Abstract
The COVID-19 pandemic has brought an unprecedented crisis to the global health sector. When discharging COVID-19 patients in accordance with throat or nasal swab protocols using RT-PCR, the potential risk of reintroducing the infection source to humans and the environment must be resolved. Here, 14 patients including 10 COVID-19 subjects were recruited; exhaled breath condensate (EBC), air samples and surface swabs were collected and analyzed for SARS-CoV-2 using reverse transcription-polymerase chain reaction (RT-PCR) in four hospitals with applied natural ventilation and disinfection practices in Wuhan. Here we discovered that 22.2% of COVID-19 patients (n = 9), who were ready for hospital discharge based on current guidelines, had SARS-CoV-2 in their exhaled breath (~105 RNA copies/m3). Although fewer surface swabs (3.1%, n = 318) tested positive, medical equipment such as face shield frequently contacted/used by healthcare workers and the work shift floor were contaminated by SARS-CoV-2 (3–8 viruses/cm2). Three of the air samples (n = 44) including those collected using a robot-assisted sampler were detected positive by a digital PCR with a concentration level of 9–219 viruses/m3. RT-PCR diagnosis using throat swab specimens had a failure rate of more than 22% in safely discharging COVID-19 patients who were otherwise still exhaling the SARS-CoV-2 by a rate of estimated ~1400 RNA copies per minute into the air. Direct surface contact might not represent a major transmission route, and lower positive rate of air sample (6.8%) was likely due to natural ventilation (1.6–3.3 m/s) and regular disinfection practices. While there is a critical need for strengthening hospital discharge standards in preventing re-emergence of COVID-19 spread, use of breath sample as a supplement specimen could further guard the hospital discharge to ensure the safety of the public and minimize the pandemic re-emergence risk.
Keywords: COVID-19, SARS-CoV-2, Exhaled breath, Airborne transmission, Surface-borne
Graphical abstract
Highlights
-
•
Recovering COVID-19 patients (22.2%) still exhale thousands of SARS-CoV-2 per minute.
-
•
Hospital air (6.8%) was shown to have SARS-CoV-2 levels of 9–219 COVID-19 viruses/m3.
-
•
Surface swabs had a level of 3–8 viruses/cm2 with a SARS-CoV-2 positive rate of 3.1%.
1. Introduction
The world has almost been brought to a standstill by the COVID-19 pandemic, and hospitals across the globe face unprecedented challenges (Wan et al., 2020). Inside COVID-19 patient care centers, the air, frequently touched object surfaces, and floors have been found to be contaminated by SARS-CoV-2 (Guo et al., 2020; Liu et al., 2020; Santarpia et al., 2020). Infections of medical staff by SARS-CoV-2 have been reported in hospitals globally; some of them unfortunately lost their lives (CCDC, 2020; Reusken et al., 2020). Additionally, the medical community is challenged with the dilemma of discharging patients to re-allocate precious resources, while accepting the risks of reintroducing the source of infection to the public. For convenient diagnosis of the disease, the hospitals use throat or nasal swabs, sometimes supplemented with computed tomography (CT) scans for enhanced screening (Xie et al., 2020; Zhao et al., 2020). After hospital discharge, however, some COVID-19 patients, have tested positive again for SARS-CoV-2 using the throat swab protocol (Peng et al., 2020; Xiao et al., 2020; Zhang et al., 2020). Despite major progress in understanding COVID-19, infection risks related to recovering patients and to contaminated surfaces and air remain uncertain. In this study, we aimed to examine the possible presence of SARS-CoV-2 in exhaled breath specimens from recovering COVID-19 patients who have repeatedly tested negative using throat swabs; and to study environmental contamination by SARS-CoV-2 within four hospitals in Wuhan, China.
2. Materials and methods
2.1 Exhaled breath sample collection
Details of ready-to-discharge COVID-19 patients and the four hospital configurations (Tables S1 and S2) were described in Supporting Information. Exhaled breath condensate (EBC) samples were collected from 13 recruited patients (9 recovering COVID-19 patients, and four with influenza symptoms who tested negative for SARS-CoV-2), using a BioScreen II device following the protocols provided by the manufacturer (Fig. S4). To avoid saliva contamination and collection of larger droplets, a long straw, made of polypropylene, was used to allow the patient to breathe into a tube that was electrically cooled for 5 min. For COVID-19 patient B-L2, who died later, EBC was not taken. The EBC sample of one non-COVID-19 patient (C–Y1) was collected twice to confirm the result. EBC sample volumes of approximately 300–500 μL were obtained for all patients. The samples were immediately pipetted into a corning tube and transported to the laboratory for SARS-CoV-2 analysis. Clinical information for non-COVID-19 patients is listed in Table S2. The throat swabs of the recovering COVID-19 patients had all tested negative for SARS-CoV-2 by the hospital (Table S2) before their EBC samples were collected. Examples of the EBC sample collection points in four different hospitals are shown in Figs. S2 and S3. A total of 14 EBC samples were collected (see Table S3 for detailed information).
2.2 Surface swab sample collection
Swab samples were collected from surfaces associated with the COVID-19 patients and medical staff, and from many other surfaces inside the four hospitals in Wuhan. Specifically, a wet cotton swab was used to scrub the surface (an area of 10 cm × 10 cm or 5 cm × 5 cm) of objects in the hospital environment and from personal items of the patients. The surface swab samples were deposited in the virus collection liquid (Jiangsu Kangjian Medical Supply, Inc, Taizhou, China), and then transported to the laboratory and stored at −20 °C for SARS-CoV-2 analysis. A total of 318 surface swabs were collected. Details are listed in Table S4.
2.3 Air sample collection
Air samples were collected from the corridors, hospital waste storage rooms, ICU rooms, toilets, medical preparation rooms, clinical observation rooms, and general wards of four hospitals in Wuhan, China. The air samples were collected using the Air-nCoV-Watch (ACW) system developed by Peking University through integrating housemade impinger samplers (Wa-15,WA-400) in collaboration with a company in Beijing and a robot (examples for onsite sampling are shown in Fig. S5). The WA-15 sampled at a flow rate of 15 L/min, while the WA-400 with a cutoff size of 0.58 μm sampled at 400 L/min. For corridor spaces or naturally ventilated environments, the WA-400 was installed on a robot for air sampling (Figs. S5A and B), while for semi-enclosed environments such as toilets or ICU rooms, the WA-15 was used for sampling (Fig. S5C). The robot was programmed to move along pre-determined routes inside the hospital (Fig. S5B; Video S1). In each case, air was sampled into 3 mL of the virus sampling liquid mentioned above for 40 min; after the sampling, the remaining volume of the collection liquid dropped to about 1.5–2 mL due to evaporation. The collected air samples were transported to the laboratory for SARS-CoV-2 analysis. The air samples collected using the robot comprised air from different areas of the interior hospital corridor, and thus were more representative than those from stationary samplers for average viral level of the corridor air. . Air samples were also collected inside intensive care units, hospital wards, and toilet room. Some examples of air sample collection points are also shown in Figs. S2 and S3. A total of 44 air samples were collected; detailed information is listed in Table S5.
The following is the supplementary data related to this article:
The collected samples (200 μL taken from the collection liquid) went through several steps for SARS-CoV-2 detection. First, an automated nucleic acid extraction device (NP968–S, Xi'an Tianlong Sci &Tech Co., Ltd., Xi'an, China) and an RNA extraction kit (Jiangsu Bioperfectus Technologies, Nanjing, China) were used to extract SARS-CoV-2 RNA, achieving a final sample RNA suspension of 70–80 μL. SARS-CoV-2 detection with targets of N and ORF1a/b genes was then performed using RT-PCR (BioRAD CFX96 Real-Time System C1000 Thermal Cycler, Hercules, California) together with a detection kit (Jiangsu Bioperfectus Technologies) (except for sample D–SS–V10 with additional E and RdRp genes-Table S4) under the following cycle conditions: 50 °C for 10 min and 97 °C for 1 min, followed by 45 cycles of 97 °C for 5 s and 58 °C for 30 s. The reaction mixture included 7.5 μL of nucleic acid amplification mix, 5 μL of Taq Enzyme Mix, 4 μL of SARS-CoV-2 reaction mix, 3.5 μL of RNA-free H2O, and 5 μL of sample RNA. In accordance with the instructions, for cycle threshold (Ct) values of less than 37, and those greater than 37 but less than 40, with an “S” shape amplification curve, the corresponding samples can be treated as positive. The RT-PCR detection limits using various primer sets were shown to be around 100 SARS-CoV-2 RNA copies/μL; those obtained by the Chinese CDC were lower (below 50 RNA copies/μL) for the N gene with a maximum Ct value of approximately 39.5 (Vogels et al., 2020), but the primer set was not disclosed by the company for the kit used here. For some air and surface swab samples were also analyzed using digital PCR (Suzhou RainSure Scientific CO.,Ltd, Suzhou, China) and a detection kit (FastPlex Triplex SARS-CoV-2 Detection Kit, Suzhou RainSure Scientific CO.,Ltd) following the same procedure described previously (Liu et al., 2020). Some of the collected samples were re-tested for SARS-CoV-2 for both N and ORF1a/b genes using the LAMP chip technology (Beijing CapitalBio Technology Co., Ltd, Beijing, China) following the manufacturer's instructions. In addition to detecting SARS-CoV-2, this technology can also detect 18 other viruses. All samplings were performed using single-use consumables and deionized (DI) water served as the negative controls.
3 Results and discussion
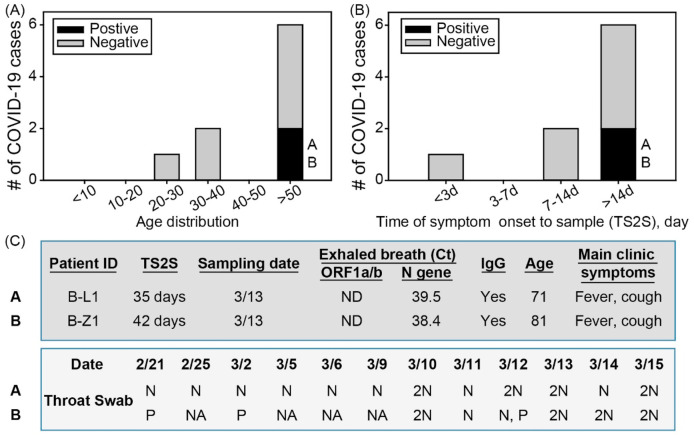
In this study, we have detected SARS-CoV-2 in exhaled breath (2 of 9, 22.2%), air samples (3 of 44, 6.8%), and surface swabs (10 of 318, 3.1%) collected from hospitals of Wuhan using both RT-PCR and digital PCR (Table 1 ). Here we discovered that two recovering COVID-19 patients, in Wuhan hospitals (Table S1), ready for hospital discharge were emitting SARS-CoV-2 RNA, about (7.35–7.77) x 104viruses per hour estimated by the method described (Ma et al., 2020), via breathing (Fig. 1 ; Table 1, Table S3). These patients were aged 71 years (BL-1) and 81 years (B-Z1) (Tables S2 and S3) (Fig. 1). The EBC samples were collected at least 14 days after they had developed clinical symptoms (Table S3); their throat swabs had repeatedly tested negative (the time line is shown in Fig. 1) at the time of the EBC collection and analysis (Fig. 1; Table S3). Their IgG tests were both positive on March 6 and 9, about 4–7 days before their EBC samples were collected (Table S3). Surprisingly, for patient B-L1(A), all tests with the throat swabs since February 21 had been negative, but the patient's EBC sample, which was collected on March 13 (about 35 days after the patient developed symptoms), tested positive for SARS-CoV-2 (Fig. 1). For patient B-Z1(B), throat swabs tested positive on February 21, and March 2, and then tested negative on March 11; however, of two tests performed on March 12, one tested positive, and the other tested negative. On the following days (March 13, 14, and 15), the patient's throat swabs repeatedly tested negative (Fig. 1; Table S3). Similar to patient B-L1 (A), patient B-Z1's EBC sample, which was collected on March 13 (42 days after the patient developed clinical symptoms), tested positive. The throat swabs from three non-COVID-19 patients (C-J1, A-U1, A-J1) all tested negative for SARS-CoV-2. For additional one non-COVID-19 patient (C–Y1), two EBC samples were collected, both of which tested negative by RT-PCR. The EBC samples from one COVID-19 subject (A-X1) and two non-COVID-19 subjects (A-J1 and A-U1) also tested negative using the chip technology as described in the Methods. Overall, the EBC sample positive rate was 22.2% among the 9 recovering COVID-19 patients (one patient B-L2 died, and the subject's EBC was thus not taken).
Table 1.
Analysis of SARS-CoV-2 and its positive rates from EBC samples collected from 9 ready-to-discharge/recovering COVID-19 patients, 44 air samples, and 318 surface swabs. Air and swab samples were directly quantified by a digital PCR. SARS-CoV-2 RNA level in exhaled breath sample was estimated based on an assumed amplification efficiency of 75%; and a RT-PCR detection limit of 100 copies/μL (Vogels et al., 2020) following a method described (Ma et al., 2020).
| Analysis of SARS-CoV-2 for different Samples |
Exhaled breath condensates from recovering COVID-19 patients (n=9) | Air samples (n=44) |
Surface swabs (n=318) |
|---|---|---|---|
| Sample SARS-CoV-2 RNA positive rate | 2/9 (22.2%) | 3/44 (6.8%) | 10/318 (3.1%) |
| Estimated SARS-CoV-2 emission rate/level | a (7.35 × 104, 7.77 × 104) RNA copies/hour |
b (9 × 10°, 2.19 × 102) RNA copies/m3 (digital PCR) |
b (3 × 10°, 8 × 10°) RNA copies/cm2 (6 of 10 by digital PCR) |
Lower Ct values from RT-PCR were used among those of N or ORF1a/b genes for presentation and viral estimation.
Air and surface swab samples were analyzed using digital PCR.
Fig. 1.
SARS-CoV-2 detection from EBC samples taken from the 9 recovering COVID-19 patients (Table S2); A) the recovering COVID-19 cases by age group; B) the recovering COVID-19 cases vs time of symptom onset to sample (TS2S) (day); C) throat swab tests for two COVID-19 patients. 2N = two negative results on the same date. P = positive result; NA = no tests available; ND = not detected; N = negative result.
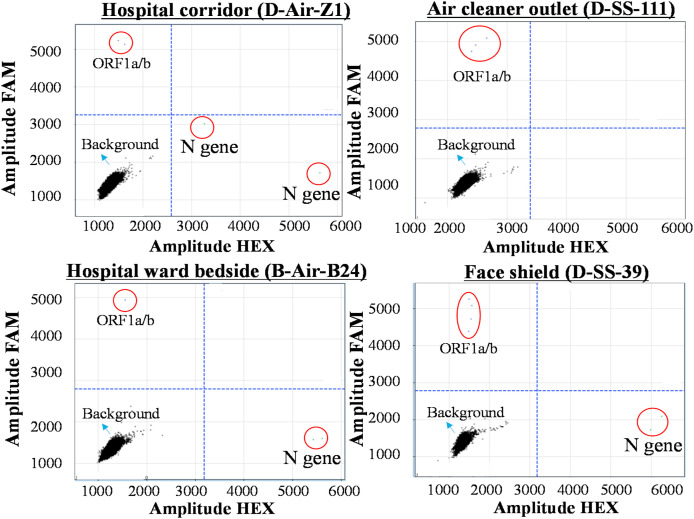
Of the 318 surface swabs collected, four samples tested positive for SARS-CoV-2 using RT-PCR, and another six samples were detected positive using digital PCR (Table 1, Fig. 2 ; Table S4). Some of example digital PCR data including hospital corridor air, ward air, air cleaner ventilation outlet, and face shield are presented in Fig. 2. More positive samples (60%) were detected among the medical touching surfaces (Table S4). The surface-borne SARS-CoV-2 concentration levels by digital PCR were shown to range from 3 to 8 viruses/cm2 (Table 1). Surprisingly, only one positive sample (bedside faucet handle- C–SS–297) (3 viruses/cm2) was detected from the 158 surfaces frequently touched by patients such as cell phones, door handles, patients' hands, or even surfaces of the masks worn by the patients (D-Y1, D-Y2) (Table S4). In line with the surface testing results, the EBC samples of the patients who were wearing the tested masks also tested negative (Table S3). However, for other mask tests we did not perform the exhaled breath analysis for their wearers due to time and resource constraints (Tables S3 and S4). One sample (D–SS–71) from the 57 hospital floor samples tested positive (4 viruses/cm2 estimated by digital PCR); and none of the 21 surface samples from clean areas, and 16 samples from other hospital surfaces tested positive for the virus (Table S4). Contrary to common belief, the observed overall positive rate for the surface swab samples was strikingly low, only 3.1% (n = 318). For all the air samples collected, none of them were detected positive by RT-PCR; however three of them were detected positive using a digital PCR, a more sensitive technology with a detection limit of 1 copy per μL (Liu et al., 2020) (Table 1, Table S5). The airborne SARS-CoV-2 concentration levels ranged from 9 viruses/m3 for the hospital corridor (D-Air-Z1) to 219 viruses/m3 for COVID-19 patient's bedside air (B-Air-B24), while the hospital ward (B-Air-A24) had a concentration level of 21 viruses/m3 (Table 1). The positive air sample from the hospital corridor was collected using the ACW as described (Video S1). In addition, the surface swab from an air cleaner was also detected positive with SARS-CoV-2 by digital PCR with a concentration level of 4 viruses/cm2. Using the described chip technology, six air samples (A-Air-1,-2,-3,-5 and -6, A-Air-Un1) (Table S5) were re-tested for both target SARS-CoV-2 genes; all of them were confirmed negative.
Fig. 2.
Examples of digital PCR data from several air and surface samples collected during the campaign, including hospital corridor air (A), air cleaner ventilation outlet surface(B) ward air (C), and face shield surface (D). ORF1a/b gene detection was performed using FAM fluorescence probe, while N gene detection was performed using HEX fluorescence probe. For a signal amplitude above 3000, the corresponding signal was treated as a positive. Cy5 fluorescence probe was used the internal control.
In our work in Beijing, we have shown that COVID-19 patients upon the onset of the disease were shown to emit ~105 viruses per min (Ma et al., 2020), two orders magnitude higher than that of the recovering stages here. Another study reported that two COVID-19 cases (one without clinical symptoms), who repeatedly tested negative using throat swabs, tested positive for SARS-CoV-2 with their bronchoalveolar lavage fluid samples (Tan et al., 2020). These findings further support our observation that SARS-CoV-2 could be emitted from the lungs via breathing, presenting a significant health risk for surrounding people and environments. This potential of hidden spread represents a serious airborne infection risk from the recovering COVID-19 patients, while deemed ready for hospital discharge using throat-swab tests. Accordingly, throat swabs and EBC might work differently with different COVID-19 patients in terms of SARS-CoV-2 screening. COVID-19 diagnosis efficacy could be further improved by using EBC specimens or additional techniques such as CT scans as a complement to current throat swab testing by RT-PCR. Because of resource constraints, we did not study the viability and biological integrity of breath-borne SARS-CoV-2. Nonetheless, one study showed that aerosolized SARS-CoV-2 could remain viable in the air for up to 3 h (van Doremalen et al., 2020). In a recent work, it was shown that in average about 60% of SARS-CoV-2 collected from a hospital air were viable (Lednicky et al., 2020). Logically, breath-emitted SARS-CoV-2, in a more favourable form of aerosolization, would be active in the air for at least the same amount of time, and would thus be capable of spreading COVID-19. On the other hand, the detected SARS-CoV-2 could be RNA-containing exosomes emitted by infected cells in viral defense (Hoen et al., 2020), dead viruses or bare RNAs in exhaled breath. Further work is needed to investigate where the detected RNA in exhaled breath originated from. Information about airborne emission and the viability of SARS-CoV-2 emitted by patients is critical to understanding COVID-19 transmission. The present work is the first to discover the presence of SARS-CoV-2 RNA in exhaled breath from recovering COVID-19 patients ready for discharge, and serves as warning for the underlying deficiency (22.2% failure rate) of the existing protocol in discharging COVID-19 patients.
In contrast to the current belief that direct surface contact represents a major route for COVID-19 transmission, we detected a very low positive rate (3.1%) for surface swabs (N = 318) from various settings in the four Wuhan hospitals. This finding implies that direct surface contact, even in high risk areas, e.g., the environments studied, may not represent a major route of COVID-19 transmission. Using conventional RT-PCR, SARS-CoV-2 levels were shown to be below the detection limits for all 44 air samples collected (Video S1) in various hospital environments. However, three of them were detected positive with SARS-CoV-2 using digital PCR (a quantitative method). The observed low positive rates for both the air and surfaces were a collective consequence of a number of factors. Firstly, virus emission dynamics from COVID-19 patients – when, how, where, and at what rate patients emit SARS-CoV-2 – are still largely unknown. The observed virus emission by at least one patient was not continuous (patient B-Z1); it may be strongly dependent on the patient's activities, e.g., coughing, sneezing, talking, or lung self-cleaning during the day. However, such activities, which are difficult to document, may have occurred before sample collection. Secondly, the hospitals applied disinfectants three times a day, possibly inactivating the virus and its RNA segments. Furthermore, all COVID-19 patients were required to wear a mask while in the hospital, reducing the release of the virus into the air or onto surfaces in the hospital environment. Lastly, natural air ventilation via open windows (every room had at least one window with an outside wind speed of up to 1.6–3.3 m/s) diluted airborne viruses. Since the time this study was conducted, no infections of medical staff have been reported from these four hospitals. Nonetheless, these data suggest that certain surfaces frequently touched by the medical staff and hospital air should be regularly disinfected to further lower related infection risks. The reported virus levels by the RT-PCR method could have been underestimated, which was previously reported to have a detection limit of 100 SARS-CoV-2 RNA copies/μL (Vogels et al., 2020). In terms of exposure to SARS-CoV-2, any single EBC collection involved high infection risks during the process, which severely limited the sample size in this work. While low infection risks were shown for surfaces and air in the evaluated hospitals, we revealed the breath emission of SARS-CoV-2 by convalescent patients otherwise ready for discharge, warranting an urgent need to revisit current hospital discharge guidelines to minimize the public risk.
CRediT authorship contribution statement
Lian Zhou: Data curation, Resources, Validation, Investigation. Maosheng Yao: Supervision, Conceptualization, Methodology, Data interpretation, Formal analysis, Resources, Investigation, Funding acquisition. Xiang Zhang: Data curation, Resources, Validation, Investigation. Bicheng Hu: Data curation, Resources, Validation, Investigation. Xinyue Li: Data curation, Resources, Validation, Investigation. Haoxuan Chen: Data curation, Resources, Validation, Investigation. Lu Zhang: Data curation, Resources, Validation, Investigation. Yun Liu: Data curation, Resources, Validation, Investigation. Meng Du: Data curation, Resources, Validation, Investigation. Bochao Sun: Data curation, Resources, Validation, Investigation. Yunyu Jiang: Data curation, Resources, Validation, Investigation. Kai Zhou: Data curation, Resources, Validation, Investigation. Jie Hong: Data curation, Resources, Validation, Investigation. Na Yu: Data curation, Resources, Validation, Investigation. Zhen Ding: Data curation, Resources, Validation, Investigation. Yan Xu: Data curation, Resources, Validation, Investigation. Min Hu: Data interpretation, Investigation, Writing - original draft, Writing - review & editing, Formal analysis, Validation. Lidia Morawska: Data interpretation, Investigation, Writing - original draft, Writing - review & editing, Formal analysis, Validation. Sergey A. Grinshpun: Data interpretation, Investigation, Writing - original draft, Writing - review & editing, Formal analysis, Validation. Pratim Biswas: Data interpretation, Investigation, Writing - original draft, Writing - review & editing, Formal analysis, Validation. Richard C. Flagan: Data interpretation, Investigation, Writing - original draft, Writing - review & editing, Formal analysis, Validation. Baoli Zhu: Supervision, Conceptualization, Methodology, Data interpretation, Formal analysis, Resources, Investigation, Funding acquisition. Wenqing Liu: Supervision, Conceptualization, Methodology, Data interpretation, Formal analysis, Resources, Investigation, Funding acquisition. Yuanhang Zhang: Supervision, Conceptualization, Methodology, Data interpretation, Formal analysis, Resources, Investigation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was equally supported by the Chinese Academy of Engineering Grant (2020-ZD-15), and a National Natural Science Foundation of China (NSFC) grant (22040101) (PI: M. Yao) dedicated to the COVID-19 pandemic. This work was also partially supported by the NSFC Distinguished Young Scholars Fund Awarded to M. Yao (21725701), and the Scientific Research Fund of Jiangsu Provincial Health Committee (S2017002).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaerosci.2020.105693.
Supporting Information
COVID-19 patient statistics; map of Hospital C (Fever clinics) and sample collection points; map of Hospital C (Wards) and air sample collection points; an example of collection of exhaled breath samples using the BioScreen II; examples of air sample collection in various hospital settings (PDF).
Hospital information; patients information; exhaled breath samples; surface swabs; air samples; pollutants and meteorological parameters information (XLSX).
Robot sampling (MP4).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England Journal of Medicine. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Prevention and Control (CCDC) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China, 2020. Chinese Journal of Epidemiology. 2020;41:145–151. [Google Scholar]
- Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., Cui Y., Fu R., Dong Y., Chi X., Zhang M., Liu K., Cao C., Liu B., Zhang K., Gao Y., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging Infectious Diseases. 2020;26(7):1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen E.N., Cremer T., Gallo R.C., Margolis L.B. Extracellular vesicles and viruses: Are they close relatives? Proceedings of the National Academy of Sciences. 2016;113(33):9155–9161. doi: 10.1073/pnas.1605146113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., Usmani M., Shankar S.N., Mohamed K., Eiguren-Fernandez A., Stephenson C.J., Alam M.M., Elbadry M.A., Loeb J.C., Subramaniam K., Waltzek T.B., Cherabuddi K., Morris G.J., Wu C.Y. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. International Journal of Infectious Diseases. 2020 doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Ma J., Qi X., Chen H., Li X., Zhan Z., Wang H., Sun L., Zhang L., Guo J., Morawska L., Grinshpun S.A., Biswas P., Flagan R.C., Yao M. Coronavirus Disease 2019 patients in early stages exhaled millions of Severe Acute Respiratory Syndrome Coronavirus 2 per hour. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa1283. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1283/5898624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Wang M., Zhang G., Lu E. Seven discharged patients turning positive again for SARS-CoV-2 on quantitative RT-PCR. American Journal of Infection Control. 2020;48:725–726. doi: 10.1016/j.ajic.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C.B., Buiting A., Bleeker-Rovers C., Diederen B., Hooiveld M., Friesema I., Koopmans M., Kortbeek T., Lutgens S.P.M., Meijer A., Murk J., Overdevest I., Trienekens T., Timen A., den Bijllaardt W.V., Disse J.V., Gageldonk-Lafeber A.V., der Vegt D.V., Wever P.C., der Hoek W.V., Kluytmans J. Rapid assessment of regional SARS-CoV-2 community transmission through a convenience sample of healthcare workers, The Netherlands, March 2020. Euro Surveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.12.2000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., Crown K.K., Brett-Major D., Schnaubelt E., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. Aerosol and surface transmission potential of SARS-CoV-2. medRxiv. 2020:2020. 03.23.20039446. [Google Scholar]
- Tan F., Qiu Y., Xu Z. Novel coronavirus pneumonia diagnosis for two cases using Bronchoalveolar lavage fluid (in Chinese) Chinese Journal of Tuberculosis and Respiratory Medicine. 2020;43:337–339. doi: 10.3760/cma.j.cn112147-20200224-00167. [DOI] [PubMed] [Google Scholar]
- Vogels C.B.F., Brito A.F., Wyllie A.L., Fauver J.R., Ott I.M., Kalinich C.C., Petrone M.E., Casanovas-Massana A., Muenker M.C., Moore A.J., Klein J., Lu P., Lu-Culligan A., Jiang X., Kim D.J., Kudo E., Mao T., Moriyama M., Oh J.E.…Grubaugh N.D. Analytical sensitivity and efficiency comparisons of SARS-COV-2 qRT-PCR primer-probe sets. Nature Microbiology. 2020 doi: 10.1038/s41564-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W., Cha A.E., Sun L.H. This is the coronavirus math that has experts so worried: Running out of ventilators, hospital beds. Washington Post. 2020 https://www.washingtonpost.com/health/2020/03/13/coronavirus-numbers-we-really-should-be-worried-about/ [Google Scholar]
- Xiao A.T., Tong Y.X., Zhang S. False‐negative of RT‐PCR and prolonged nucleic acid conversion in COVID‐19: Rather than recurrence. Journal of Medical Virology. 2020 doi: 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: Relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yan K., Ye H.H., Lin J., Zheng J.J., Cai T. SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standard for discharge. International Journal of Infectious Diseases. 2020;97:212–214. doi: 10.1016/j.ijid.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: A multicenter study. American Journal of Roentgenology. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.