Abstract
For the past 10 years, the main efforts of most bioprinting research teams have focused on creating new bioink formulations, rather than inventing new printing set-up concepts. New tissue-specific bioinks with good printability, shape fidelity, and biocompatibility are based on “old” (well-known) biomaterials, particularly fibrin. While the interest in fibrin-based bioinks is constantly growing, it is essential to provide a framework of material’s properties and trends. This review aims to describe the fibrin properties and application in three-dimensional bioprinting and provide a view on further development of fibrin-based bioinks.
Keywords: Fibrin, Bioink, Tissue engineering, Regenerative medicine, Bioprinting, Biofabrication
1 Introduction
Tissue engineering, particularly three-dimensional (3D) bioprinting, is one of the most rapidly developing fields in biomedicine. As any cutting-edge technology, 3D bioprinting requires both complex equipment and novel materials. Hence, its development can be divided into at least two steps: Technical and material.
To date, the technical step has almost passed, and the main approaches in printing set-ups have been already presented and are based on extrusion, droplet deposition, stereolithography, and laser-induced forward transfer[1-4]. However, the material step involving bioinks is in progress.
Bioinks consisting of cells (or spheroids) and biomaterials are an essential element of 3D bioprinting, and their development should ensure both precise deposition and tissue specificity. For the last decade, there is a bioink boom, and the efforts of many research teams focus on not inventing new set-ups, but creating new bioink formulations. New tissue-specific bioinks with good printability, shape fidelity, and biocompatibility can be based on “old” biomaterials. Among their huge variety, fibrin is of particular interest.
Despite its long history of use, fibrin is still highly in demand that is ensured by its unique properties. Except its biocompatibility, it is biodegradable, and the degradation products are not toxic. Moreover, compared to other biomaterials, fibrin properties (fiber morphology, stability, mechanics, etc.) can be simply tuned by varying component concentrations, buffers, etc.[5-9] While the interest in fibrin-based bioinks is constantly growing, it is essential to provide a framework of material’ properties and trends. This review focuses on describing the fibrin properties and application in 3D bioprinting and providing a view on further development of fibrin-based bioinks.
2 Fibrin overview
2.1 Classification and structure
Fibrin is a fibrillar protein formed from fibrinogen circulating in blood. It may have different origin and can be derived from salmon, bovine, porcine, and human blood plasma. Fibrinogen is an elongated dimeric glycoprotein (inactive fibrin monomer) which consists of two-dimensional domains bound by a coiled-coil segment to the central E domain. The fibrinogen molecule is formed by three polypeptide chains Aα, Bβ, and γ connected to each other in the N-terminal E domain by disulfide bridges[10,11]. It is synthetized by hepatocytes[12] that makes the liver to be the main source of fibrinogen. Fibrinogen is mostly distributed in circulating blood plasma; however, it can also be found in platelets, lymph, and interstitial fluid. Fibrinogen synthesis can be stimulated by injury and/or inflammation which causes a ten-fold increase in concentration[7]. Such activation is induced by interleukin-6 (IL-6) which triggers intercellular signaling pathways in hepatocytes and modulates gene expression through various transcription factors[13].
2.2 Fibrinogenesis
Fibrin formation from fibrinogen is one of the essential steps in the enzymatic cascade of blood coagulation pathway to stop bleeding. This process can be divided into two stages: Enzymatic and non-enzymatic. In the first stage, thrombin (Factor II) induces proteolytic cleavage and fibrinopeptide release from Aα and Bβ chains. Hence, two polymerization regions, α and β, are formed and spontaneously interact with complementary polymerization centers a- and b- in γC and βC regions on the D knot of another fibrin monomer. This leads to the gradual formation of protofibrils. Protofibrils’ aggregation in lateral and longitudinal directions ensures the formation of fibers, which branch and form a fibrin network providing structural stability[12,14]. Transglutaminase (Factor XIIIa) stabilizes this fibrillar network.
2.3 Fibrinolysis
Fibrinolysis is controlled by various cofactors, inhibitors, and receptors[15]. The main enzyme which lyses fibrin to fragments known as D-dimers is plasmin activated by plasminogen[16]. Plasminogen is a physiological substrate for two serine proteases, tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). The first one is synthetized and released by endothelial cells; the second one by monocytes, macrophages, and urothelial cells[17]. Both activators have a short half-life period (4 – 8 min) because of high concentrations of inhibitors (e.g. plasminogen activator inhibitor-1 (PAI-1)) in blood plasma. Compared to tPA, uPA has low affinity to plasminogen and does not require fibrin as a cofactor; normally, it functions in extravascular regions[17]. Both tPA and uPA are eliminated by the liver after the formation of a complex with low density lipoprotein (LDL)-receptor-like protein[18]. Moreover, fibrin can be easily lysed by other proteolytic enzymes, for example, proteinase K, collagenase, trypsin, accutase, and metalloproteinases.
2.4 Mechanical properties
Pure fibrinogen solutions show a nonlinear increase in viscosity with increasing concentration, with the values ranging from ones to hundreds of cP[19]. Moreover, the concentration of the fibrinogen in blood plasma correlates with plasma viscosity[20]. The drastic changes in mechanical properties occur with the onset of the fibrin clot formation (gelation), which could be traced by a change of turbidity[21] and an increase in the elastic or shear modulus in rheological measurements[22-24]. In vitro, gelation time which can take from several seconds to several minutes is mostly controlled by the concentration of thrombin and temperature[21,25].
The resulted fibrin gel has a set of remarkable and unique viscoelastic properties among polymers which are related to its molecular structure with complex multi-scale hierarchy[7]. Fibrin fibers might constitute <1% of the gel volume, yet it will have measurable elastic modulus and strength. The gel also has a high water-uptake ratio of 30 – 50[21]. The fibrin fibers of the gel can have different length, thickness, and density and type of branching points, which generally made up of three fibers at a junction[17,23]. These parameters are strongly dependent on the polymerization conditions, including a concentration of fibrinogen, thrombin, additional factors such as Factor XIII and CaCl2, and physical factors such as temperature and external tension or compression forces. Several models for fibrin mechanics have been suggested that take into account its filamentous nature and interactions between the fibers at different hierarchy levels[7,8,17].
The storage modulus of the gel only weakly depends on frequency, while the loss modulus increases with frequency[23]. Thus, at low frequencies (<10 – 100 Hz), the behavior is mostly elastic and could be efficiently characterized by elastic modulus only, but the viscous component is pronounced at high frequencies. The shear and elastic moduli show non-linear behavior with relation to strains, the so-called strain hardening or stiffening[23]. Shear modulus increases up to a factor of twenty-fold at large strains[18]. The elastic modulus initially decreases (up to strain = 0.5), but then dramatically increases by a factor of 100 (compressive strains >0.8)[26]. Strain hardening might be of biological importance since it allows fibrin clots to sustain larger deformations without significant integrity loss.
The main parameter that controls the gel stiffness appears to be fibrinogen concentration. By varying it in a range from 1 to 50 mg/mL, the elastic modulus of the resulted gel from several Pa to several hundred Pa might be achieved[27-29]. Another important modulator is Factor XIII, its addition substantially increases the elastic modulus of the gel by incorporating fibrin covalent crosslinking and compacting fibers[6]. The cell embedded into the gel might also induce its stiffening through myosin-driven cell contraction[30].
The low viscosity of pure fibrinogen solution makes it suitable for inkjet bioprinting methods[31]. However, shape fidelity and mechanical properties of such gels are relatively poor. Due to irreversible and fast fibrin gelation at physiological temperatures, bioprinting with fibrinogen/thrombin mixture might be performed at low temperatures, or thrombin can be added to the construct after bioprinting[32]. The gelated fibrin cannot be printed with standard extrusion-based techniques without damaging its structure. To improve or modify the mechanical properties of the gel construct, the composite bioinks of fibrin with other components were used. Combinations with gelatin[33], alginate[34], collagen[35], hyaluronic acid[36], or more complex formulations[37] were used for different applications. Some biochemical modifications were also introduced to the fibrinogen to modulate the structural and mechanical properties of the gel[27,29,38].
3 Biological properties and their tuning
3.1 Wound healing
The formation of fibrin which is known as fibrinogenesis is associated with hemostasis, one of the main stages in wound healing. By forming an interconnected porous network, fibrin fibers act as a temporary scaffold for migrating and proliferating cells. Fibrin provides an angiogenic environment that enables the growth of capillaries’ sprouts. Together with fibroblasts and macrophages, they form the mature granulation tissue, essential for the following re-epithelialization. Hence, its angiogenic (will be discussed in the next section) and healing potential are physiologically determined, and it is not surprising that fibrin is widely applied in vascular tissue engineering and improvement of wound healing.
Due to its tunable properties that can guide cells and determine substance release kinetics, fibrin is commonly used in skin equivalent design or cell/bioactive substance delivery for the defect site treatment[40]. Even only by adjusting, for example, its mechanical properties, one can tailor its biological properties. For instance, Murphy et al.[41] varied the component concentrations to reveal their correlation with gel stiffness, degradation rate, and vascular endothelial growth factor (VEGF), and prostaglandin (PGE2) secretion by encapsulated mesenchymal stromal cell (MSC) spheroids. They showed that the secretion of both factors was the highest in hydrogels with medium values of compressive and storage moduli.
To improve its innate healing potential, fibrin can be combined with cells, functionalized particles, or bioactive compounds (Table 2). In the first case, various cell types, for example, keratinocytes[42], fibroblasts[43], bone-marrow derived,[44] and adipose-derived[45] MSC, have already been tested. In the second case, for instance, platelet-like particles prepared from functionalized ultralow crosslinked poly (N-isopropylacrylamide-co-acrylic acid) microgels were offered to improve wound healing. In the third case, growth factors are usually applied, which can be physically entrapped within a fibrin mesh or affinely or covalently bonded. For instance, fibrin can be mixed with growth factor-loaded nanoparticles that promoted wound healing. Losi et al.[46] tested poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with VEGF and bFGF and showed that they can significantly promote wound closure and facilitate the re-epithelialization and granulation tissue formation. Growth factors can be linked to fibrin, for example, by transglutaminase-assisted binding of their recombinant modifications that ensures their prolonged release. Mittermayr et al.[47] showed the efficacy of such approach. In their study, they achieved a controllable release of platelet-derived growth factor AB (PDGF.AB) from fibrin that enabled the acceleration and improvement of wound healing in severe burns[47]. Muhamed et al.[48] fabricated fibrin nanoparticles, which were modified with keratinocyte growth factor (KGF) by its coupling using carbodiimide derivative and N-hydroxysulfosuccinimide. These particles had higher healing potential that those from non-loaded ones.
Table 2.
Possible fibrin modifications to tune its biological properties.
| Modifying agent | Type of IM | Type of experiments | Outcomes | Ref. |
|---|---|---|---|---|
| PLGA nanoparticles loaded with VEGF and bFGF | Physical | In vivo (diabetic mice) | Promoted wound closure Accelerated re-epithelialization Increased formation of granulation tissue Enhanced collagen synthesis |
[46] |
| Platelet-like particles | Physical | In vitro | Promoted cell migration | [55] |
| In vivo (mice) | Improved wound healing | |||
| TG-PDGF.AB | Covalent | In vivo (pigs) | Enhanced wound healing | [47] |
| KGF | Covalent | In vitro | Increased cell migration | [48] |
| In vivo (mice) | Improved wound healing | |||
| RGD, IKVAV, YIGSR, and RNIAEIIKDI | Cocrosslinking | In vitro (dorsal root ganglia dissected from chicken embryos) | Enhanced neurite outgrowth | [56] |
| In vivo (rats) | Improved axons regeneration | |||
| Bifunctional carboxylated N-hydroxysulfosuccinimide-active ester PEG | Covalent | In vitro | Maintained cell proliferation Increased ALP activity Up-regulated osteoblast-specific genes | [57] |
| In vivo (nude mice) | Formation of soft vascularized connective tissue | |||
| O,O′-bis[2-(N-succinimidyl-succinylamino)ethyl]PEG | Covalent | In vitro (MSC spheroids) | Promoted better sprouting | [58] |
| TG-VEGF121 | Covalent | In vivo (VEGFR2-luc mice) | Enhanced vessel formation SMC stabilization | [59] |
| Fusion proteins LN-TGF-β1 and LNG-TGF-β1 | Covalent | In vitro | Enhanced contractile function of vascular constructs | [60] |
| T1 peptide sequence from CCN1 | Covalent | In vitro | Improved cellular sprouting without adding VEGF Increased effects when VEGF is added | [61] |
| In vivo (CAM) | Stimulated formation of new vessels | |||
| Anti-VEGF aptamer and anti-PDGF-BB aptamer | Covalent Affinity | In vivo (mice) | Enhanced blood vessel growth | [62] |
| TG-PDGF.AB | Covalent | In vivo (rats) | Decreased flap tissue necrosis Enhanced perfusion Maturation of new vessels | [63] |
| Vector containing the VEGF-A cDNA | Physical | In vivo (rats) | Prolonged flap survival for 7 days after surgery Increased perfusion of tissues Higher VEGF-A expression | [64] |
Abbreviations. Ref: References; ALP: Alkaline phosphatase activity; bFGF: Basic fibroblast growth factor; CAM: Chorioallantoic membrane; IM: Immobilization; KGF: Keratinocyte growth factor; MSC: Mesenchymal stromal cells; PDGF: Platelet-derived growth factor; PEG: Polyethylene glycol; PLGA: Poly(lactic-co-glycolic acid); SMC: Smooth muscle cells; TG: Transglutaminase; TGF-β: Transforming growth factor beta; VEGF: Vascular endothelial growth factor
Table 1.
Mechanical properties of pure fibrinogen and fibrin.
| Components concentrations | Viscosity (cP) | E(Pa) | G’ (Pa) | Comments | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fibrin (mg/ml) | Thrombin (U/ml) | Ca+ (mM) | Factor XIII (µg/ml) | Buffer | |||||
| 10–150 | – | – | – | PBS | 2–43 | n/a | n/a | – | [19] |
| 25 | 100 | – | – | PBS | n/a | 580-640 | – | – | [27] |
| 1, 2, 4, 8 | 0.1–6.4 | – | – | – | n/a | n/a | 3.1-247.5 | – | [28] |
| 6, 7, 8, 9 | – | – | – | – | n/a | n/a | 4-147 | PEGylated fibrinogen, polymerized by photo-initiator using a UV light | [29] |
| 2–50 | 2–100 | 40 | – | – | n/a | 0.058–4000 | n/a | – | [39] |
| 2 | 1 | 2 | 0–20 | HEPES 23 mM NaCl 175 mM pH 7.4 |
n/a | n/a | 33-150 | – | [6] |
Ref.: References; E: Young’s modulus; n/a: Not available; PBS: Phosphate buffer saline; HEPES: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
Except pure fibrin and the mentioned above modifications, there is also platelet-rich fibrin (PRF) that is so-called the “second-generation platelet concentrate”[49]. This review does not go into details regarding this type of fibrin-based products. The reader can learn more about it from the following publications[49-54].
3.2 Angiogenesis
As mentioned above, fibrin has intrinsic angiogenic properties and provides relevant microenvironment determined by structure-depended chemical, physical, and biochemical cues. Its fibrillar structure serves as a scaffold for invading cells which bind to its fibers through cellular receptors and form capillaries. It was showed that the fiber network morphology can be significantly influenced by fibrinogen and thrombin concentrations[65-67], pH[68], buffers[5], incorporation of extra molecules[27], etc.
On invading cells, receptors bind to specific sites on fibrin fibers that not only ensures cell adhesion but also triggers various intracellular pathways due to their biochemical interaction and formed tensional forces. Such cues determine position-assisted cell response to the external stimulation by cytokines and growth factors. The cell adhesion to fibrin is mainly ensured by two arginylglycylaspartic acid (RGD) sites located on the α-chain through integrins (αvβ3, α5β1, etc.). Integrin αvβ3 and integrin α5β1 were proven to control vacuolation and lumen formation by endothelial cells[69]. Interestingly, the insertion of additional selective binding sites for αvβ3 integrin (the sixth immunoglobulin-like (Ig-like) domain of the cell adhesion molecule L1 (L1Ig6)) provided the increase in vessel formation by them[68]. Except RGD sites, endothelial cells interact with β15–42 sequence of fibrin where VE-cadherin serves as a specific cell receptor[70]. The adhesion of MSC used to stabilize the newly forming vessels and induce their formation is ensured through the interaction with another type of integrins – α6β1 – to fibrin fibers[71].
However, in angiogenesis, fibrin is not a stable scaffold for migrating cells; it is a highly responsive system that remodels providing the required environment for forming vessels. Its degradation and remodeling are critical in the new vessel formation and mainly orchestrated by matrix metalloproteinases (MMP), including membrane-type MMP (MT-MMP). When cells migrate within a fibrin network, they degrade it facilitating their invasion and making space for lumenogenesis that causes the heterogeneity in local ECM stiffness and changes in its bulk structure[72]. Among the MMP variety, the MMP2, MMP9, and MT1-MMP are considered to play the most important role. In endothelial cells, VEGF, well-known angiogenic factor, is proven to induce the MMP9 and MT1-MMP expression through Notch signaling that regulates cell morphogenesis[73]. It was observed that initial stages of capillarogenesis by endothelial cells corresponds with the rise of proenzyme proMMP-2 and drop of proMMP-9; however, MMP-2 was not revealed and MMP-9 was low[74]. MT1-MMP was proven to regulate vessel formation by both EC and MSC and more strongly affected it than MMP-2 and MMP-9[75,76]. Interestingly, compared to fibroblast-assisted one, MSC-induced vessel formation is totally controlled by MT-MMP[77]. Hence, by tuning fibrin gel properties through its modification that changes its mechanical properties and degradability the tissue engineers can significantly influence angiogenesis in vitro[78]. Moreover, fibrin ensures the synthesis of extracellular matrix (ECM) proteins such as laminin, and collagen type IV[66,79,80] that stabilizes the formed microvasculature.
Angiogenesis can be also promoted by fibrin degradation products. It was showed that fibrin fragment E undergone thrombin-assisted proteolytic cleavage led to the increase in the endothelial cell proliferation, migration an differentiation in vitro[81] and in the vessel number while applied on the chorioallantoic membrane (CAM) model[82].
To increase its angiogenic properties, several structural modifications which can be divided into two main groups: Inert or active substance loading/binding were offered. For instance, it was showed that the PEGylation of fibrin can ensure the enhanced endothelial and mesenchymal stromal cells’ migration and spreading followed with the formation of cell extensions and intercellular junctions and expression of specific MMP[66,79]. Moreover, compared to the native fibrin gel, the PEGylated fibrin promoted the increased growth rate, branching, and length of tubules formed by encapsulated spheroids from adipose-derived MSC[58] (Figure 1). However, the most trivial approach to improve fibrin angiogenic properties is to use single or multiple pro-angiogenic factors such as VEGF or bFGF. They can be entrapped within a fiber network or immobilized using release systems (e.g. nanoparticles) or through affinity or covalent binding (Table 2). Moreover, the binding of some peptides such as RGD, LN-TGF-β1, or T1 peptide sequence from CCN1 can significantly promote vessel formation. The efficiency of plasmids delivered by fibrin to improve angiogenesis is questionable and requires further investigation. On the one hand, Michlits et al.[64] showed that VEGF plasmid-laden fibrin gel increased skin flap survival. On the other hand, Jozkowicz et al.[83] revealed that all carrier types used for the VEGF plasmid delivery (water, phosphate buffer saline, and fibrin glue) stimulated similar effects on capillaries growth.
Figure 1.
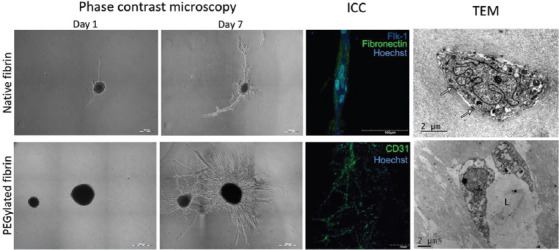
Tubulogenesis within native and PEGylated fibrin gels ICC – immunocytochemical staining; L – lumen; TEM – transmission electron microscopy. Copyright permission provided by IOP Publishing[58].
3.3 Application
Fibrin is a commonly used biomaterial and widely applied in medicine from the 70 to 80s as a surgical sealant (fibrin glue). Due to its flexible properties, fibrin has become a versatile tool in engineering of various tissues, for example, skin, blood vessels, and bone.[84-86] Fibrin and its blends with other biomaterials, such as collagen, alginate, and hyaluronic acid, are applied in both scaffold and scaffold-free technologies.
Since the advent of bioprinting and its influence started to take hold of the field of tissue engineering, fibrin (fibrinogen) has become a biomaterial of choice due to its good biocompatibility, biodegradability, and other described biological properties in Table 3. The experience gained in cell encapsulation was transferred and adapted to bioprinting.
Table 3.
Bioprinting with fibrin-based bioinks.
| Components | Bioprinting | Origin | Cells | Outcomes | Ref | ||
|---|---|---|---|---|---|---|---|
| Fibrinogen | Cross-linking agents | Additives | |||||
| 60 mg/ml | Thrombin 50 U/ml CaCl2 80 mM | Furfuril-gelatin 155 mg/ml RB | Extrusion-based | n/a | iPSC-derived cardiomyocytes | Printed structures were porous and showed good stability Cells remained viable, proliferated, and expressed cardiac marker (troponin I) | [89] |
| 30 mg/ml | Thrombin n/a | Gelatin 35 mg/ml Hyaluronic acid 3 mg/ml | Extrusion-based | n/a | bladder urothelial cells, bladder smooth muscle cells | Cells maintained high viability for a week after printing, actively proliferated and expressed specific biomarkers | [94] |
| 50 mg/ml | NaCl 150 mM CaCl2 5 mM PVA 1.4%w/v Thrombin 50 U/ml | Hyaluronic acid 4 mg/ml Factor XIII 1 U/ml Aprotinin 0.5 mg/ml | Extrusion-based | Bovine | primary Schwann cells | Cells were viable and proliferated Directed cell alignment was observed | [36] |
| 10 % w/v | Thrombin 100 U | Gelatin 5 % w/v | Extrusion-based | Bovine | HUVECs, MSCs | EC-MSC distance defining cell-cell communication regulates angiogenesis | [93] |
| 20 mg/ml | Thrombin 50 U/ml | Gelatin 30 mg/ml Aprotinin 20 µg/ml Hyaluronic acid 3 mg/ml Glycerol 10% | Extrusion-based | n/a | neonatal rat ventricular cardiomyocytes | Bioprinted constructs contracted, were formed by aligned and electromechanically coupled cells and responsive to drugs | [90] |
| 20 mg/ml | Thrombin 2000 U/ml | Gelatin 7.5 % w/v | Extrusion-based, rotary | Bovine | primary neonatal human dermal fibroblast | Heat-treated gelatin increased fibrinogen-based bioink printability Tensile mechanical properties of printed constructs induced the rise in circumferential and axial elastic moduli | [95] |
| 20 mg/ml | Thrombin 4 U/ml | Hyaluronic acid 1% w/v | Laser-assisted | Human | iPSCs | Cells were sensitive to biomaterials used as a bioink base (not printing) Hyaluronic-based blends ensured better cell survivability without pluripotency loss | [96] |
| 20 mg/ml | Thrombin 40 U/ml CaCl2 50 mM | Hyaluronic acid 1% w/v | Laser-assisted | Human | ASCs, ECFCs | Cell-cell contacts regulate the formation of vessel networks | [97] |
Ref: References; n/a: Not available; RB: Rose bengal; PVA: Polyvinyl alcohol; iPSC: Induced pluripotent cells; HUVEC: Human umbilical vein endothelial cells; MSC: Mesenchymal stromal cells; ASC: Adipose-derived stromal cells; ECFC: Endothelial colony-forming cells
Fibrin-based bioinks were successfully applied to print skin, heart, and neural constructs. Particularly, Cubo et al.[87] fabricated a bioprinted skin substitute from plasma-derived fibrin and primary fibroblasts and keratinocytes that were tested in vivo and showed to be similar to the native skin. Fibrin-collagen bioinks provided favorable environment for cells bioprinted using a mobile skin bioprinting system.[88] Hence, the in situ fabricated constructs accelerated wound closure and re-epithelialization. Moreover, Kumar et al.[89] revealed that cardiomyocytes printed using a fibrin-based bioink not only were viable and proliferating but also expressed a specific cardiac marker and coupled with cardiac fibroblasts. Wang et al.[90] also successfully applied a fibrin-based bioink to fabricate functional cardiac tissue constructs that contracted synchronously and responded to epinephrine and carbachol. Being the same complex as cardiac one, neural tissue was achieved using fibrin which structure ensured cell alignment and guided Schwann cells’ growth.
Bioprinting tumors is a novel interesting direction in not only fibrin application but also in bioprinting in general.[91] The fabricated tumor models are positioned mostly to be used as a more relevant platform for drug screening and personalized patient’s therapy. For instance, Lee et al.[92] printed an in vitro glioblastoma model using a fibrin-based bioink. Cells remained viable for more than 1 week after printing and formed spheres expressing cancer stem cells and metastatic invasiveness markers. Moreover, the printed constructs treated with a novel method were shown to be more in vivo relevant than a monolayer culture. Using a fibrin blend, Zhao et al.[32] described a method to print a 3D cervical tumor model that also was more resistant to chemotherapy than two-dimensional culture.
Despite the biofabrication of tissues and organs, fibrin-based bioinks are demanded to study cell-cell interactions, mainly for deeper understanding of cell biology features related to vascularization, innervation, etc. For instance, by regulating the distance between ECs and MSCs with bioprinting, Piard et al.[93] showed that angiogenesis can be significantly modulated: When endothelial cells were placed closer to stromal ones (≤200 μm), the better angiogenesis promotion was observed.
3.4 Trends
As it is clear from above, the use of fibrin as a bioink base is only growing that corresponds with an increasing trend to bioprinting in general. Undoubtedly, the development of bioprinting is strongly connected with the development of new bioinks, particularly fibrin-based ones; and widening its applications will the applications of bioinks. Therefore, fibrin as one of biomaterials of choice will be used to print not only tissues and organs but also tumor models, organ-on-a-chip, etc.
To improve fibrin (fibrinogen) mechanical properties, shape fidelity, etc., for bioprinting, the main strategy is blending with other biomaterials such as gelatin, collagen, and alginate. Despite its simplicity, it will be further used because it does not need strong structural changes in protein molecule requiring deep knowledge of biomaterial chemistry and a bioink can be easier roughly tuned to a particular protocol.
However, blending consumes too much time and labor when fine tuning both mechanical and biological properties is required. Therefore, the number of modifications has been already offered that were described above. Moreover, such biomaterials give a birth to a new class of bioink – “smart bioinks.” These bioinks with finely tuned mechanical and biological properties provide not only a favorable microenvironment supporting cell survivability, proliferation, and differentiation but also the information on cell functioning, for example, oxygen consumption, and changes in pH level. This approach was previously realized using scaffolds, but no study regarding bioinks, particularly fibrin-based bioinks, has been performed. For instance, O’Donnell fabricated pH-sensitive cellulose-based scaffolds labeled through cellulose-binding domain with enhanced cyan fluorescent protein[98]. Such scaffolds ensured the analysis of extracellular acidification combined with probe-based monitoring of cell oxygenation. Moreover, being “smart,” such bioinks may adapt to meet cell requirements that include not only matrix re-modeling but also bioactive substance release. Hence, researchers will have a unique in vitro platform for organ and tissue fabrication.
Compared to the majority of biomaterials, fibrin can be autologously derived that is a significant advantage for further clinical translation of the bioprinted constructs. However, the fibrinogen concentration in blood is relatively low in comparison with the used one for bioink preparation (2 mg/ml[99] vs. 20 mg/ml[95]). Therefore, in recent papers[87,100], such bioinks were prepared not from pure fibrinogen, but blood plasma.
4 Conclusions
The development of bioprinting has inspired new applications of fibrin as a bioink. Compared to other biomaterials, fibrin can be autologously derived that facilitates its clinical translation and has significant intrinsic properties such as induction of wound healing and angiogenesis that are highly valuable in tissue engineering. It also provides a possibility for fine tuning both mechanical and biological properties. Fibrin and its blends can be pioneering in the development of smart bionks that provide not only an adaptable cell-friendly microenvironment but also the information on cell functioning.
Authors’ contributions
AS, VM, and PS outlined the manuscript. DO contributed to “Fibrin overview,” YE – “Mechanical properties,” PB, EB, and NK – “Wound healing,” AS and ASo - “Trends”, and RS, ML, and MV – “Angiogenesis.” AS drafted the manuscript with primary editing and revision support from PS, RS, and EB. PS and VM coordinated the manuscript preparation. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by the Russian Science Foundation (18-15-00407, general information, applications, biological properties and their tuning) and Russian academic excellence project 5–100 (trends).
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Antoshin AA, Churbanov SN, Minaev NV, et al. LIFT-bioprinting, is it Worth it? Bioprinting. 2019;15:e00052. DOI:10.1016/j.bprint.2019 e00052. [Google Scholar]
- 2.Jiang T, Munguia-Lopez JG, Flores-Torres S, et al. Extrusion Bioprinting of Soft Materials:An Emerging Technique for Biological Model Fabrication. Appl Phys Rev. 2019;6:011310. DOI:10.1063/1.5059393. [Google Scholar]
- 3.Gudapati H, Dey M, Ozbolat I. A Comprehensive Review on Droplet-based Bioprinting:Past, Present and Future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. DOI:10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Unagolla JM, Jayasuriya AC. Hydrogel-based 3D Bioprinting:A Comprehensive Review on Cell-laden Hydrogels, Bioink Formulations, and Future Perspectives. Appl Mater Today. 2020;18:100479. doi: 10.1016/j.apmt.2019.100479. DOI:10.1016/j.apmt.2019.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurniawan NA, Van Kempen TH, Sonneveld S, et al. Buffers Strongly Modulate Fibrin Self-Assembly into Fibrous Networks. Langmuir. 2017;33:6342–52. doi: 10.1021/acs.langmuir.7b00527. DOI:10.1021/acs.langmuir.7b00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurniawan NA, Grimbergen J, Koopman J, et al. Factor XIII Stiffens Fibrin Clots by Causing Fiber Compaction. J Thromb Haemost. 2014;12:1687–96. doi: 10.1111/jth.12705. DOI:10.1111/jth.12705. [DOI] [PubMed] [Google Scholar]
- 7.Weisel JW, Litvinov RI. Fibrin Formation, Structure and Properties. Subcell Biochem. 2017;82:405–56. doi: 10.1007/978-3-319-49674-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E, Kim OV, Machlus KR, et al. Correlation between Fibrin Network Structure and Mechanical Properties:An Experimental and Computational Analysis. Soft Matter. 2011;7:4983–92. DOI:10.1039/c0sm01528h. [Google Scholar]
- 9.Brown AE, Litvinov RI, Discher DE, et al. Multiscale Mechanics of Fibrin Polymer:Gel Stretching with Protein Unfolding and Loss of Water. Science. 2009;325:741–4. doi: 10.1126/science.1172484. DOI:10.4016/12254.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosesson MW. Fibrinogen and Fibrin Structure and Functions. J Thromb Haemost. 2005;3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 11.Fuss C, Palmaz JC, Sprague EA. Fibrinogen:Structure, Function, and Surface Interactions. J Vasc Interv Radiol. 2001;12:677–82. doi: 10.1016/s1051-0443(07)61437-7. [DOI] [PubMed] [Google Scholar]
- 12.Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler Thromb Vasc Biol. 2017;37:e13–e21. doi: 10.1161/ATVBAHA.117.308564. DOI:10.1161/atvbaha.117.308564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fish RJ, Neerman-Arbez M. Fibrinogen Gene Regulation. Thromb Haemost. 2012;108:419–26. doi: 10.1160/TH12-04-0273. DOI:10.1160/th12-04-0273. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Mochalkin I, Doolittle RF. A Model of Fibrin Formation Based on Crystal Structures of Fibrinogen and Fibrin Fragments Complexed with Synthetic Peptides. Proc Natl Acad Sci U S A. 2000;97:14156–61. doi: 10.1073/pnas.97.26.14156. DOI:10.1073/pnas.97.26.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapin JC, Hajjar KA. Fibrinolysis and the Control of Blood Coagulation. Blood Rev. 2015;29:17–24. doi: 10.1016/j.blre.2014.09.003. DOI:10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesarman-Maus G, Hajjar KA. Molecular Mechanisms of Fibrinolysis. Br J Haematol. 2005;129:307–21. doi: 10.1111/j.1365-2141.2005.05444.x. DOI:10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 17.Litvinov RI, Weisel JW. Fibrin Mechanical Properties and their Structural Origins. Matrix Biol. 2017;60–61:110–23. doi: 10.1016/j.matbio.2016.08.003. DOI:10.1016/j.matbio.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janmey PA, Amis EJ, Ferry JD. Rheology of Fibrin Clots. VI Stress Relaxation, Creep, and Differential Dynamic Modulus of Fine Clots in Large Shearing Deformations. J Rheol. 1983;27:135–53. DOI:10.1122/1.549722. [Google Scholar]
- 19.Martens TP, Godier AF, Parks JJ, et al. Percutaneous Cell Delivery into the Heart Using Hydrogels Polymerizing In Situ. Cell Transplant. 2009;18:297–304. doi: 10.3727/096368909788534915. DOI:10.3727/096368909788534915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metry G, Adhikarla R, Schneditz D, et al. Effect of Changes in the Intravascular Volume during Hemodialysis on Blood Viscoelasticity. Indian J Nephrol. 2011;21:95. doi: 10.4103/0971-4065.82139. DOI:10.4103/0971-4065.82139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Ma L, Zhou J, et al. Fabrication and Physical and Biological Properties of Fibrin gel Derived from Human Plasma. Biomed Mater. 2008;3:1–10. doi: 10.1088/1748-6041/3/1/015001. DOI:10.1088/1748-6041/3/1/015001. [DOI] [PubMed] [Google Scholar]
- 22.Roberts WW, Lorand L, Mockros LF. Viscoelastic Properties of Fibrin Clots. Biorheology. 1973;10:29–42. doi: 10.3233/bir-1973-10105. DOI:10.3233/bir-1973-10105. [DOI] [PubMed] [Google Scholar]
- 23.Weisel JW. The Mechanical Properties of Fibrin for Basic Scientists and Clinicians. Biophys Chem. 2004;112:267–76. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Carr ME, Shen LL, Hermans J. A Physical Standard of Fibrinogen:Measurement of the Elastic Modulus of Dilute Fibrin Gels with a New Elastometer. Anal Biochem. 1976;72:202–11. doi: 10.1016/0003-2697(76)90522-4. DOI:10.1016/0003-2697(76)90522-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaibara M. Dynamic Viscoelastic Study of the Formation of Fibrin Networks in Fibrinogen-Thrombin Systems and Plasma. Biorheology. 1973;10:61–73. doi: 10.3233/bir-1973-10109. DOI:10.3233/bir-1973-10109. [DOI] [PubMed] [Google Scholar]
- 26.Kim OV, Litvinov RI, Weisel JW, et al. Structural Basis for the Nonlinear Mechanics of Fibrin Networks under Compression. Biomaterials. 2014;35:6739–49. doi: 10.1016/j.biomaterials.2014.04.056. DOI:10.1016/j.biomaterials.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shpichka AI, Konarev PV, Efremov YM, et al. Digging Deeper:Structural Background of PEGylated Fibrin Gels in Cell Migration and Lumenogenesis. RSC Adv. 2020;10:4190–200. doi: 10.1039/c9ra08169k. DOI:10.1039/c9ra08169k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaramillo M, Singh SS, Velankar S, et al. Inducing Endoderm Differentiation by Modulating Mechanical Properties of Soft Substrates. J Tissue Eng Regen Med. 2015;9:1–12. doi: 10.1002/term.1602. DOI:10.1002/term.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapira-Schweitzer K, Seliktar D. Matrix Stiffness Affects Spontaneous Contraction of Cardiomyocytes Cultured within a PEGylated Fibrinogen Biomaterial. Acta Biomater. 2007;3:33–41. doi: 10.1016/j.actbio.2006.09.003. DOI:10.1016/j.actbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Jansen KA, Bacabac RG, Piechocka IK, et al. Cells Actively Stiffen Fibrin Networks by Generating Contractile Stress. Biophys J. 2013;105:2240–51. doi: 10.1016/j.bpj.2013.10.008. DOI:10.1016/j.bpj.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panwar A, Tan LP. Current Status of Bioinks for Micro-extrusion-based 3D Bioprinting. Molecules. 2016;21:685. doi: 10.3390/molecules21060685. DOI:10.3390/molecules21060685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Yao R, Ouyang L, et al. Three-Dimensional Printing of Hela Cells for Cervical Tumor Model In Vitro. Biofabrication. 2014;6:035001. doi: 10.1088/1758-5082/6/3/035001. DOI:10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Wang X, Yan Y, et al. Rapid Prototyping Three-Dimensional Cell/Gelatin/Fibrinogen Constructs for Medical Regeneration. J Bioact Compat Polym. 2007;22:363–77. [Google Scholar]
- 34.Shikanov A, Xu M, Woodruff TK, et al. Interpenetrating Fibrin Alginate Matrices for in Vitro Ovarian Follicle Development. Biomaterials. 2009;30:5476–85. doi: 10.1016/j.biomaterials.2009.06.054. DOI:10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai VK, Lake SP, Frey CR, et al. Mechanical Behavior of Collagen-Fibrin Co-Gels Reflects Transition From Series to Parallel Interactions With Increasing Collagen Content. J Biomech Eng. 2012;134:011004. doi: 10.1115/1.4005544. DOI:10.1115/1.4005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.England S, Rajaram A, Schreyer DJ, et al. Bioprinted Fibrin-factor XIII-Hyaluronate Hydrogel Scaffolds with Encapsulated Schwann Cells and their In Vitro Characterization for use in Nerve Regeneration. Bioprinting. 2017;5:1–9. DOI:10.1016/j.bprint.2016.12.001. [Google Scholar]
- 37.Han J, Kim DS, Jang H, et al. Bioprinting of Three-dimensional Dentin Pulp Complex with Local Differentiation of Human Dental Pulp Stem Cells. J Tissue Eng. 2019;10:2041731419845849. doi: 10.1177/2041731419845849. DOI:10.1177/2041731419845849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigandt KM, White N, Chung D, et al. Fibrin Clot Structure and Mechanics Associated with Specific Oxidation of Methionine Residues in Fibrinogen. Biophys J. 2012;103:2399–407. doi: 10.1016/j.bpj.2012.10.036. DOI:10.1016/j.bpj.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duong H, Wu B, Tawil B. Modulation of 3D Fibrin Matrix Stiffness by Intrinsic Fibrinogen-thrombin Compositions and by Extrinsic Cellular Activity. Tissue Eng Part A. 2009;15:1865–76. doi: 10.1089/ten.tea.2008.0319. DOI:10.1089/ten.tea.2008.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shpichka A, Butnaru D, Bezrukov EA, et al. Skin Tissue Regeneration for Burn Injury. Stem Cell Res Ther. 2019;10:1–16. doi: 10.1186/s13287-019-1203-3. DOI:10.1186/s13287-019-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy KC, Whitehead J, Zhou D, et al. Engineering Fibrin Hydrogels to Promote the Wound Healing Potential of Mesenchymal Stem Cell Spheroids. Acta Biomater. 2017;64:176–86. doi: 10.1016/j.actbio.2017.10.007. DOI:10.1016/j.actbio.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krasna M, Planinsek F, Knezevic M, et al. Evaluation of a Fibrin-based Skin Substitute Prepared in a Defined Keratinocyte Medium. Int J Pharm. 2005;291:31–7. doi: 10.1016/j.ijpharm.2004.07.040. DOI:10.1016/j.ijpharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 43.Idrus RB, Rameli MA, Low KC, et al. Full-thickness Skin wound Healing Using Autologous Keratinocytes and Dermal Fibroblasts with Fibrin:Bilayered Versus Single-Layered Substitute. Adv Ski Wound Care. 2014;27:171–80. doi: 10.1097/01.ASW.0000445199.26874.9d. DOI:10.1097/01.asw.0000445199.26874.9d. [DOI] [PubMed] [Google Scholar]
- 44.Falanga V, Iwamoto S, Chartier M, et al. Autologous Bone Marrow-derived Cultured Mesenchymal Stem Cells Delivered in a Fibrin Spray Accelerate Healing in Murine and Human Cutaneous Wounds. Tissue Eng. 2007;13:1299–312. doi: 10.1089/ten.2006.0278. DOI:10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 45.Mendez JJ, Ghaedi M, Sivarapatna A, et al. Mesenchymal Stromal Cells form Vascular Tubes when Placed in Fibrin Sealant and Accelerate wound Healing In Vivo. Biomaterials. 2015;40:61–71. doi: 10.1016/j.biomaterials.2014.11.011. DOI:10.1016/j.biomaterials.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Losi P, Briganti E, Errico C, et al. Fibrin-based Scaffold Incorporating VEGF and bFGF-Loaded Nanoparticles Stimulates wound Healing in Diabetic Mice. Acta Biomater. 2013;9:7814–21. doi: 10.1016/j.actbio.2013.04.019. DOI:10.1016/j.actbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Mittermayr R, Branski L, Moritz M, et al. Fibrin Biomatrix-conjugated Platelet-derived Growth Factor AB Accelerates wound Healing in Severe Thermal Injury. J Tissue Eng Regen Med. 2016;10:E275–85. doi: 10.1002/term.1749. DOI:10.1002/term.1749. [DOI] [PubMed] [Google Scholar]
- 48.Muhamed I, Sproul EP, Ligler FS, et al. Fibrin Nanoparticles Coupled with Keratinocyte Growth Factor Enhance the Dermal Wound-Healing Rate. ACS Appl Mater Interfaces. 2019;11:3771–80. doi: 10.1021/acsami.8b21056. DOI:10.1021/acsami.8b21056. [DOI] [PubMed] [Google Scholar]
- 49.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich Fibrin (PRF):A Second-generation Platelet Concentrate. Part I:Technological Concepts and Evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:37–44. doi: 10.1016/j.tripleo.2005.07.008. DOI:10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich Fibrin (PRF):A Second-generation Platelet Concentrate Part IV:Clinical Effects on Tissue Healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006:101, 56–60. doi: 10.1016/j.tripleo.2005.07.011. DOI:10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich Fibrin (PRF):A Second-generation Platelet Concentrate Part II:Platelet-related Biologic Features. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006;101:45–50. doi: 10.1016/j.tripleo.2005.07.009. DOI:10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Dohan DM, Choukroun J, Diss A, et al. Platelet-rich Fibrin (PRF):A Second-generation Platelet Concentrate Part III:Leucocyte Activation:A New Feature for Platelet Concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2006;101:51–5. doi: 10.1016/j.tripleo.2005.07.010. DOI:10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Miron RJ, Fujioka-Kobayashi M, Bishara M, et al. Platelet-Rich Fibrin and Soft Tissue Wound Healing:A Systematic Review. Tissue Eng Part B Rev. 2017;23:83–99. doi: 10.1089/ten.TEB.2016.0233. DOI:10.1089/ten.teb.2016.0233. [DOI] [PubMed] [Google Scholar]
- 54.Anitua E, Nurden P, Prado R, et al. Autologous Fibrin Scaffolds:When Platelet and Plasma-derived Biomolecules Meet Fibrin. Biomaterials. 2019;192:440–60. doi: 10.1016/j.biomaterials.2018.11.029. DOI:10.1016/j.biomaterials.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 55.Nandi S, Sproul EP, Nellenbach K, et al. Platelet-like Particles Dynamically Stiffen Fibrin Matrices and Improve Wound Healing Outcomes. Biomater Sci. 2019;7:669–82. doi: 10.1039/c8bm01201f. DOI:10.1039/c8bm01201f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schense JC, Bloch J, Aebischer P, et al. Enzymatic Incorporation of Bioactive Peptides into Fibrin Matrices Enhances Neurite Extension. Nat Biotechnol. 2000;18:415–9. doi: 10.1038/74473. DOI:10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 57.Galler KM, Cavender AC, Koeklue U, et al. Bioengineering of Dental Stem Cells in a PEGylated Fibrin Gel. Regen Med. 2011;6:191–200. doi: 10.2217/rme.11.3. DOI:10.2217/rme.11.3. [DOI] [PubMed] [Google Scholar]
- 58.Gorkun AA, Shpichka AI, Zurina IM, et al. Angiogenic Potential of Spheroids from Umbilical Cord and Adipose-derived Multipotent Mesenchymal Stromal Cells within Fibrin Gel. Biomed Mater. 2018;13(4):44108. doi: 10.1088/1748-605X/aac22d. DOI:10.1088/1748-605x/aac22d. [DOI] [PubMed] [Google Scholar]
- 59.Ehrbar M, Zeisberger SM, Raeber GP, et al. The Role of Actively Released Fibrin-Conjugated VEGF for VEGF Receptor 2 Gene Activation and the Enhancement of Angiogenesis. Biomaterials. 2008;29:1720–9. doi: 10.1016/j.biomaterials.2007.12.002. DOI:10.1016/j.biomaterials.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Liang MS, Andreadis ST. Engineering Fibrin-binding TGF-β1 for Sustained Signaling and Contractile Function of MSC Based Vascular Constructs. Biomaterials. 2011;32:8684–93. doi: 10.1016/j.biomaterials.2011.07.079. DOI:10.1016/j.biomaterials.2011.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loureiro J, Torres AL, Neto T, et al. Conjugation of the T1 Sequence from CCN1 to Fibrin Hydrogels for Therapeutic Vascularization. Mater Sci Eng C. 2019;104:109847. doi: 10.1016/j.msec.2019.109847. DOI:10.1016/j.msec.2019.110514. [DOI] [PubMed] [Google Scholar]
- 62.Zhao N, Suzuki A, Zhang X, et al. Dual Aptamer-Functionalized In Situ Injectable Fibrin Hydrogel for Promotion of Angiogenesis via Codelivery of Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor-BB. ACS Appl Mater Interfaces. 2019;11:18123–32. doi: 10.1021/acsami.9b02462. DOI:10.1021/acsami.9b02462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mittermayr R, Slezak P, Haffner N, et al. Controlled Release of Fibrin Matrix-Conjugated Platelet Derived Growth Factor Improves Ischemic Tissue Regeneration by Functional Angiogenesis. Acta Biomater. 2016;29:11–20. doi: 10.1016/j.actbio.2015.10.028. DOI:10.1016/j.actbio.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Michlits W, Mittermayr R, Schäfer R, et al. Fibrin-embedded Administration of VEGF Plasmid Enhances Skin Flap Survival. Wound Repair Regen. 2007;15:360–7. doi: 10.1111/j.1524-475X.2007.00238.x. DOI:10.1111/j.1524-475x.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 65.Mooney R, Tawil B, Mahoney M. Specific Fibrinogen and Thrombin Concentrations Promote Neuronal Rather than Glial Growth when Primary Neural Cells are Seeded within Plasma-derived Fibrin Gels. Tissue Eng Part A. 2010;16:1607–19. doi: 10.1089/ten.TEA.2009.0372. DOI:10.1089/ten.tea.2009.0372. [DOI] [PubMed] [Google Scholar]
- 66.Shpichka AI, Koroleva AV, Deiwick A, et al. Evaluation of the Vasculogenic Potential of Hydrogels Based on Modified Fibrin. Cell Tissue Biol. 2017;11:81–7. DOI:10.1134/s1990519x17010126. [Google Scholar]
- 67.Shpichka AI, Revkova VA, Aksenova NA, et al. Transparent PEG-fibrin Gel as a Flexible Tool for Cell Encapsulation. Sovrem Tehnol Med. 2018;10:64–9. DOI:10.17691/stm2018.10.1.08. [Google Scholar]
- 68.Hall H, Baechi T, Hubbell JA. Molecular Properties of Fibrin-based Matrices for Promotion of Angiogenesis In Vitro. Microvasc Res. 2001;62:315–26. doi: 10.1006/mvre.2001.2348. DOI:10.1006/mvre.2001.2348. [DOI] [PubMed] [Google Scholar]
- 69.Bayless KJ, Salazar R, Davis GE. RGD-dependent Vacuolation and Lumen Formation Observed During Endothelial Cell Morphogenesis in Three-dimensional Fibrin Matrices Involves the Alpha(v) Beta(3) and Alpha(5)beta(1) Integrins. Am J Pathol. 2000;156:1673–83. doi: 10.1016/s0002-9440(10)65038-9. DOI:10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bach TL, Barsigian C, Yaen CH, et al. Endothelial Cell VE-cadherin Functions as a Receptor for the β15-42 Sequence of Fibrin. J Biol Chem. 1998;273:30719–28. doi: 10.1074/jbc.273.46.30719. DOI:10.1074/jbc.273.46.30719. [DOI] [PubMed] [Google Scholar]
- 71.Carrion B, Kong YP, Kaigler D, et al. Bone Marrow-derived Mesenchymal Stem Cells Enhance Angiogenesis via their α6β1 Integrin Receptor. Exp Cell Res. 2013;319:2964–76. doi: 10.1016/j.yexcr.2013.09.007. DOI:10.1016/j.yexcr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Juliar BA, Keating MT, Kong YP, et al. Sprouting Angiogenesis Induces Significant Mechanical Heterogeneities and ECM Stiffening Across Length Scales in Fibrin Hydrogels. Biomaterials. 2018;162:99–108. doi: 10.1016/j.biomaterials.2018.02.012. DOI:10.1016/j.biomaterials.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Funahashi Y, Shawber CJ, Sharma A, et al. Notch Modulates VEGF Action in Endothelial Cells by Inducing Matrix Metalloprotease Activity. Vasc Cell. 2011;3:2. doi: 10.1186/2045-824X-3-2. DOI:10.1186/2045-824x-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thi MU, Trocmé C, Montmasson MP, et al. Investigating Metalloproteinases MMP-2 and MMP-9 Mechanosensitivity to Feedback Loops Involved in the Regulation of In Vitro Angiogenesis by Endogenous Mechanical Stresses. Acta Biotheor. 2012;60:21–40. doi: 10.1007/s10441-012-9147-3. DOI:10.1007/s10441-012-9147-3. [DOI] [PubMed] [Google Scholar]
- 75.Lafleur MA, Handsley MM, Knäuper V, et al. EC tubulogenisis in fibrin requires MT-MMPs. J Cell Sci. 2002;115:3427–38. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- 76.Kachgal S, Carrion B, Janson IA, et al. Bone Marrow Stromal Cells Stimulate an Angiogenic Program that Requires Endothelial MT1-MMP. J Cell Physiol. 2012;227:3546–55. doi: 10.1002/jcp.24056. DOI:10.1002/jcp.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghajar CM, Kachgal S, Kniazeva E, et al. Mesenchymal Cells Stimulate Capillary Morphogenesis via Distinct Proteolytic Mechanisms. Exp Cell Res. 2010;316:813–25. doi: 10.1016/j.yexcr.2010.01.013. DOI:10.1016/j.yexcr.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urech L, Bittermann AG, Hubbell JA, et al. Mechanical Properties, Proteolytic Degradability and Biological Modifications Affect Angiogenic Process Extension Into Native and Modified Fibrin Matrices In Vitro. Biomaterials. 2005;26:1369–79. doi: 10.1016/j.biomaterials.2004.04.045. DOI:10.1016/j.biomaterials.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 79.Koroleva A, Deiwick A, Nguyen A, et al. Hydrogel-based Microfluidics for Vascular Tissue Engineering. BioNanoMaterials. 2016;17:19–32. DOI:10.1515/bnm-2015-0026. [Google Scholar]
- 80.Morin KT, Smith AO, Davis GE, et al. Aligned Human Microvessels Formed in 3D Fibrin Gel by Constraint of Gel Contraction. Microvasc Res. 2013;90:12–22. doi: 10.1016/j.mvr.2013.07.010. DOI:10.1016/j.mvr.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bootle-Wilbraham CA, Tazzyman S, Thompson WD, et al. Fibrin Fragment E Stimulates the Proliferation, Migration and Differentiation of Human Microvascular Endothelial Cells In Vitro. Angiogenesis. 2001;4:269–75. doi: 10.1023/a:1016076121918. DOI:10.1023/a:1016076121918. [DOI] [PubMed] [Google Scholar]
- 82.Thompson WD, Smith EB, Stirk CM, et al. Angiogenic Activity of Fibrin Degradation Products is Located in Fibrin Fragment E. J Pathol. 1992;168:47–53. doi: 10.1002/path.1711680109. DOI:10.1002/path.1711680109. [DOI] [PubMed] [Google Scholar]
- 83.Jozkowicz A, Fügl A, Nanobashvili J, et al. Delivery of High dose VEGF Plasmid Using Fibrin Carrier does Not Influence its Angiogenic Potency. Int J Artif Organs. 2003;26(2):161–9. doi: 10.1177/039139880302600211. DOI:10.1177/039139880302600211. [DOI] [PubMed] [Google Scholar]
- 84.Noori A, Ashrafi SJ, Vaez-Ghaemi R, et al. A Review of Fibrin and Fibrin Composites for Bone Tissue Engineering. Int J Nanomed. 2017;12:4937–61. doi: 10.2147/IJN.S124671. DOI:10.2147/ijn.s124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blache U, Ehrbar M. Inspired by Nature:Hydrogels as Versatile Tools for Vascular Engineering. Adv Wound Care. 2018;7:232–46. doi: 10.1089/wound.2017.0760. DOI:10.1089/wound.2017.0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y, Meng H, Liu Y, et al. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci World J. 20152015:685690. doi: 10.1155/2015/685690. DOI:10.1155/2015/685690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cubo N, Garcia M, Del Cañizo JF, et al. 3D Bioprinting of Functional Human Skin:Production and In Vivo Analysis. Biofabrication. 2017;9:15006. doi: 10.1088/1758-5090/9/1/015006. DOI:10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- 88.Albanna M, Binder KW, Murphy SV, et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci Rep. 2019;9:1–15. doi: 10.1038/s41598-018-38366-w. DOI:10.1038/s41598-018-38366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar SA, Alonzo M, Allen SC, et al. A Visible Light-Cross-Linkable, Fibrin-Gelatin-Based Bioprinted Construct with Human Cardiomyocytes and Fibroblasts. ACS Biomater Sci Eng. 2019;5:4551–63. doi: 10.1021/acsbiomaterials.9b00505. DOI:10.1021/acsbiomaterials.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Z, Lee SJ, Cheng H, et al. 3D Bioprinted Functional and Contractile Cardiac Tissue Constructs. Acta Biomater. 2018;70:48–56. doi: 10.1016/j.actbio.2018.02.007. DOI:10.1016/j.actbio.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oztan YC, Nawafleh N, Zhou Y, et al. Recent Advances on Utilization of Bioprinting for Tumor Modeling. Bioprinting. 2020;18:e00079. doi: 10.1016/j.bprint.2020.e00079. DOI:10.1016/j.bprint.2020 e00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee C, Abelseth E, de la Vega L, et al. Bioprinting a Novel Glioblastoma Tumor Model Using a Fibrin-based Bioink for Drug Screening. Mater Today Chem. 2019;12:78–84. DOI:10.1016/j.mtchem.2018.12.005. [Google Scholar]
- 93.Piard C, Jeyaram A, Liu Y, et al. 3D Printed HUVECs/MSCs Cocultures Impact Cellular Interactions and Angiogenesis Depending on Cell-cell Distance. Biomaterials. 2019;222:119423. doi: 10.1016/j.biomaterials.2019.119423. DOI:10.1016/j.biomaterials.2019.119423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang K, Fu Q, Yoo J, et al. 3D Bioprinting of Urethra with PCL/PLCL Blend and Dual Autologous Cells in Fibrin Hydrogel:An In Vitro Evaluation of Biomimetic Mechanical Property and Cell Growth Environment. Acta Biomater. 2017;50:154–64. doi: 10.1016/j.actbio.2016.12.008. DOI:10.1016/j.actbio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 95.Freeman S, Ramos R, Chando PA, et al. A Bioink Blend for Rotary 3D Bioprinting Tissue Engineered Small-diameter Vascular Constructs. Acta Biomater. 2019;95:152–64. doi: 10.1016/j.actbio.2019.06.052. DOI:10.1016/j.actbio.2019.06.052. [DOI] [PubMed] [Google Scholar]
- 96.Koch L, Deiwick A, Franke A, et al. Laser Bioprinting of Human Induced Pluripotent Stem Cells the Effect of Printing and Biomaterials on Cell Survival, Pluripotency, and Differentiation. Biofabrication. 2018;10:35005. doi: 10.1088/1758-5090/aab981. DOI:10.1088/1758-5090/aab981. [DOI] [PubMed] [Google Scholar]
- 97.Gruene M, Pflaum M, Hess C, et al. Laser Printing of Three-dimensional Multicellular Arrays for Studies of Cell-cell and Cell-environment Interactions. Tissue Eng Part C Methods. 2011;17:973–82. doi: 10.1089/ten.tec.2011.0185. DOI:10.1089/ten.tec.2011.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O'Donnell N, Okkelman IA, Timashev P, et al. Cellulose-based Scaffolds for Fluorescence Lifetime Imaging-assisted Tissue Engineering. Acta Biomater. 2018;80:85–96. doi: 10.1016/j.actbio.2018.09.034. DOI:10.1016/j.actbio.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 99.McQuilten ZK, Bailey M, Cameron PA, et al. Fibrinogen Concentration and Use of Fibrinogen Supplementation with Cryoprecipitate in Patients with Critical Bleeding Receiving Massive Transfusion:A Bi-national Cohort Study. Br J Haematol. 2017;179:131–41. doi: 10.1111/bjh.14804. DOI:10.1111/bjh.14804. [DOI] [PubMed] [Google Scholar]
- 100.Ahlfeld T, Cubo-Mateo N, Cometta S, et al. A Novel Plasma-based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl Mater Interfaces. 2020;12:12557–72. doi: 10.1021/acsami.0c00710. DOI:10.1021/acsami.0c00710. [DOI] [PubMed] [Google Scholar]


