08/03-04/04/2020 | Poor |
| Maatman et al59 (2020) | United States | Retrospective cohort | 109 | 12/03-31/03/2020 | Poor |
| Mitra et al60 (2020) | Canada | Case series | 117 | 21/02-14/04/2020 | Fair |
| Myers et al61 (2020) | United States | Retrospective cohort | 113 | 01/03-31/03/2020 | Poor |
| Pavoni et al32 (2020) | Italy | Case series | 40 | 28/02-10/04/2020 | Fair |
| Pedersen et al33 (2020) | Denmark | Case series | 16 | 11/03-01/04/2020 | Poor |
| Richardson et al62 (2020) | United States | Case series | 373 | 01/03-04/04/2020 | Fair |
| Rodriguez et al34 (2020) | Spain | Prospective cohort | 43 | 14/03-16/04/2020 | Poor |
| Simonnet et al35 (2020) | France | Retrospective cohort | 124 | 27/02-05/04/2020 | Good |
| Thomas et al36 (2020) | United Kingdom | Case series | 63 | 15/03-14/04/2020 | Poor |
| Wang Y et al41 (2020) | China | Case series | 344 | 25/01-25/02/2020 | Good |
| Wei et al42 (2020) | China | Retrospective case control subject | 14 | 27/01-11/03/2020 | Poor |
| Wu et al43 (2020) | China | Retrospective case control subject | 83 | 20/01-19/02/2020 | Good |
| Xu B et al44 (2020) | China | Retrospective case control subject | 107 | 26/12-01/03/2020 | Good |
| Xu J et al45 (2020) | China | Retrospective cohort | 239 | 12/01-03/02/2020 | Good |
| Yang L et al46 (2020) | China | Retrospective case control subject | 29 | 30/01-08/02/2020 | Good |
| Yu et al47 (2020) | China | Prospective cross-sectional | 226 | 26/02-26/02/2020 | Poor |
| Zhang G et al48 (2020) | China | Case series | 55 | 02/01-10/02/2020 | Fair |
| Zhang J et al49 (2020) | China | Case series | 19 | 16/01-20/02/2020 | Poor |
| Zheng et al50 (2020) | China | Case series | 34 | 22/01-05/03/2020 | Fair |
| Zhou Y et al51 (2020) | China | Retrospective case control subject | 21 | 28/01-02/03/2020 | Poor |
Table 2.
Pooled Prevalence of Patient Characteristics, Presenting Symptoms, Interventions, Treatment, and Disposition
| Variable | Studies | Total Sample Size | Patients | Crude Prevalence (%) |
Pooled Prevalence (%), or Pooled Mean, Unit | 95% CI (Upper-Lower) | Heterogeneity |
|
|---|---|---|---|---|---|---|---|---|
| I2 (%) | P Value | |||||||
| Demographics | ||||||||
| Age | 38 | 15,654 | ... | ... | 62.6 y | (60.4-64.7) | 98 | <.01 |
| BMI | 12 | 1,391 | ... | ... | 30.1 kg/m2 | (28.7-31.4) | 93 | <.01 |
| Male | 41 | 14,431 | 9,925 | 68.8% | 65.6% | (62.7-68.5) | 80 | <.01 |
| Current smoker | 19 | 1,321 | 218 | 16.5% | 17.4% | (11.8-23.8) | 87 | <.01 |
| Comorbidities | ||||||||
| Hypertension | 34 | 3,283 | 1,631 | 49.7% | 49.5% | (44.9-54.0) | 84 | <.01 |
| Diabetes mellitus | 35 | 3,345 | 907 | 27.1% | 26.6% | (22.7-30.8) | 84 | <.01 |
| Cardiovascular disease | 31 | 13,604 | 766 | 5.6% | 22.2% | (13.9-31.8) | 98 | <.01 |
| OSA | 5 | 287 | 54 | 18.8% | 20.0% | (12.0-29.5) | 64 | .03 |
| Chronic kidney disease | 25 | 12,786 | 431 | 3.4% | 10.0% | 6.2-14.6) | 95 | <.01 |
| COPD | 21 | 2,053 | 183 | 8.9% | 9.4% | (7.3-11.8) | 59 | <.01 |
| Asthma | 11 | 985 | 89 | 9.0% | 9.2% | (7.0-11.6) | 27 | .18 |
| Malignancy | 21 | 1,925 | 125 | 6.5% | 6.5% | (5.0-8.1) | 42 | .02 |
| Chronic liver disease | 17 | 1,744 | 78 | 4.5% | 4.7% | (2.9-6.8) | 69 | <.01 |
| Organ transplantation | 4 | 499 | 21 | 4.2% | 4.4% | (2.7-6.3) | 0 | .51 |
| Immunosuppressed | 10 | 11,437 | 402 | 3.5% | 4.1% | (2.5-6.0) | 60 | <.01 |
| Presenting symptoms | ||||||||
| Fever | 17 | 1,377 | 1,071 | 77.8% | 78.9% | (68.6-87.6) | 94 | <.01 |
| Dyspnea | 19 | 1,386 | 935 | 67.5% | 70.0% | (59.7-79.4) | 93 | <.01 |
| Cough | 20 | 1,503 | 1,001 | 66.6% | 68.1% | (58.5-77.0) | 93 | <.01 |
| Anorexia | 3 | 473 | 166 | 35.1% | 46.8% | (21.7-72.8) | 95 | <.01 |
| Fatigue | 10 | 794 | 364 | 45.8% | 37.6% | (24.3-51.8) | 92 | <.01 |
| Sputum | 10 | 633 | 221 | 34.9% | 34.2% | (24.9-44.1) | 77 | <.01 |
| Myalgia | 12 | 781 | 192 | 24.6% | 23.2% | (14.4-33.4) | 87 | <.01 |
| Diarrhea | 15 | 1,278 | 236 | 18.5% | 15.5% | (10.9-20.7) | 79 | <.01 |
| Rhinorrhea | 5 | 393 | 38 | 9.7% | 11.2% | (6.3-17.1) | 42 | .14 |
| Sore throat | 11 | 701 | 71 | 10.1% | 10.9% | (5.5-17.7) | 82 | <.01 |
| Nausea | 8 | 455 | 42 | 9.2% | 9.5% | (6.1-13.6) | 35 | .16 |
| Chest pain | 3 | 211 | 19 | 9.0% | 9.3% | (5.7-13.6) | 0 | .70 |
| Headache | 12 | 663 | 38 | 5.7% | 6.5% | (4.2-9.1) | 24 | .21 |
| Hemoptysis | 4 | 151 | 8 | 5.3% | 4.5% | (0.6-11.2) | 49 | .12 |
| Symptoms onset to ICU admission | 8 | 2,030 | ... | ... | 9.0 d | (7.9-10.0) | 91 | <.01 |
| Laboratory results on hospital admission | ||||||||
| C-Reactive Protein | 7 | 732 | ... | ... | 170.0 mg/L | (113.6-226.3) | 99 | <.01 |
| D-Dimer | 8 | 929 | ... | ... | 3.1 mg/L | (2.0-4.1) | 95 | <.01 |
| Ferritin | 2 | 37 | ... | ... | 1,968.3 μg/mL | (660.4-3276.1) | 91 | .02 |
| Lactate | 4 | 377 | ... | ... | 1.3 mmol/L | (1.1-1.6) | 88 | <.01 |
| Lymphocyte count | 9 | 745 | ... | ... | 0.8 × 109/L | (0.8-0.9) | 54 | .03 |
| Procalcitonin | 3 | 448 | ... | ... | 1.5 ng/L | (1.0-2.0) | 72 | .03 |
| Imaging | ||||||||
| Chest radiography: bilateral chest infiltrates | 10 | 735 | 381 | 51.8% | 71.7% | (48.1-90.7) | 97 | <.01 |
| CT chest: ground glass opacity | 5 | 495 | 267 | 53.9% | 65.5% | (23.7-97.7) | 98 | <.01 |
| Disease severity on ICU admission | ||||||||
| Sequential Organ Failure Assessment score | 12 | 1,391 | ... | ... | 6.3% | (5.1-7.6) | 99 | <.01 |
| Acute Physiology and Chronic Health Evaluation II score | 7 | 11,099 | ... | ... | 16.8% | (14.9-18.8) | 98 | <.01 |
| Organ dysfunction | ||||||||
| ARDS | 13 | 1,260 | 819 | 65.0% | 76.1% | (65.7-85.2) | 93 | <.01 |
| Acute kidney injury | 13 | 1,287 | 380 | 30.2% | 27.1% | (20.6-34.2) | 84 | <.01 |
| Acute liver injury | 6 | 715 | 270 | 37.8% | 25.8% | (1.3-61.6) | 98 | <.01 |
| Shock | 7 | 895 | 230 | 25.7% | 25.3% | (16.7-35.0) | 88 | <.01 |
| Acute cardiac injury | 11 | 1,326 | 357 | 26.9% | 24.2% | (13.5-36.7) | 95 | <.01 |
| Arrhythmia | 3 | 302 | 49 | 16.2% | 22.7% | (3.1-50.5) | 93 | <.01 |
| Thrombotic event | 7 | 852 | 195 | 22.9% | 22.6% | (16.3-29.5) | 76 | <.01 |
| Secondary infection | 9 | 873 | 159 | 18.2% | 18.4% | (14.0-23.2) | 60 | <.01 |
| Interventions | ||||||||
| Invasive mechanical ventilation | 28 | 13,543 | 9,247 | 68.3% | 67.7% | (59.1-75.7) | 98 | <.01 |
| Vasopressors | 12 | 1,052 | 581 | 55.2% | 65.9% | (52.4-78.4) | 94 | <.01 |
| Renal replacement therapy | 18 | 12,276 | 3,017 | 24.6% | 16.9% | (12.1-22.2) | 92 | <.01 |
| Noninvasive mechanical ventilation | 15 | 1,519 | 276 | 18.2% | 16.6% | (9.4-25.3) | 93 | <.01 |
| Extracorporeal membrane oxygenation | 18 | 1,828 | 103 | 5.6% | 6.4% | (4.1-9.1) | 76 | <.01 |
| Treatment | ||||||||
| Antimicrobial therapy | 18 | 1,677 | 1,526 | 91.0% | 94.6% | (90.6-97.6) | 88 | <.01 |
| Antiviral therapy | 18 | 1,580 | 791 | 50.1% | 74.3% | (51.9-91.9) | 99 | <.01 |
| Intravenous immunoglobulin | 8 | 917 | 365 | 39.8% | 50.1% | (17.8-82.3) | 99 | <.01 |
| Glucocorticoid | 17 | 1,617 | 704 | 43.5% | 43.2% | (24.8-62.5) | 98 | <.01 |
| Disposition | ||||||||
| ICU length of stay | 11 | 2,484 | ... | ... | 10.8 d | (9.3-18.4) | 94 | <.01 |
| Hospital length of stay | 10 | 2,518 | ... | ... | 19.1 d | (16.3-21.9) | 95 | <.01 |
| In-hospital deaths | 45 | 16,561 | 6,783 | 41.0% | 28.1% | (23.4-33.0) | 96 | <.01 |
| Remain in hospital | 32 | 15,842 | 1,590 | 10.0% | 22.6% | (16.8-28.9) | 98 | <.01 |
| Discharged from hospital | 33 | 15,896 | 7,689 | 48.4% | 43.9% | (38.9-48.9) | 95 | <.01 |
Critically ill patients with COVID-19 had a mean age of 62.6 years (95% CI, 60.4-64.7), a mean BMI of 30.1 kg/m2 (95% CI, 28.7-31.4) and more than two-thirds were male (65.6%; 95% CI, 62.7-68.5). Mortality rates did not differ between sex (OR [men vs women], 1.20; 95% CI, 0.93-1.55; P = .16) (Table 2; e-Appendixes 5, 6). Common comorbidities included hypertension (49.5%; 95% CI, 44.9-54.0), diabetes mellitus (26.6%; 95% CI, 22.7-30.8), and cardiovascular disease (22.2%; 95% CI, 13.9-31.8). Nearly one-fifth of patients were current smokers (17.4%; 95% CI, 11.8-23.8). The most frequent symptoms on presentation were fever (78.9%), dyspnea (70.0%), cough (68.1%), and anorexia (46.8%). The median time from onset of symptoms to ICU admission was 9.0 days (95% CI, 7.9-10.0) (Table 2; e-Appendix 7).
On admission, inflammatory markers were elevated: C-reactive protein 170.0 mg/L (95% CI, 113.6-226.3), ferritin 1968.3 μg/mL (95% CI, 660.4-3276.1), procalcitonin 1.5 ng/L (95% CI, 1.0-2.0), and D-Dimer 3.1 mg/L (95% CI, 2.0-4.1). Lactate 1.3 mmol/L (95% CI, 1.1-1.6) was not raised markedly. In ten studies that reported chest radiography findings, bilateral infiltrates were seen in 72% (95% CI, 48.1-90.7) of patients.40 , 41 , 43 , 48 , 52 , 55 , 57 , 61 , 63 , 65 In five studies that reported CT findings, ground glass opacities were reported in 66% (95% CI, 23.7-97.7) of patients39 , 41 , 42 , 52 , 63 (Table 2; e-Appendix 8).
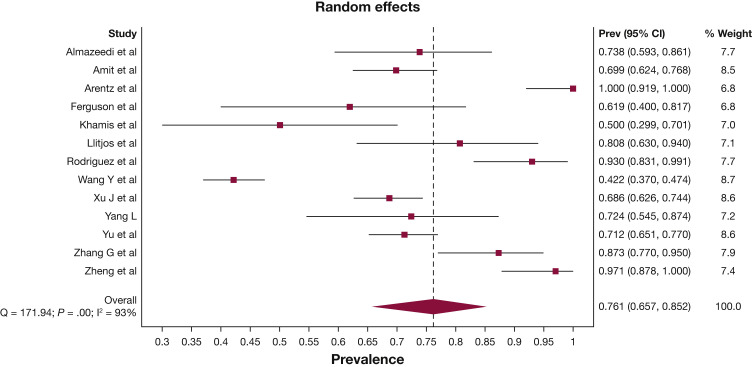
More than three-quarters of patients were diagnosed with ARDS during their ICU admission (76.1%; 95% CI, 65.7-85.2) (Fig 2 ). Approximately one-quarter of patients were reported to have acute kidney injury (27.1%; 95% CI, 20.6-34.2), shock (25.3%; 95% CI, 16.7-35.0), acute cardiac injury (24.2%; 95% CI, 13.5-36.7), arrhythmia (22.7%; 95% CI, 3.1-50.5), and/or a thrombotic event (22.6%; 95% CI, 16.3-29.5). The pooled initial Sequential Organ Failure Assessment (SOFA) score was 6.3 (95% CI, 5.1-7.6) and Acute Physiology and Chronic Health Evaluation (APACHE) II score was 16.8 (95% CI, 14.9-18.8) (Table 2; e-Appendix 9). Invasive mechanical ventilation was required in 67.7% (95% CI, 59.1-75.7) of patients; 65.9% (95% CI, 52.4-78.2) of patients required vasopressor support; 16.9% (95% CI, 12.1-22.2) of patients received renal replacement therapy; 16.6% (95% CI, 9.4-25.3) of patients received noninvasive ventilation, and 6.4% (95% CI, 4.1-9.1) of patients received extracorporeal membrane oxygenation (Table 2, e-Appendix 10).
Figure 2.
Forest plot of prevalence of ARDS. Prev = Prevalence.
Antimicrobial therapy was administered to 94.6% (95% CI, 90.6-97.6) of patients with severe-to-critical COVID-19 infection. Antiviral therapy use was reported in 18 studies, with a pooled prevalence of 74.3% (95% CI, 51.9-91.9). Ten studies reported using lopinavir/rotinavir,20 , 37 , 39 , 43 , 45, 46, 47 , 50 , 64 , 65 and five studies reported using remdesivir54, 55, 56, 57 , 64 (Table 2, e-Appendix 10). Glucocorticoid was prescribed to 43.2% (95% CI, 24.8-62.5) of patients.
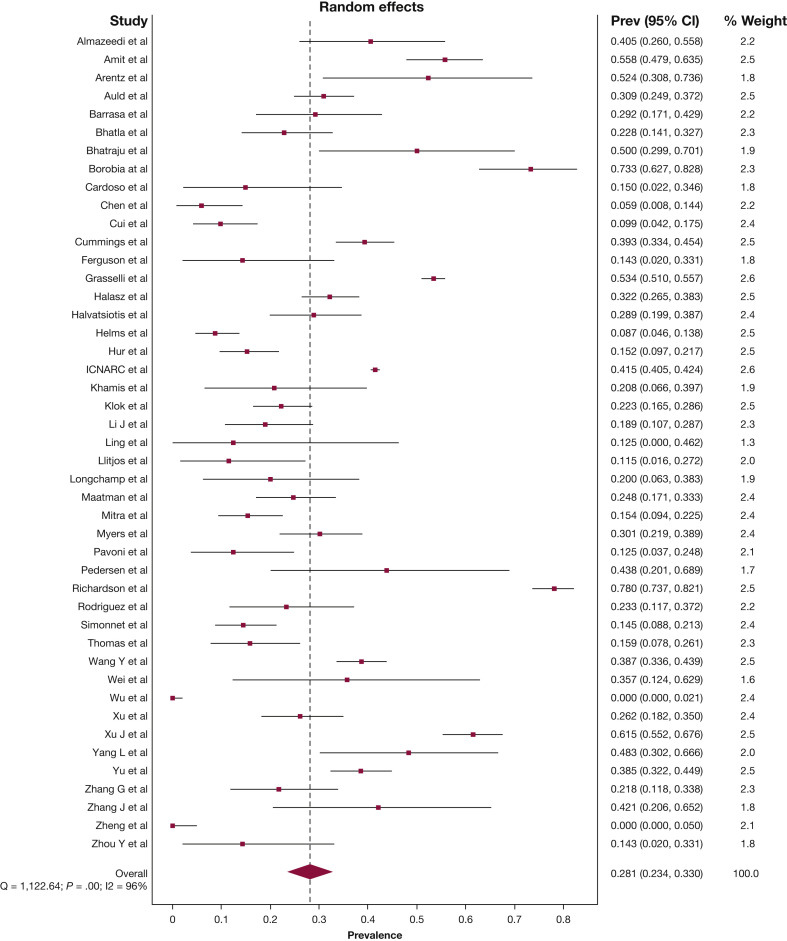
The pooled mean duration of ICU admission was 10.8 days (95% CI, 9.3-18.4), and the pooled mean hospital duration of admission was 19.1 days (95% CI, 16.3-21.9). The pooled estimate for patients who remained in hospital with uncertain outcomes at the study end point was 22.6% (95% CI, 16.8-28.9). The pooled estimate for in-hospital mortality rate was 28.1% (95% CI, 23.4-33.0) (Fig 3 ), and discharge from hospital alive was 43.9% (95% CI, 38.9-48.9), albeit with considerable statistical heterogeneity (I2 96%, P < .01; I2 95%, P < .01, respectively). The funnel plot of all 45 studies was asymmetric, which suggests possible publication bias (Table 2; e-Appendixes 11-13).
Figure 3.
Forest plot of prevalence of in-hospital death. Prev = Prevalence.
Subgroup analyses revealed that mortality rate did not differ by geographic distribution, study type or quality, sample size, center type, end of study date, or patient disposition. There is no evidence of substantial difference in the mortality rates across the prespecified subgroups. (e-Appendix 14).
Discussion
This systematic review and meta-analysis of 45 studies includes 16,561 patients from 17 countries across four continents. The main findings are that patients who were admitted to ICU with COVID-19 required considerable organ support, with point estimates that more than three-quarters were diagnosed with ARDS and one-quarter were recorded as having shock and/or acute kidney injury. Invasive mechanical ventilation and vasopressor support were required in more than two-thirds of patients; renal replacement therapy was required in one-fifth of patients, and more than one in 20 of the patients received extracorporeal membrane oxygenation. The in-hospital mortality rate was between 23.4% and 33.0%.
More than 80% of coronavirus cases experience mild-to-moderate symptoms; approximately 15% have severe disease that requires hospitalization, and around 5% require intensive care support.66 , 67 Previous meta-analyses have examined risk factors for mortality rates in COVID-19 infections; however, these studies included a majority of data within a certain region68, 69, 70, 71, 72 or included patients with COVID-19 infections but less severe symptoms.73, 74, 75, 76, 77, 78, 79 To address this limitation, the current systematic review and meta-analysis provides a comprehensive global overview of patient demographic, comorbidities, signs and symptoms, initial laboratory and imaging results, treatment, organ dysfunction, and outcomes in adults with severe and critical COVID-19.
More than two-thirds of patients with severe COVID-19 were men, with a mean age of 62.6 years and a mean BMI of 30.1 kg/m2, which confirms previous reports that there is a preponderance to severe disease with male sex, older age, and obesity.69 , 73 In keeping with an older overweight population, we describe a comorbid demographic that progress to severe COVID-19, with one-half of patients having arterial hypertension and approximately one-quarter having diabetes mellitus and cardiovascular disease.
This analysis confirms that, across the globe, once admitted to ICU with COVID-19 infection, the duration of admission to ICU and hospital is protracted. The mean ICU length of stay of 10.8 days was approximately double the duration of admission for severe community-acquired pneumonia80 and longer than observed with H1N1 influenza pneumonia.81 However, the upper 95% CI for in-hospital mortality rates was only 33.0%. Given that both the duration of admission and the intensity of ICU-level supports have led to enormous strain on critical care provision, particularly in geographic areas that experienced rapid community transmission,82, 83, 84 this may have contributed to the heterogeneity of mortality data.
These estimates of global death for severe COVID-19 (in-hospital mortality rate between 23.4% and 33.0%) may impact the interpretation of existing trial data. For example, data from the RECOVERY trial indicated that dexamethasone caused a marked reduction in deaths (in-hospital censored at day 28) in patients with COVID-19 whose condition required mechanical ventilation (dexamethasone 29.3% vs usual care 41.4%).85 However, the current meta-analysis of 16,561 patients with severe COVID-19 infection suggests that the magnitude of benefit observed with dexamethasone during the RECOVERY trial may not be reproducible across all settings.
Furthermore, given that global data suggest that less than one-third of patients die once admitted to ICU, the implication of this meta-analysis is that provision of sufficient ICU level service capacity is a global health priority to prevent inequalities in outcomes from this disease.
With 16,561 critically ill patients across 17 countries and four continents, this review is the largest and most granular assessment of outcomes of severe COVID-19 to date. Limitations of this systematic review include the presence of publication bias and that most included studies were case series and retrospective in design. There is a risk of survivor bias, with nearly one-quarter of patients remaining in hospital. The pooled prevalence for patients discharged alive was only 43.9% (95% CI, 38.9-48.9). Studies varied with the censor date for the identification of death. We specified the outcome measure as “death,” “remained in hospital,” and “discharged alive,” and it is possible that all-cause death that is censored at some later landmark (eg, day 90) may be greater than reported in this meta-analysis. There was considerable statistical heterogeneity found for many results. However, despite exploring the cause of heterogeneity through extensive subgroup analyses, there was no singular cause identified. Finally, studies were excluded that were not written in English. Although the risk of bias from excluding studies not published in English is considered low,86 it is uncertain how the additional patients would have affected the 95% CIs.
This systematic review provides the most expansive snapshot to date of the international experience of COVID-19 that requires critical care support. Advanced age, male sex, obesity, smoking, hypertension, diabetes mellitus, and cardiovascular disease are major risk factors for severe COVID-19. More than two-thirds of patients require invasive mechanical ventilation; approximately 20% of patients require renal replacement therapy, and the mean duration of ICU admission was 11 days. There was marked heterogeneity in mortality rate; however, the global mortality rate point estimate for patients with COVID-19 who are admitted to an ICU was 28%.
Acknowledgments
Author contributions: E. T. is the guarantor of the content of this manuscript, including the data and analysis. E. T. contributed to protocol design, search, data extraction, quality assessment, statistical analysis, and writing the first draft of the report. J. S. contributed to search, data extraction, and quality assessment. M. P. P. contributed to protocol design, interpretation of data and critical revision of the report. A. M. D. contributed to interpretation of data and critical revision of the report. All authors have seen and approved the final version.
Financial/nonfinancial disclosures: None declared.
Additional information: The e-Appendixes can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;7(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Rojek A., Dutch M., Peyton D., et al. Patients presenting for hospital-based screening for COVID-19: risk of disease, and healthcare access preferences. Emerg Med Australas. 2020;32(5):809–813. doi: 10.1111/1742-6723.13589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ANZIC Influenza Investigators. Webb S.A., Pettila V., et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361(20):1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen-Van-Tam J.S., Openshaw P.J., Hashim A., et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009) Thorax. 2010;65(7):645–651. doi: 10.1136/thx.2010.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagiannidis C., Mostert C., Hentschker C., et al. Case characteristics, resource use, and outcomes of 10,021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitra A.R., Fergusson N.A., Lloyd-Smith E., et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ. 2020;192(26):E694–E701. doi: 10.1503/cmaj.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adhikari N.K., Fowler R.A., Bhagwanjee S., Rubenfeld G.D. Critical care and the global burden of critical illness in adults. Lancet. 2010;376(9749):1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2020;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standardized deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2) doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Savovic J., Page M.J., Elbers R.G. In: Cochrane Handbook for Systematic Reviews of Interventions: Cochrane. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., editors. 2020. Overview of RoB 2. [Google Scholar]
- 17.Wells G., Shea D., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-analysis. 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed August 3, 2020.
- 18.Kontopantelis E., Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a stimulation study. Stat Methods Med Res. 2010;21(4):409–426. doi: 10.1177/0962280210392008. [DOI] [PubMed] [Google Scholar]
- 19.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 20.Barrasa H., Rello J., Tejada S., et al. SARS-CoV-2 in Spanish Intensive Care Units: Early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39(5):553–561. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borobia A.M., Carcas A.J., Arnalich F., et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. 2020;9(6):1733. doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso F.S., Pereira R., Germano N. Liver injury in critically ill patients with COVID-19: a case series. Crit Care. 2020;24(1):190. doi: 10.1186/s13054-020-02924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasselli G., Greco M., Zanella A., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halasz G., Leoni M.L., Villani G.Q., Nolli M., Villani M. Obesity, overweight and survival in critically ill patients with SARS-CoV-2 pneumonia: is there an obesity paradox? Preliminary results from Italy. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320939675. 2047487320939675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halvatsiotis P., Kotanidou A., Tzannis K., et al. Demographic and clinical features of critically ill patients with COVID-19 in Greece: the burden of diabetes and obesity. Diabetes Res Clin Pract. 2020;166:108331. doi: 10.1016/j.diabres.2020.108331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Intensive Care National Audit & Research Centre . ICNARC; London: 2020. ICNARC Report on COVID-19 in Critical Care 31 July 2020. https://www.icnarc.org/DataServices/Attachments/Download/42ceb4d2-3dd3-ea11-9128-00505601089b. Accessed August 3, 2020. [Google Scholar]
- 28.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klok F.A., Kruip M., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llitjos J.F., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longchamp A., Longchamp J., Manzocchi-Besson S., et al. Venous thromboembolism in critically ill patients with COVID-19: results of a screening study for deep vein thrombosis. Res Pract Thromb Haemost. 2020;4(5):842–847. doi: 10.1002/rth2.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50(2):281–286. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen H.P., Hildebr T., Poulsen A., et al. Initial experiences from patients with COVID-19 on ventilatory support in Denmark. Dan Med J. 2020;67(5):A04200232. [PubMed] [Google Scholar]
- 34.Rodriguez A., Moreno G., Gomez J., et al. Severe infection due to the SARS-CoV-2 coronavirus: experience of a tertiary hospital with COVID-19 patients during the 2020 pandemic. Med Intensiva. 2020;44(9):525–533. doi: 10.1016/j.medin.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonnet A., Chetboun M., Poissy J., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas W., Varley J., Johnston A., et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y., Zhang K., Zhu G., et al. Clinical characteristics and treatment of critically ill patients with COVID-19 in Hebei. Ann Palliat Med. 2020;9(4):2118–2130. doi: 10.21037/apm-20-1273. [DOI] [PubMed] [Google Scholar]
- 38.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J., Xu G., Yu H., Peng X., Luo Y., Cao C. Clinical characteristics and outcomes of 74 patients with severe or critical COVID-19. Am J Med Sci. 2020;360(3):229–235. doi: 10.1016/j.amjms.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling L., So C., Shum H.P., et al. Critically ill patients with COVID-19 in Hong Kong: a multicentre retrospective observational cohort study. Crit Care Resusc. 2020;22(2):119–125. doi: 10.51893/2020.2.oa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Wang Z., Tse G., et al. Cardiac arrhythmias in patients with COVID-19. J Arrhythm. 2020;36(5):827–836. doi: 10.1002/joa3.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Y., Zeng W., Huang X., et al. Clinical characteristics of 276 hospitalized patients with coronavirus disease 2019 in Zengdu District, Hubei Province: a single-center descriptive study. BMC Infect Dis. 2020;20(1):549. doi: 10.1186/s12879-020-05252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J., Li W., Shi X., et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288(1):128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 44.Xu B., Fan C., Wang A., et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J., Yang X., Yang L., et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. 2020;24(1):394. doi: 10.1186/s13054-020-03098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L., Liu J., Zhang R., et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol. 2020;129:104475. doi: 10.1016/j.jcv.2020.104475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y., Xu D., Fu S., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24(1):219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang G., Hu C., Luo L., et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J., Liu P., Wang M., et al. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single-centred, retrospective, observational study. Z Gesundh Wiss. 2020:1–4. doi: 10.1007/s10389-020-01291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y., Sun L.J., Xu M., et al. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B. 2020;21(5):378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y., Han T., Chen J., et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13(6):1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. J Emerg Med. 2020;58(4):710. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auld S.C., Caridi-Scheible M., Blum J.M., et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48(9):e799–e804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. COVID-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson J., Rosser J.I., Quintero O., et al. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, northern California, USA, March-April 2020. Emerg Infect Dis. 2020;26(8):14. doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hur K., Price C.P.E., Gray E.L., et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngology Head Neck Surg. 2020;163(1):170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maatman T.K., Jalai F., Feizpour C., et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48(9):e783–e790. doi: 10.1097/CCM.0000000000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitra A.R., Fergusson N.A., Llyod-Smith E., et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ. 2020;29(192):E694–E701. doi: 10.1503/cmaj.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almazeedi S., Al-Youha S., Jamal M.H., et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amit M., Sorkin A., Chen J., et al. Clinical course and outcomes of severe Covid-19: a national scale study. J Clin Med. 2020;9(7):18. doi: 10.3390/jcm9072282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khamis F., Al-Zakwani I., Al Naamani H., et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13(7):906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). 2020. https://stacks.cdc.gov/view/cdc/88624
- 67.Wu Z., McGoogan J.M. Characteristics of and important lessons from coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese centre for Disease Control and Prevention. JAMA. 2020;7(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 68.Taylor E.H., Hofmeyr R., Torborg A., et al. Risk factors and interventions associated with mortality or survival in adult covid-19 patients admitted to critical care: A systematic review and meta-analysis. Southern African Journal of Anaesthesia and Analgesia. 2020;26(3):116–127. [Google Scholar]
- 69.Zhang J.Y., Lee K.S., Ang L.W., Leo Y.S., Young B.E. Risk factors for severe disease and efficacy of treatment in patients infected wiht COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020;81(12):e16–e25. doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Z., Peng F., Xu B., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(12):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasiri M.J., Haddadi S., Tahvildari A., et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med (Lausanne) 2020;7:459. doi: 10.3389/fmed.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quah P., Li A., Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24(1):285. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jutzeler C.R., Bourguignon L., Weis C.V., et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;(37):2020. doi: 10.1016/j.tmaid.2020.101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu L., Wang B., Yuan T., et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Potere N., Valeriani E., Candeloro M., et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. 2020;24(1):389. doi: 10.1186/s13054-020-03022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao Y., Liu X., Xiaong L., Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: z systematic review and meta-analysis. J Med Virol. 2020;92(9):1449–1459. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 80.Woodhead M., Welch C.A., Harrison D.A., Bellingan G., Ayres J.G. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10(suppl 2):S1. doi: 10.1186/cc4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.ANZIC Influenza Investigators. Webb S.A., Aubron C., et al. Critical care services and the H1N1 (2009) influenza epidemic in Australia and New Zealand in 2010: the impact of the second winter epidemic. Crit Care. 2011;15(3):R143. doi: 10.1186/cc10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 83.Xie J., Tong Z., Guan X., Du B., Qiu H., Slutsky A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46(5):837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. 2020;323(15):1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 85.Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19: preliminary report [published online ahead of print July 17, 2020]. N Engl J Med. 10.1056/NEJMoa2021436. [DOI]
- 86.Morrison A., Polisena J., Husereau D., et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.