Abstract
Background
The risk of pulmonary embolism (PE) in patients with Coronavirus Disease 2019 (COVID-19) is recognized. The prevalence of PE in patients with respiratory deterioration at the Emergency Department (ED), the regular ward, and the Intensive Care Unit (ICU) are not well-established.
Objectives
We aimed to investigate how often PE was present in individuals with COVID-19 and respiratory deterioration in different settings, and whether or not disease severity as measured by CT-severity score (CTSS) was related to the occurrence of PE.
Patients/methods
Between April 6th and May 3rd, we enrolled 60 consecutive adult patients with confirmed COVID-19 from the ED, regular ward and ICU who met the pre-specified criteria for respiratory deterioration.
Results
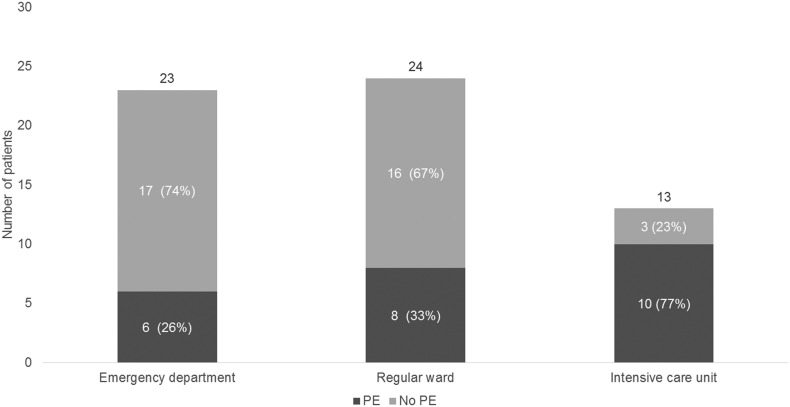
A total of 24 (24/60: 40% (95% CI: 28–54%)) patients were diagnosed with PE, of whom 6 were in the ED (6/23: 26% (95% CI: 10–46%)), 8 in the regular ward (8/24: 33% (95% CI: 16–55%)), and 10 in the ICU (10/13: 77% (95% CI: 46–95%)). CTSS (per unit) was not associated with the occurrence of PE (age and sex-adjusted OR 1.06 (95%CI 0.98–1.15)).
Conclusion
The number of PE diagnosis among patients with COVID-19 and respiratory deterioration was high; 26% in the ED, 33% in the regular ward and 77% in the ICU respectively. In our cohort CTSS was not associated with the occurrence of PE. Based on the high number of patients diagnosed with PE among those scanned we recommend a low threshold for performing computed tomography angiography in patients with COVID-19 and respiratory deterioration.
Keywords: COVID-19 (coronavirus disease 2019), Pulmonary embolism, Thromboprophylaxis, Computed tomography angiography
Highlights
-
•
The high prevalence of pulmonary embolism in COVID-19 is not limited to the ICU
-
•
Respiratory deterioration in COVID-19 patients should prompt a low threshold CTPA
-
•
Regular thromboprophylaxis may be insufficient to prevent PE in COVID-19 patients
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has claimed many lives, and the number of deaths continues to rise [1]. Although the majority of patients with COVID-19 develop only mild symptoms, some develop respiratory failure. Per individual, it remains unclear what underlies this respiratory deterioration, but the development of acute respiratory distress syndrome (ARDS), progression of viral pneumonia and pulmonary emboli (PE) seem to be major contributing factors. To distinguish between ARDS, progression of pneumonia and/or PE in patients with COVID-19 poses a diagnostic challenge with important therapeutic implications. Current guidelines recommend the use of non-contrast enhanced chest CT for severity assessment and monitoring of disease [2], hereby discarding PE as the cause of sudden-onset respiratory deterioration. Also, in patients with severe pneumonia there may be a lack of additional signs and symptoms to trigger diagnostic evaluation for PE. Since many COVID-19 patients have elevated d-dimer levels [3], diagnostic algorithms, like Well's criteria or Years [4,5], may not be helpful. Taken together, a vigilant approach to detection of PE and consecutive treatment may be one of the ways to improve outcomes in hospitalized symptomatic COVID-19 patients. The optimal diagnostic regimen to achieve this goal is unclear, and therefore prevalence studies of PE at the time of respiratory deterioration are urgently needed.
Several studies have shown thrombotic complications varying from 23% to as high as 69% in ICU patients with COVID-19 when systematic screening for DVT was performed [[6], [7], [8], [9], [10]]. While most studies focussed predominantly on the critically ill ICU population, these data also raise concern for COVID-19 patients in clinical non-ICU settings, such as low-to-medium care wards or emergency departments (ED). Furthermore, it is currently unknown whether disease severity is a risk factor for developing PE, a vital statistic that could help clinicians in the diagnostic decision-making process. Recent autopsy studies in patients with COVID-19 have shown histopathologic patterns of diffuse alveolar damage consistent with ARDS, but also widespread thrombosis, frequent lethal PE and deep venous thrombosis (DVT) without obvious clinical preceding symptoms of these conditions [11,12]. However, contradictory autopsy findings (no thromboembolic events at all), have also been shown in a case series of 10 deceased patients [13]. Therefore, the prevalence of PE at the moment of respiratory deterioration in COVID-19 remains unknown, yet it is a vital statistic in caring for COVID-19 patients. A timely diagnosis of PE might change an otherwise unfavourable course by the administration of anticoagulants. Furthermore, the prevalence of PE is essential in designing the clinical trials needed to establish the optimal diagnostic and therapeutic strategy in hospitalized COVID-19 patients. We hypothesized that the typical sudden deterioration in oxygen saturation is not always caused by the progression of COVID-19 pneumonia, but is often related to the occurrence of PE. Given these considerations, the aim of the current study was first, to investigate how often respiratory deterioration, as the main diagnostic trigger, would be associated with PE in three different settings; ED, regular ward and ICU; and, secondly, whether there was an association between disease severity on CT and the occurrence of PE.
2. Methods
2.1. Patients and data collection
In a Dutch university hospital we consecutively enrolled 60 hospitalized adult patients with COVID-19 between April 6th and May 3rd who met the pre-specified criteria of respiratory deterioration or had a clinical suspicion of PE and underwent a computer tomography pulmonary angiography (CTPA). Initially, the primary focus was on optimal and, where applicable, standardized care. The criteria were part of our local hospital COVID-19 guidelines that evolved to include a low threshold for suspicion of PE in COVID-19 patients. We included patients from the ED, regular ward and ICU. All CTPAs performed in COVID-19 patients were separately coded, a list of included patients was later provided to the study team. Then, informed consent was obtained retrospectively from all surviving participants. No informed consent was deemed necessary for the deceased patients. All patients were followed up for mortality until June 3rd. This study was approved by the ethics committee of the Maastricht University Medical Centre (METC 2020–1572). COVID-19 was confirmed by a positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2 RNA on a nasal and pharyngeal swab or sputum. COVID-19 was also considered confirmed in patients with a negative RT-PCR but a disease course consistent with COVID-19 and a chest-CT showing pulmonary abnormalities consistent with COVID-19 (CO-RADS 4 or 5 as defined by the Dutch Radiology Society [14,15]).
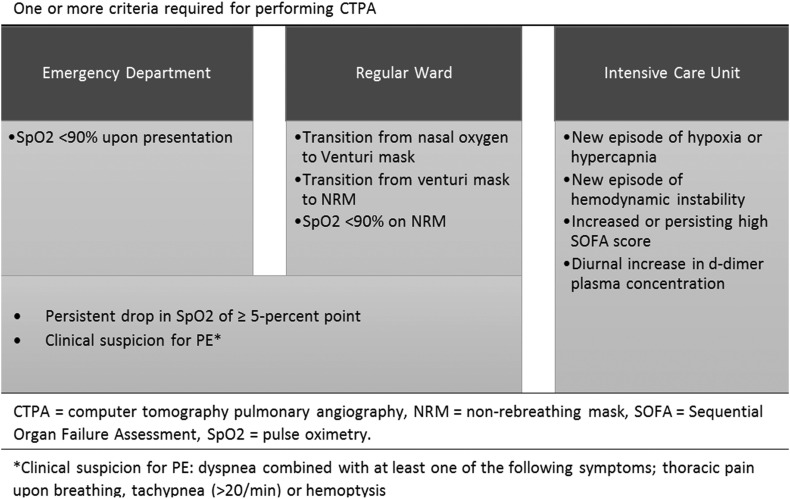
Respiratory deterioration was defined as an oxygen saturation measured by pulse oximetry (SpO2) <90% on ambient air upon presentation to the ED or a persistent (>1 h) drop in SpO2 of ≥5‐percent point at any time during admission. We also included patients that required transition from nasal oxygen to Venturi mask, from Venturi mask to non-rebreathing mask, or respiratory failure while on a non-rebreathing mask (SpO2 < 90%); i.e. the need for mechanical ventilation. Clinical suspicion for PE was defined as dyspnea combined with at least one of the following symptoms; thoracic pain upon breathing, tachypnea (>20/min) or hemoptysis. In the ICU, where all patients were mechanically ventilated during the course of their disease, we included all patients who showed one or more of the following clinical characteristics: a new episode of hypoxia or hypercapnia that persisted after optimization of both mechanical ventilator settings and overall fluid balance; a new episode of hemodynamic instability; an increased or persisting high Sequential Organ Failure Assessment (SOFA) [16]; or a diurnal increase in d-dimer plasma concentration [17] (Fig. 1 ). For example, in patients with a d-dimer of <1000–2000 μg/L at admission, a progressive rise to >2000–4000 μg/L was a reason to perform CTPA, even when no other apparent clinical signs or symptoms were present.
Fig. 1.
Criteria for respiratory deterioration per setting.
All patients admitted to the regular ward who did not use therapeutic anticoagulants upon admission received thromboprophylaxis adjusted for body weight (Nadroparine 2850 IU if <70 kg, Nadroparine 3800 IU if 70–90 kg, Nadroparine 5700 IU if >90 kg). During the COVID pandemic, incident thrombosis appeared frequently. From April 1st thromboprophylaxis dosage in the ICU was increased (Nadroparine 3800 IU if <70 kg, Nadroparine 5700 IU if 70–90 kg, Nadroparine 7600 IU if >90 kg). After the release of a new national guidance [18], patients in the ICU received a double dose starting April 23rd (i.e. Nadroparine 5700 IU if <70 kg, Nadroparine 7600 IU if 70–90 kg, Nadroparine 11.400 IU if >90 kg) as compared to patients on the regular ward.
2.2. Assessment of PE and pulmonary evolvement by COVID-19
All patients admitted through the ED of our hospital underwent an unenhanced CT-scan at admission in a triage setting using a mobile CT unit (Lightspeed 16; GE; Alliance Medical). The CT scans were interpreted by the attending chest radiologist, who scored the likelihood of COVID-19 based on a 5-point scale (CO-RADS [14]). In patients who presented with an oxygen saturation < 90%, the unenhanced chest CT was directly followed by a CTPA. All patients underwent a dedicated CTPA with intravenous contrast on a 2nd generation Dual-Source CT (Somatom Flash; Siemens).
Patients who were already admitted to the regular ward or ICU only underwent a CTPA, and the lung window reconstructions were used for assessment of extent of parenchymal involvement, since in this population the COVID-19-related lung abnormalities had evolved to consolidations and a separate unenhanced CT scan for pulmonary evolvement was considered unnecessary. CT scans were reconstructed at a soft tissue window for assessment of PE and at a lung window for assessment of the extent of parenchymal abnormalities. The extent of parenchymal involvement on CTPA was separately assessed using a CT severity score (CTSS) by a dedicated chest radiologist (HAG) [19]. For each lobe involved, a score 1–5 was given: 1:1–5%, 2:6–25%, 3: 56–50%, 4: 51–75%: 5: >75% involved, resulting in a maximum score of 25. The primary outcome was confirmed PE in patients with respiratory failure in the different settings. CT scans were systematically scored for PE, and classified as central, lobular, segmental or subsegmental.
2.3. Covariates
Demographic, clinical, laboratory and treatment data were collected at the time of inclusion. We recorded sex, age, medical history significant for VTE or active malignancy and the use of anticoagulants.
3. Statistical analysis
Statistical analyses were performed using SPSS statistics version 25 (IBM, Armonk, New York, USA). Patient characteristics were compared between ED, regular ward and ICU using the Chi-square test for categorical variables. Continuous variables were described by means with standard deviation (SD) or medians with interquartile range (IQR) depending on the distribution. In addition, the Mann-Whitney U test, the Kruskal-Wallis test, t-test, or ANOVA (analysis of variance) were used, as appropriate. Univariable and multivariable logistic regression analyses were performed to estimate the potential confounding effect of age and sex. A two-sided p-value < 0.05 was considered statistically significant.
4. Results
Table 1 shows the patient characteristics of our cohort with COVID-19 patients and respiratory deterioration. In this population, 24 (40%) were diagnosed with PE, of whom 6 were in the ED (6/23: 26% (95% CI: 10–46%)), 8 in the regular ward (8/24: 33% (95% CI: 16–55%)), and 10 in the ICU (10/13: 77% (95% CI: 46–95%)) (Fig. 2 ). Only one patient was included for having a clinical suspicion for PE alone, all other included patients also met the criteria for respiratory deterioration. Patients who developed PE, were on average, 68 (SD 11.7) years old and most (62%) did not use anticoagulation (VKA/ DOAC/ therapeutic LMWH) or antiplatelet therapy upon admission. Notably, 3 patients already on therapeutic anticoagulants developed PE, and 15 patients developed PE in spite of in-hospital treatment with prophylactic LMWH. The location of PE was segmental (46%) or subsegmental (33%) in most patients; however, we also diagnosed 2 central PE (8%) and 3 lobar PE (13%). D-dimers were elevated in all patients (median 2.410 IQR 1.272-5929) but were significantly higher in patients with PE (3.463 IQR 3.059-10.000) as compared to patients without PE (median 1668 IQR 998–2996). Next, we investigated whether the severity of COVID-19 pneumonia was associated with the occurrence of PE. Median (IQR) CTSS were 21 (19.3–23.0), 11 (1.8–16.8) and 15 (7.5–19.5) in the ICU, Ward and ED, respectively. Although median CTSS was higher in the ICU, logistic regression analysis also showed no statistically significant association between CTSS and occurrence of PE (age and sex-adjusted OR 1.06 (95%CI 0.98–1.15), per unit CTSS). Patients with higher CTSS were however more likely to be in ICU (Table 1).
Table 1.
Characteristics of the study population.
| Characteristics | Total cohort N = 60 |
ED N = 23 |
Regular ward N = 24 |
ICU N = 13 |
|---|---|---|---|---|
| Mean age in years – SDa | 68 (11.7) | 69 (12.6) | 70 (10.2) | 62 (11.8) |
| Male sex – No. (%) | 42 (70.0) | 16 (69.6) | 15 (62.5) | 11 (84.6) |
| COVID-19 PCR confirmed – No. (%) | 53 (88.3) | 18 (78.3) | 22 (91.7) | 13 (100) |
| CTSS – median (IQR)ab | 15.5 (9.3–19.8) |
12.0 (5.0–17.0) |
14.5 (7.3–17.0) |
21.0 (18.5–23.0) |
| Hospital length of stay in days – median (IQR)abc | 16.0 (8.0–32.0) |
8.0 (4.8–21.5) |
13.5 (9.3–31.8) |
34.0 (22.0–44.5) |
| Follow-up period in days – median (IQR)ac | 44 (11–49) |
45 (41–50) |
35 (3.3–45.5) |
46 (11–52) |
| Antithrombotic therapy – No. (%) | ||||
| None | 37 (61.6) | 12 (52.2) | 16 (66.6) | 9 (69.2) |
| Antiplatelet therapy | 15 (25.0) | 8 (34.8) | 4 (16.7) | 3 (23.1) |
| VKA/DOAC | 7 (11.7) | 3 (13.0) | 4 (16.7) | 0 |
| Therapeutic LMWH | 1 (1.7) | 0 | 0 | 1 (7.7) |
| Prophylactic LMWH – No. (%)b | 33 (55) | 1 (4.3) | 20 (83.3) | 13 (100) |
| Malignancy – No. (%) | 8 (13.3) | 4 (17.4) | 4 (16.7) | 0 |
| Prior history VTE – No. (%) | ||||
| No VTE | 56 (93.3) | 21 (91.3) | 23 (95.8) | 12 (92.3) |
| Previous VTE | 4 (6.7) | 2 (8.7) | 1 (4.2) | 1 (7.7) |
| D-dimer in ug/L – mediana | 2410 | 3276 | 1369 | 3643 |
| (IQR) | (1272–5929) | (1077–9747) | (605–2358) | (1980–5929) |
| Missing – No (%) | 23 (38.3) | 7 (30.4) | 16 (66.7) | 0 |
| hsTNT in ng/L – median | 18 | 24 | 24 | 16 |
| (IQR) | (11.3–45.3) | (12–78) | (14.8–41.8) | (8.5–23.5) |
| Missing – No (%) | 24 (40) | 8 (33.3) | 16 (66.7) | 0 |
| NT-proBNP in pmol/L – median | 124 | 117 | 188 | 65 |
| (IQR) | (36.4–249.5) | (36–357) | (77.5–359) | (42.4–186.5) |
| Missing – No (%) | 23 (38.3) | 8 (34.8) | 15 (62.5) | 0 |
| Outcome – No (%)c | ||||
| Discharged alive | 42 (70.0) | 20 (87.0) | 13 (54.8) | 9 (69.2) |
| Died | 17 (28.3) | 2 (8.7) | 11 (45.2) | 4 (30.8) |
| Still hospitalized | 1 (1.7) | 1 (4.3) | 0 | 0 |
SD = standard deviation; CT = Computed tomography; CTSS = CT severity score; LMWH = Low Molecular Weight Heparin; VKA = vitamin K antagonist; DOAC = direct oral anticoagulant; IQR = interquartile range; NT-proBNP = N-terminal pro hormone B-type natriuretic peptide; hs-TNT = high-sensitive troponin T.
p < 0.05 between ICU and ward.
p < 0.05 between ICU and ED
p < 0.05 between ward and ED.
Fig. 2.
Development of pulmonary embolism subdivided by setting.
Median follow-up was 44 days (IQR 11–49), and 17 (28.3% (95% CI:18–41%)) patients died. In these 17 patients, we observed no difference in mortality between individuals with (n = 9) and without PE (n = 8) (p logrank = 0.272). Importantly, no bleeding complications were reported in our cohort.
5. Discussion
Our main findings were that, at the occurrence of respiratory deterioration in COVID-19 the prevalence of PE is high and different for the three settings, and is not associated with disease severity on CT (CTSS). To our knowledge, this is the first study to report the prevalence of PE in the setting of respiratory deterioration, which is a more clinically meaningful circumstance than screening or autopsy-detected PE in previous studies [9,11,13]. As the prevalence of PE in our cohort was higher than observed in previous studies, we postulated that PE directly contributes to respiratory deterioration in COVID-19 rather than being a bystander of severe pulmonary inflammation. This is further substantiated by the fact that we found no noticeable difference in CTSS between individuals with and without PE in our overall cohort, or when subdivided by setting. However, this contrasts with the fact that only 21% of the PEs were central or lobar, which are more prone to cause hemodynamic instability. Nevertheless, and although our data do not directly support this hypothesis, this is an important observation, as timely diagnosis of PE may potentially alter an otherwise unfavourable clinical course, by the administration of therapeutic doses of anticoagulation. Therefore, we advocate the use of a low threshold of CTPA to detect PE in the setting of unexplained respiratory deterioration in the hospitalized non-ICU population.
An earlier study, performed in the ICU, reported a cumulative incidence of 31% for thrombotic complications [7] and after an updated analysis 49%, of which the majority was PE [20]. Diagnostic tests were performed in this study if thrombotic complications were clinically suspected. Another study observed a very high cumulative incidence for VTE (47%) in ICU patients using a screening approach [9]. Interestingly, this study showed only 3% symptomatic VTE in the ward and zero DVTs when screening ward patients. Recently, studies have been advocating the use of duplex ultrasound as a method of systematic screening for thromboembolic events in COVID-19 pneumonia [21]. However, we believe that a vigilant approach with regard to respiratory deterioration in patients is preferable over screening for VTE in hospitalized individuals with COVID-19 for several reasons. Firstly, in COVID-19, PE is not always preceded by DVT [11,22], implying that screening with duplex ultrasound would be insufficient. Also, an earlier prospective study showed that the incidence of asymptomatic DVT was similar to other series [23]. Moreover, the importance of diagnosing asymptomatic DVT can be questioned, especially when in situ immunothrombosis plays a pivotal role in the pathophysiology of PE in COVID-19 [24,25]. Finally, in a pandemic with hospital resources being stretched to their limits, a screening routine using a labour-intensive method (e.g. duplex ultrasound) is not feasible and also not in line with measures taken to spare personal protective equipment. Other studies previously reported on the prevalence or incidence of PE in other settings than the ICU. A French study examined the prevalence of PE in 100 COVID-19 patients with clinical features of severe COVID-19 (e.g. the requirement for mechanical ventilation or underlying comorbidities), in both the ICU and regular ward, and they found PE in 23 (23%) patients of whom 17 resided in the ICU and 6 on the regular ward [6]. A retrospective cohort study in Italy found 10 (2.8%) PE in 362 patients of whom 2 (4.2% of ICU population) resided in the ICU and 8 (2.5% of general ward population) in the general ward. These incidences are probably highly underestimated, since they only performed imaging tests in 44 (11% of total) patients [26]. The difference in PE prevalence between our study and previous studies can be explained by the algorithms used to perform a CTPA, thereby increasing the a-priori likelihood of PE. This also ensures optimal use of the resources available. In patients with proven COVID-19, a clinical suspicion for PE is often lacking when a reassuring differential diagnosis is present (e.g. progression of pneumonia). This might suggest that caution is needed when employing standard diagnostic PE-algorithms in COVID-19 as they have not been validated in this population, and relevant studies are currently lacking. This is further highlighted by the fact that three patients developed PE in our study, although already being treated with therapeutic anticoagulants (because of atrial fibrillation) upon admission. Moreover, 15 patients diagnosed with PE received thromboprophylaxis, suggesting that the current standard thromboprophylaxis is insufficient to prevent thrombotic complications in patients with COVID-19, however recommendations on thromboprophylaxis await the results of further research specifically designed to assess the effectiveness of the current thromboprophylaxis in COVID-19. These data also underline the need for including unselected patients in RCT's addressing the benefit of increased doses of thromboprophylaxis and not only patients from high risk settings.
Our study has some strengths and limitations. Strengths include the use of simple, clearly defined and pre-specified criteria for respiratory deterioration, making it directly applicable to day-to-day care for COVID-19 patients. Furthermore, we included patients from the ED and regular ward, which are both underrepresented in available studies, adding valuable information to the expanding COVID-19 literature. Limitations include a small sample size. Additionally, even though inclusion criteria were clearly defined, we cannot rule out the possibility that clinical judgement might have also influenced the decision to order scans. Selection bias might have been introduced as patients that were deemed unfit for transportation to the CT scanner, and patients started on palliative care as a result of their respiratory deterioration, did not undergo CTPA, possibly missing some of the sickest patients. Not including these patients most likely will have led to an underestimation of the prevalence of PE as these are among the sickest patients with a high a priori probability for PE, further highlighting the extremely high prevalence of PE in this population. Since some patients already resided in the ICU at the start of this study, PE might have initially been missed when they were first transported to the ICU, possibly leading to an overestimation of the amount of PEs that occurred in the ICU. Furthermore, we acknowledge that the criteria used to determine clinical deterioration in the ICU are at least partly subjective and therefore difficult to apply uniformly. Nevertheless, we believe these criteria do justice to the difficult real-life clinical setting.
In conclusion, we found PE in a substantial part of patients hospitalized for COVID-19 with respiratory deterioration. This study has several important implications, including a low clinical threshold for performing CTPA in patients with rapid respiratory deterioration. Further research should focus on exploring the mechanisms behind the development of PE in COVID-19 to optimize prophylactic treatment and prophylactic strategies for all COVID-19 patients.
Funding
This research did not receive any specific grand from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare no relevant conflicts of interest for the submitted work.
References
- 1.University JH Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://coronavirus.jhu.edu.map.html Available at:
- 2.ACR recommendations for the use of Chest Radiography and Computed Tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection Available at:
- 3.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb. Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Maanen R., Rutten F.H., Klok F.A. Validation and impact of a simplified clinical decision rule for diagnosing pulmonary embolism in primary care: design of the PECAN prospective diagnostic cohort management study. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2019-031639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf S.J., McCubbin T.R., Feldhaus K.M. Prospective validation of Wells criteria in the evaluation of patients with suspected pulmonary embolism. Ann. Emerg. Med. 2004;44(5):503–510. doi: 10.1016/j.annemergmed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Grillet F., Behr J., Calame P. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;201544 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llitjos J.F., Leclerc M., Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bompard F., Monnier H., Saab I. Pulmonary embolism in patients with Covid-19 pneumonia. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wichmann D., Sperhake J.P., Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaller T., Hirschbuhl K., Burkhardt K. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prokop M., van Everdingen W., van Rees Vellinga T. CO-RADS - a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020;201473 doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tas J., van Gassel R.J.J., Heines S.J.H. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht Intensive Care COVID cohort. MaastrICCht. medRxiv. 2020;10 doi: 10.1136/bmjopen-2020-040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent J.L., Moreno R., Takala J. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Oudkerk M., Buller H.R., Kuijpers D. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for public health of the Netherlands. Radiology. 2020;201629 doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leidraad COVID-19 coagulopathie. https://www.demedischspecialist.nl/sites/default/files/Leidraad%20COVID-19%20coagulopathie.pdf Available at:
- 19.Chest CT severity score: an imaging tool for assessing severe COVID-19. https://radiologyassistant.nl/chest/lk-jg-1 Available at: [DOI] [PMC free article] [PubMed]
- 20.Klok F.A., Kruip M., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandmaison G., Andrey A., Periard D. Systematic screening for venous thromboembolic events in COVID-19 pneumonia. TH Open. 2020;4(2) doi: 10.1055/s-0040-1713167. (e113-e5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cattaneo M., Bertinato E.M., Birocchi S. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120:1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demelo-Rodriguez P., Cervilla-Munoz E., Ordieres-Ortega L. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb. Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desborough M.J.R., Doyle A.J., Griffiths A. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb. Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dam L.F., Kroft L.J.M., van der Wal L.I. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb. Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]