Abstract
Aims
Endothelin-1 (ET-1) is a potent vasoconstrictor peptide linked to vascular diseases through a common intronic gene enhancer [(rs9349379-G allele), chromosome 6 (PHACTR1/EDN1)]. We performed a multimodality investigation into the role of ET-1 and this gene variant in the pathogenesis of coronary microvascular dysfunction (CMD) in patients with symptoms and/or signs of ischaemia but no obstructive coronary artery disease (CAD).
Methods and results
Three hundred and ninety-one patients with angina were enrolled. Of these, 206 (53%) with obstructive CAD were excluded leaving 185 (47%) eligible. One hundred and nine (72%) of 151 subjects who underwent invasive testing had objective evidence of CMD (COVADIS criteria). rs9349379-G allele frequency was greater than in contemporary reference genome bank control subjects [allele frequency 46% (129/280 alleles) vs. 39% (5551/14380); P = 0.013]. The G allele was associated with higher plasma serum ET-1 [least squares mean 1.59 pg/mL vs. 1.28 pg/mL; 95% confidence interval (CI) 0.10–0.53; P = 0.005]. Patients with rs9349379-G allele had over double the odds of CMD [odds ratio (OR) 2.33, 95% CI 1.10–4.96; P = 0.027]. Multimodality non-invasive testing confirmed the G allele was associated with linked impairments in myocardial perfusion on stress cardiac magnetic resonance imaging at 1.5 T (N = 107; GG 56%, AG 43%, AA 31%, P = 0.042) and exercise testing (N = 87; −3.0 units in Duke Exercise Treadmill Score; −5.8 to −0.1; P = 0.045). Endothelin-1 related vascular mechanisms were assessed ex vivo using wire myography with endothelin A receptor (ETA) antagonists including zibotentan. Subjects with rs9349379-G allele had preserved peripheral small vessel reactivity to ET-1 with high affinity of ETA antagonists. Zibotentan reversed ET-1-induced vasoconstriction independently of G allele status.
Conclusion
We identify a novel genetic risk locus for CMD. These findings implicate ET-1 dysregulation and support the possibility of precision medicine using genetics to target oral ETA antagonist therapy in patients with microvascular angina.
Trial registration
ClinicalTrials.gov: NCT03193294.
Keywords: Endothelin-1, Single-nucleotide polymorphism, Stable angina pectoris, Coronary microvascular dysfunction, Microvascular angina, Precision medicine
Introduction
The coronary microcirculation has been implicated in the pathogenesis of angina for over 50 years, however, disease mechanisms remain incompletely understood.1 Coronary microvascular dysfunction (CMD) is associated with adverse outcomes in angina and a plethora of other cardiovascular disorders.2–5 Standardized diagnostic criteria for microvascular dysfunction6 underpin recent studies which have identified the disease prevalence affecting two-thirds of angina patients without obstructive epicardial coronary artery disease (CAD).7–10 These patients present a diagnostic and therapeutic challenge with up to one in four experiencing a major adverse cardiac event after 5 years of follow-up.11,12 The syndrome of ischaemia and no obstructive CAD (INOCA) is particularly important in women,13 whose elevated cardiac risk is mostly driven by impaired coronary flow reserve (CFR) (and not obstructive coronary disease).11
Endothelin-1 (ET-1) is a highly potent endogenous vasoconstrictor of human coronary arteries14 and has been implicated in the pathogenesis of microvascular dysfunction.15,16 Endothelin-1-mediated activation of the G protein-coupled endothelin A (ETA) receptor on vascular smooth muscle cells induces endothelial dysfunction, inflammation, and vasoproliferative effects. Circulating concentrations of serum ET-1 are inversely associated with coronary flow responses in patients with CMD.14,16 Recently, a common (39%) genetic locus in chromosome 6p24 (PHACTR1/EDN1) has been shown to be a distal regulator of endothelin gene expression.17 The allele, rs9349379-G, is associated with an increased risk for atherosclerotic epicardial CAD and myocardial infarction.18 This functional single-nucleotide polymorphism (SNP: rs9349379-G) is associated with increased endothelin gene expression resulting in a lifetime’s exposure of at least 20% higher ET-1 precursor levels in the plasma.17 Endothelin-1 dysregulation is implicated in coronary vascular disease, however, the role of rs9349379 in the pathogenesis of CMD has not been examined.
We investigated the association, if any, of the rs9349379-G allele with CMD in angina patients undergoing invasive coronary function testing. Our secondary objectives were to investigate whether the G allele associates with non-invasive parameters of myocardial ischaemia. Our final objective was to examine vascular mechanisms using isometric tension recordings in small peripheral resistance vessels isolated from patients according to genotype. We evaluated ETA receptor-mediated vasoconstriction in subjects according to rs9349379-G allele status. These included zibotentan, an ETA receptor-selective antagonist, which is available for repurposing following neutral results in phase 3 oncology trials.
Methods
Study population
We prospectively enrolled patients with stable angina. We screened elective adult referrals to two hospitals serving a population of ∼2.5 million in the West of Scotland. Patients were scheduled to undergo clinically indicated invasive coronary angiography for the investigation of suspected CAD. The participants were enrolled into the Coronary Microvascular Angina (CorMicA) study (ClinicalTrials.gov: NCT03193294), which was a randomized, controlled, strategy trial of stratified medicine in angina patients without obstructive CAD.19 Rose-Angina questionnaire was administered on the day of the angiogram and only patients with definite or possible angina were eligible to participate.20 Exclusion criteria included a non-coronary indication for invasive angiography, e.g. valve disease, severe renal dysfunction (glomerular filtration rate < 30 mL/min), inability to give informed consent and obstructive coronary disease determined during invasive coronary angiography [≥50% diameter stenosis and/or fractional flow reserve (FFR) ≤ 0.80]. All coronary vasodilating drugs were discontinued at least 24 h before the procedure. Pooled control genotype frequencies were ascertained from a contemporary medical genome reference cohort.21
Definitions: coronary microvascular dysfunction
We defined CMD using invasive coronary function testing and the Coronary Vasomotion Disorders International Study Group (COVADIS) diagnostic criteria.20 These physiological criteria included an abnormal response to adenosine [raised index of microcirculatory resistance (IMR) (≥25) and/or abnormal CFR (<2.0)]. In addition, CMD also included subjects with microvascular spasm during acetylcholine (ACh) provocation [reproduction of angina symptoms, ischaemic electrocardiogram changes (≥1 mm ST-segment deviation), but <90% epicardial spasm during ACh testing].22 Coronary microvascular dysfunction is frequently associated with epicardial vasospasm and hence patients with abnormal vasoreactivity during adenosine assessment (abnormal IMR and/or CFR) and coexistent epicardial vasospasm during ACh provocation were included within the CMD group. Fractional flow reserve was measured to rule-out flow limiting CAD as an alternative explanation for myocardial ischaemia (INOCA subjects had an FFR >0.80 in target artery).
Measurement of coronary vascular function in vivo
We used an interventional diagnostic procedure (IDP) that combined guidewire-based direct measurement of coronary vascular function followed by pharmacological vasoreactivity testing. Specifically, the IDP included a guidewire-based measurement of coronary vascular function [FFR, CFR and IMR] followed by pharmacological vasoreactivity testing with ACh and glyceryl trinitrate (GTN) and has been previously described.19,23
In brief, an intravenous infusion of adenosine (140 μg/kg/min) was administered via a large peripheral vein to induce steady-state maximal hyperaemia for a period of at least 90 s with a target time of 180 s. A pressure–temperature sensitive guidewire was placed into the distal third of a major epicardial coronary artery (typically the left anterior descending). The myocardial FFR was calculated by the ratio of mean distal coronary pressure to mean aortic pressure during maximal hyperaemia. A FFR ≤0.80 was taken as abnormal and indicative of flow-limiting CAD.24 Coronary flow reserve was calculated using thermodilution as resting mean transit time divided by hyperaemic mean transit time.25 A CFR <2.0 was defined as abnormal representing impaired vasodilator reserve.26 The IMR was calculated as the product of mean hyperaemic transit time and mean distal coronary pressure at hyperaemia.27 An IMR ≥25 was defined as abnormal and indicative of increased microcirculatory resistance.28 These invasive parameters were simultaneously derived in real time using dedicated software (Coroventis, Uppsala, Sweden). We assessed endothelium-dependent coronary vasomotor function using intracoronary infusions of ACh via the guiding catheter at concentrations of 0.182, 1.82, and 18.2 µg/mL (10−6, 10−5, and 10−4 mol/L, respectively) at 1 mL/min for 2 min via a mechanical infusion pump.29 Patients who had CMD (e.g. abnormal CFR and/or IMR) but co-existent epicardial vasospasm during ACh bolus (100 μg bolus of ACh; 5.5 mL of 10−4 mol/L over 20 s) were considered in the CMD group.30 In order to assess non-endothelial dependent vasodilatation, 300 µg of GTN was administered by manual intracoronary bolus injection. Detailed methods are reported in the Supplementary material online, Appendix.
Blood and tissue analysis
Serum ET-1 was determined using blood obtained on the day of coronary function testing (Quantikine ® ELISA, R&D Systems® Europe, Abington, UK). Blood was obtained from participants following an overnight fast in a recumbent position.
Ex vivo pharmacological assessment of peripheral vascular function was performed on patients who volunteered to undergo a gluteal skin fat biopsy within 4 weeks of the invasive coronary function assessment. The biopsy was obtained under sterile conditions using local anaesthesia with lidocaine (2%). Small peripheral resistance vessels (<400 µm) were carefully dissected from fresh biopsies using a light microscope. About 2 mm length vessels were mounted on 40-μm stainless steel wires for isometric myography in multi-channel myograph chambers (DMT, Denmark) filled with physiological saline solution. Isometric tension recordings followed-on directly using the technique of wire myography to study small peripheral resistance arteries with paired cumulative concentration response curves (CCRCs) to ET-1 in the presence or absence of an ETA receptor antagonist, either BQ123 or zibotentan (AstraZeneca, UK; Open Innovation). This vascular biology sub-study was an extension of our work in INOCA subjects that was previously published in this journal.31 The detailed methods are described in the Supplementary material online, Appendix. The peripheral vascular sensitivity to ET-1 (pEC50) and maximum vasoconstriction to ET-1 (Emax) were determined.
For the antagonist studies, the affinity (KB) of BQ123 was first determined in paired vessels from individuals and calculated using Schild regression. The pKB (−log10 KB) values were compared between each genotype as an indicator of whether or not patients of different genotypes are likely to respond equally well to an ETA antagonist used clinically. A final series of experiments involved paired vessel experiments using ET-1 CCRCs in the presence and absence of a highly selective ETA receptor antagonist, zibotentan to determine a pKB value and assess whether zibotentan could reverse an established ET-1-mediated vessel constriction.
Cardiac magnetic resonance imaging and ischaemia testing protocol
Patients were prospectively invited to undergo quantitative perfusion cardiac magnetic resonance (CMR) imaging at 1.5 T using pharmacological stress testing with intravenous adenosine (140 µg/kg/min) within 6 weeks of the index coronary angiogram. CMR studies were performed using a standardized CMR protocol (Siemens MAGNETOM Avanto, Erlangen, Germany). The CMR scans were interpreted by two experienced observers (D.C., C.B.) with Level III accreditation of the European Association of Cardiovascular Imaging (EACVI), blind to diagnostic findings and genotype. The raw stress and rest perfusion images were qualitatively assessed for inducible or fixed perfusion defects. The perfusion was classified as either normal, abnormal, or equivocal. If a perfusion defect was present, it was reported as having an epicardial, microvascular or equivocal pattern. Perfusion defects were then reported on a segmental basis according to the American Heart Association 16-segment model32 and were classified according to the transmurality of the perfusion defect (<50% or >50%), and the number of segments with qualitatively abnormal perfusion was defined. Dark rim artefact was adjudicated based on standardized criteria.33
The first-pass perfusion images were then post-processed to derive quantitative pixel perfusion maps to derive absolute myocardial blood flow and myocardial perfusion reserve (MPR) (further detail in Supplementary material online).34
Treadmill exercise stress electrocardiography using the Bruce protocol was analysed from the subgroup of patients who had been pre-selected for this procedure on clinical grounds prior to invasive coronary angiography. We used the Duke treadmill score (DTS) which is a validated metric with established prognostic cardiovascular utility.35 The exercise treadmill test analysis included (i) exercise duration and (ii) the DTS36 by a cardiology researcher (EY) blinded to genotype and invasive physiology. The DTS is based on the occurrence of angina during treadmill exercise testing, ST-segment depression during the test and peak exercise duration (or metabolic equivalent of task achieved). Specifically, the DTS equals the maximum exercise time in minutes − (5 × the maximal net ST-segment deviation in mm during or after exercise) − (4 × the treadmill angina index (where 0 = no angina, 1 = non-limiting angina, 2 = exercise limiting angina).
All subjects were asked to abstain from caffeine-containing beverages or foodstuffs for 24 h, and vasoactive medications for 48 h prior to the CMR examination. All scan acquisitions were spatially co-registered. All CMR analyses were performed by a blinded analyst with Level 3 EACVI accreditation.
Statistical analysis
The main hypothesis in our study was that regulation of ET-1 gene expression reflected by the presence of the intronic ET-1 gene enhancer, rs9349379-G, associates with invasive tests of CMD. We tested the association of genotype (SNP rs9349379 G-A allele status) with CMD on invasive coronary vasoreactivity testing by calculating the odds ratio (OR) and its 95% confidence intervals (CIs). Multivariable logistic regression was used to determine whether genotype was independently associated with CMD (as defined by abnormal response to intracoronary ACh and/or systemic adenosine) adjusting for overall cardiac risk (ASSIGN score) including previous cardiac events.37
Categorical data are presented as percentages and continuous parameters are shown as means with standard deviation values or medians with interquartile ranges. For secondary analyses, subjects were divided into three genotype groups. Kruskal–Wallis test was used to test whether distribution of non-parametric variables is the same between the groups. Subgroup analysis of A vs. G genotypes was determined a priori to evaluate any differences between the two most differentiated groups. The least squares (LS) mean of serum ET-1 levels was compared between the groups derived using analysis of co-variance with serum ET-1 as dependent variable and adjusted for age, sex, body mass index, genotype and cardiovascular risk as covariates and possible confounders. Linear associations with invasive and non-invasive metrics of microvascular disease were performed by analysis of variance (ANOVA) with P for linear trend for continuous parameters and χ2 test with P for linear-by-linear test for categorical variables. Statistical analyses were performed with Prism 7.0 (GraphPad, La Jolla, CA, USA) and SPSS 25.0 (SPSS, Chicago, IL, USA).
Results
We prospectively enrolled 391 patients with angina between 25 November 2016 and 11 December 2017 at two hospitals serving a population of ∼2.5 million in the West of Scotland (CorMicA: ClinicalTrials.gov NCT03193294).19 Invasive coronary angiography revealed obstructive disease in 206 (53.7%) participants who were then excluded from further study. One hundred and fifty-one participants with no obstructive coronary disease continued in the study (Take home figure, Table 1). Evidence of CMD was found in 109 (72%) of 151 subjects undergoing invasive coronary vasoreactivity testing (Table 2). An overview of the study and investigations is illustrated in Take home figure. Genetic analysis was completed in 140 subjects (93%) using baseline venous blood samples. The mean age of patients in this analysis 61.1 ± 10.1 years. There was a predominance of women [103 (74%)] and the estimated 10-year risk of cardiovascular events (ASSIGN) was appreciable at 25% (±20).
Take home figure.
Study overview: endothelin-1 gene enhancer in microvascular angina. Three hundred and ninety-one patients with stable angina were prospectively enrolled without prior knowledge of coronary anatomy. One hundred and eighty-five (47%) had no obstructive coronary artery disease and thus eligible for invasive coronary vasoreactivity testing and further sub-studies. One hundred and fifty-one of 185 (82%) were able to undergo adjunctive invasive tests for coronary microvascular dysfunction. One hundred and nine (72%) subjects tested had evidence of coronary microvascular dysfunction. One hundred and forty subjects underwent genetic analysis for rs9349379-G allele with an allele frequency of 46% (129/280 alleles). The frequency of detrimental G alleles was higher than reference genome bank control subjects (46% vs. 39%; P = 0.013). Patients with rs9349379-G allele had higher serum endothelin-1 and over double the odds of coronary microvascular dysfunction (odds ratio 2.33, 95% confidence interval 1.10–4.96; P = 0.027). In addition, subjects were more likely to have impaired myocardial perfusion (P = 0.04) and exercise tolerance (−3.0 units in Duke Exercise Treadmill Score; P = 0.045). Peripheral small artery reactivity to endothelin-1 and affinity of ETA receptor antagonists were preserved in the rs9349379-G allele group (P = 0.209). Crucially, zibotentan tested at clinically relevant concentrations, fully reversed an established endothelin-1 vasoconstriction, indicative of efficacy in conditions associated with vasospasm. This suggests that ETA receptor antagonism in this group of patients may have therapeutic benefit.
Table 1.
Baseline demographics by genotype
| SNP (rs9349379) genotype (n = 140) |
P-valuea | |||
|---|---|---|---|---|
| AA (N = 50) | AG (N = 51) | GG (N = 39) | ||
| Clinical features | ||||
| Age (years) | 60.6 (±11) | 61.1 (±10) | 61.6 (±10) | 0.649 |
| Female | 36 (72%) | 36 (71%) | 31 (80%) | 0.607 |
| ASSIGN scoreb | 24 (±21) | 27 (±23) | 25 (±19) | 0.811 |
| Dyslipidaemia | 12 (24%) | 10 (20%) | 8 (21%) | 0.671 |
| Hypertension | 30 (60%) | 32 (63%) | 27 (69%) | 0.382 |
| Previous cardiovascular eventc | 10 (20%) | 10 (20%) | 13 (33%) | 0.239 |
| Diabetic | 9 (18%) | 11 (22%) | 6 (15%) | 0.794 |
| Smoker | 6 (12%) | 8 (16%) | 9 (23%) | 0.169 |
| Family history | 17 (34%) | 13 (26%) | 13 (33%) | 0.886 |
| Peripheral vascular disease | 2 (4%) | 3 (6%) | 2 (5%) | 0.789 |
| Atrial fibrillation | 5 (10%) | 4 (8%) | 1 (3%) | 0.195 |
| Pulse (rate/min) | 69 (±11) | 67 (±11) | 71 (±11) | 0.697 |
| Systolic blood pressure (mmHg) | 138 (±22) | 136 (±31) | 138 (±25) | 0.951 |
| Diastolic blood pressure (mmHg) | 73 (±11) | 74 (±15) | 70 (±12) | 0.260 |
| Body mass index (kg/m2) | 30.4 (±8) | 30.4 (±6) | 29.4 (±7) | 0.515 |
| Laboratory investigations | ||||
| Cholesterol (mmol/L) | 3.5 (±1) | 3.5 (±1) | 3.6 (±1) | 0.904 |
| Glucose (mmol/L) | 4.6 (±1) | 5.0 (±2) | 4.7 (±2) | 0.774 |
| C-reactive protein (mg/L) | 3.2 (±5) | 3.2 (±5) | 3.1 (±4) | 0.920 |
| N-terminal brain natriuretic peptide (pg/mL) | 140 (±187) | 157 (±197) | 135 (±153) | 0.937 |
| Endothelin-1 (pg/mL)d | 1.27 (0.42) | 1.41 (0.63) | 1.46 (0.56) | 0.097 |
Data are expressed as mean (standard deviation) or number (%).
ACE-I, angiotensin converting enzyme inhibitor; ACh, acetylcholine; BMI, body mass index; CCB, calcium channel blocker; CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; LVEDP, left ventricular end-diastolic pressure; MI, myocardial infarction.
P-value represents between group ANOVA for linear trend (continuous data) or Pearson χ2 test for linear trend (categorical data) or Kruskal–Wallis testing probability that the distribution of non-parametric variables are the same across the groups.
ASSIGN risk—predicted 10-year risk of cardiovascular event.
Previous myocardial infarction or cerebrovascular event (including transient ischaemic attack).
Endothelin-1 levels were available in 137 genotyped subjects with significance determined using one-way ANOVA (linear trend).
Table 2.
Invasive coronary physiology and non-invasive stress testing
| SNP (rs9349379) genotype |
P-valuea | |||
|---|---|---|---|---|
| AA (N = 50) | AG (N = 51) | GG (N = 39) | ||
| Minor non-obstructive CADb | 25 (50%) | 30 (59%) | 24 (62%) | 0.265 |
| Coronary atheroma burden (Gensini score)c | 0 (0.2) | 2 (0.5) | 1 (0.6) | 0.037 |
| Left ventricular end-diastolic pressure (mmHg) | 10 (±4) | 10 (±5) | 9 (±3) | 0.520 |
| Fractional flow reserve (FFR) | 0.88 (0.05) | 0.88 (0.06) | 0.88 (0.05) | 0.977 |
| Coronary microvascular dysfunction (any) | 30 (60%) | 38 (75%) | 32 (82%) | 0.021 |
| Abnormal CFR (<2.0) | 10 (20%) | 18 (36%) | 16 (41%) | 0.030 |
| Coronary flow reserve (CFR) | 3.0 (2.1–3.7) | 2.7 (1.8–3.5) | 2.1 (1.7–3.2) | 0.046 |
| Abnormal IMR (≥25) | 12 (24%) | 17 (33%) | 18 (46%) | 0.029 |
| Microcirculatory resistance (IMR) | 18.9 (15.2–24.2) | 18.6 (14.2–29.3) | 22.1 (13.8–29.3) | 0.879 |
| Abnormal CFR or IMR | 20 (40%) | 26 (51%) | 27 (69%) | 0.007 |
| Microvascular spasm (during acetylcholine) | 15 (30%) | 21 (42%) | 12 (31%) | 0.385 |
| Exercise treadmill testing (N = 87) | 28 (56%) | 34 (67%) | 25 (64%) | |
| Duration (s) | 393 (±124) | 352 (±157) | 384 (±162) | 0.827 |
| METs | 7.8 (±2.1) | 7.4 (±2.6) | 7.6 (±2.1) | 0.786 |
| Angina on treadmill | 16 (59%) | 23 (68%) | 20 (87%) | 0.036 |
| Peak systolic blood pressure (mmHg) | 178 (±30) | 173 (±34) | 182 (±25) | 0.688 |
| Duke Treadmill Score | −0.3 (±6.0) | −0.6 (±4.7) | −3.3 (±4.2) | 0.045 |
| Stress perfusion magnetic resonance imaging (N = 107) | ||||
| Inducible myocardial perfusion defect | 11 (31%) | 17 (43%) | 18 (56%) | 0.042 |
| Inducible myocardial perfusion defect with CMD | 4 (13%) | 14 (37%) | 15 (47%) | 0.016 |
| Myocardial perfusion reserve (global) | 1.8 (±0.4) | 1.7 (±0.4) | 1.6 (±0.4) | 0.154 |
| Myocardial perfusion reserve (endocardium) | 1.7 (±0.4) | 1.6 (±0.4) | 1.5 (±0.4) | 0.162 |
| Left ventricular end diastolic volume (indexed, mL/m2) | 68.5 (±13.6) | 70.1 (±13.2) | 70.2 (±11.9) | 0.591 |
| Left ventricular end systolic volume (indexed, mL/m2) | 23.4 (±6.0) | 25.4 (±8.8) | 23.1 (±5.8) | 0.848 |
| Left ventricular ejection fraction (%) | 65.9 (±4.4) | 64.5 (±6.5) | 67.3 (±5.2) | 0.321 |
| Stroke volume (indexed, mL/m2) | 45.0 (±8.8) | 44.7 (±7.0) | 47.1 (±8.2) | 0.298 |
| Left ventricular mass (indexed, mL/m2) | 42.0 (±7.0) | 42.3 (±8.1) | 42.1 (±7.8) | 0.924 |
Data are expressed as mean (±SD), median (IQR), or N (%).
CAD, coronary artery disease; CFR, coronary flow reserve; FFR, fractional flow reserve; LVEDP, left ventricular end-diastolic pressure; IMR, index of microcirculatory resistance; METS, metabolic equivalent of task.
P-value represents between group ANOVA for linear trend (continuous data) or Pearson χ2 test for linear trend (categorical data), Kruskal–Wallis test of probability that the distribution of non-parametric variables are the same across the groups.
Core-laboratory adjudication of any angiographic evidence of coronary atherosclerosis including any minimal angiographic luminal irregularity.
Gensini angiographic score is a metric of angiographic disease severity incorporating lesion severity and location. Detailed MRI methodology available in Supplementary material online, Appendix.
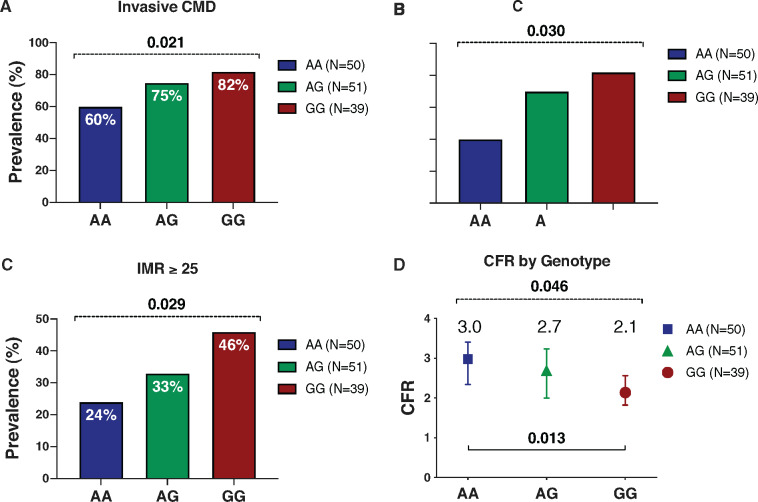
The genotype distribution of rs9349379 was AA (N = 50, 36%), AG (N = 51, 36%), and GG (N = 39, 28%). This SNP did not fulfil Hardy Weinberg equilibrium (P = 0.0015) reflecting biologic ascertainment of genotypes. One hundred and forty subjects underwent genetic analysis for (rs9349379-G allele) with an allele frequency of 46% (129/280 alleles). The allele frequency was increased in our angina cohort compared to that of genome bank control subjects [rs9349379-G allele frequency 39% (5551/14380); χ2 = 6.15, P = 0.013].21 The rs9349379-G allele was associated with over double the odds of CMD (OR 2.33, 95% CI 1.10–4.96; P = 0.027; Figure 1A). Subjects with G allele had higher circulating serum ET-1 concentration (LS mean 1.59 pg/mL vs. 1.28 pg/mL; difference 0.31 pg/mL; 0.10–0.52; P = 0.005; Figure 1B). Each additional G allele was linearly associated with CMD on invasive interrogation (Figure 2A; P = 0.021). On multivariable analysis, the G allele remained associated with CMD (OR per G allele 2.31; 1.08–4.91; P = 0.030; Supplementary material online, Table S1).
Figure 1.
Detrimental effects of rs9349379-G allele on coronary microvascular function and endothelin-1. (A) Patients with G allele were over twice as likely to have underlying microvascular dysfunction (odds ratio per G allele 2.33, 95% confidence interval 1.10–4.96; P = 0.027) Even after adjustment for other risk factors the G allele was predictive of microvascular disease (odds ratio 2.31; 95% confidence interval 1.0–4.91). This finding supports a detrimental impact on the coronary microcirculation of a lifetime of increased endothelin gene expression. (B) In a multivariable regression model adjusting for baseline group differences, patients with rs9349379-G allele had higher plasma endothelin-1 (least squares mean 1.59 pg/mL vs. 1.28 pg/mL; 95% confidence interval 0.10–0.53; P = 0.005).
Figure 2.
Genotype: phenotype association of G allele with invasive coronary microvascular dysfunction. (A–C) The prevalence of microvascular dysfunction detected during invasive coronary testing was associated with genotype status (AA 60%, AG 75%, GG 83%; P = 0.021). Presence of abnormal coronary flow reserve and microcirculatory resistance were linearly associated with each additional G allele. P-value represents Pearson χ2 test for linear trend (categorical data). (D) Coronary flow reserve was lower amongst subjects with two high-risk G alleles (rs9349379) consistent with detrimental effects of increased endothelin gene expression on the coronary microcirculation (Kruskal–Wallis between groups dotted line, P = 0.046). A priori subgroup analysis (AA vs. GG group—solid line) showed lower CFR in the GG group (P = 0.013). Data are median CFR plus error bars represent 95% confidence intervals for the median. P = 0.021, P = 0.030, P = 0.029 and P = 0.046.
Considering diagnostic subtypes of microvascular dysfunction, the vast majority had CMD during adenosine interrogation (73% abnormal CFR and/or IMR) and only 27% of the genotyped population had isolated microvascular spasm (isolated CMD to ACh only). There was a statistically significant relationship between genotype and CMD, as reflected by an impaired coronary vasodilator reserve (abnormal CFR: AA 20%, AG 35%, and GG 41%;Figure 2B; P = 0.030). A similar relationship was noted for prevalence of abnormal microvascular resistance in each genotype (abnormal IMR: AA 24%, AG 33%, and GG 46%; Figure 2C; P = 0.029). Coronary flow reserve decreased linearly with each additional rs9349379-G allele [AA 3.0 (2.1–3.7); AG 2.7 (1.8–3.5); GG 2.1 (1.7–3.2); overall P = 0.046; Figure 2D; Table 2]. The highest risk group (GG) had a significantly lower CFR than the AA group (median difference 0.84, 95% CI 0.1–1.1). The prevalence of abnormal invasive ACh response was not statistically different between the groups (any G allele 36% vs. no G allele 30%, P = 0.463). Patients with isolated CMD to ACh (microvascular spasm) had similar ET-1 levels to those without (1.33 ng/mL vs. 1.28 ng/mL; P = 0.769). The highest serum ET-1 levels were seen in subjects with concordant abnormalities in both CFR and IMR with linear stepwise reduction compared to those with only one index of CMD and lowest in those without any abnormalities [mean 1.67 ng/mL (both) vs. 1.39 ng/mL (one) vs. 1.31 ng/mL (none); P trend = 0.041].
The Gensini angiographic score reflecting the extent (or burden) of coronary atherosclerosis was higher in the rs9349379-GG group [median score 1.0 (0.0–6.0)] compared to the AA group [median score 0.0 (0.0–2.0); P = 0.037; Table 2]. As might be expected in this population of INOCA patients, the physiological burden of epicardial CAD was similar between the groups [myocardial FFR, AA 0.88 (±0.05); AG 0.88 (0.06); GG 0.88 (±0.05); P = 0.977].
One hundred and seven subjects underwent an adenosine stress perfusion cardiac magnetic resonance imaging (MRI) within 6 weeks of the invasive angiogram. Forty-six (43%) patients had evidence of a sub-endocardial circumferential abnormality of myocardial perfusion attributable to CMD (Table 2). The rs9349379-G allele was associated with abnormal myocardial perfusion disclosed by stress perfusion MRI (AA 31%, AG 43%, GG 56%; P = 0.042, Figure 3A). The association of genotype with CMD was more robust when considering subjects with either a circumferential subendocardial perfusion defect disclosed by MRI or invasive evidence of CMD (AA 65%, AG 85%, GG 91%; P < 0.001; Figure 3B). The absolute global and subendocardial perfusion reserve (MPR) was numerically lower with each G allele; however, the differences were not statistically significant (Table 2; Figure 3C and D).
Figure 3.
Genotype: phenotype association of G allele with non-invasive ischaemia testing. (A) Cardiovascular stress magnetic resonance imaging at 1.5 T (N = 107). There was a linear relationship between the G allele and presence of an inducible perfusion defect on cardiac magnetic resonance (χ2 test for linear trend P = 0.042). (B) The relationship was more robust when considering with invasive evidence of coronary microvascular dysfunction and/or inducible perfusion defect. Over 90% of GG subjects had at least one abnormality compared with only 65% of AA subjects (P < 0.001). (C and D) Myocardial perfusion reserve was numerically reduced in AG and GG subjects compared with AA subjects; however, this was not statistically significant (P-value represents analysis of variance test for trend). Error bars represent 95% confidence intervals for the mean. (E) Invasive evidence of microvascular dysfunction (defined by abnormal response to intracoronary acetylcholine and/or systemic adenosine) was functionally significant and associated with ischaemic burden on symptom limited exercise treadmill testing (coronary microvascular dysfunction −2.3 vs. no coronary microvascular dysfunction +3.5; difference −5.8 units; −8.2 to −3.3; P < 0.001). (F) Exercise treadmill testing (n = 84). There was a relationship between genotype group and worsening ischaemia on stress testing (analysis of variance P-trend = 0.045). The mean difference in ischaemia by Duke treadmill score between group GG and group AA was −3.0 units (95% confidence interval −5.8 to −0.1; P = 0.045). Error bars represent 95% confidence intervals for the mean.
We then assessed relationships between exercise treadmill testing, invasive measures of coronary vascular function and genotype. Ninety subjects prospectively completed exercise treadmill testing during standard care diagnostic work up prior to invasive coronary angiography, 84 of these subjects were included in the study with the remainder being excluded due to lack of genotype or exercise data. The mean exercise duration was 367 s (±156 s) and similar between the groups (Table 2). The mean DTS was −1.0 (±5.3) units. The presence of CMD was associated with reduced DTS (CMD −2.3 vs. no CMD +3.5; difference −5.8 units, 95% CI −8.2 to −3.3; P < 0.001; Figure 3E). Overall, there was a moderate inverse correlation between presence of CMD and the DTS (Spearman’s rho = −0.42; P < 0.001). Considering the cohort of 84 patients in whom genotype and DTS were both available, there was a lower DTS for each additional G allele consistent with increasing ischaemia with ET-1 gene enhancement. A priori analysis of high-risk subjects (homozygous for the minor G allele) compared to the AA group revealed a mean difference of −3.0 units in DTS (95% CI −5.8 to −0.1; P = 0.045) (Figure 3F). There was a modest correlation between the continuous DTS and genotype (Spearman’s rho −0.21; P = 0.055), that was not statistically significant. The angina index during exercise was linearly associated with G allele status (non-limiting or limiting angina AA 59% vs. AG 68% vs. GG 87%; P trend =0.036). The exercise time was not significantly lower amongst subjects with the G allele (365 vs. 392 s; P = 0.423).
Sixty-eight genotyped subjects agreed to participate in a vascular biology sub-study, providing written informed consent for a gluteal subcutaneous biopsy within 4 weeks of coronary angiography. Subjects who volunteered to have a biopsy were of similar age and cardiac risk to those who declined to participate in the sub-study [biopsy participants mean age 62 ± 9 years vs. 61 ± 11 years (P = 0.134), ASSIGN score 23% ± 18 vs. 28% ± 23 (P = 0.198)]. Forty-four (65%) of these patients had biopsies with a sufficient number of small arteries to undergo paired CCRCs to ET-1 in the presence and absence of an ETA receptor antagonist, either BQ123 or zibotentan (ZD4054; AstraZeneca, Cambridge, UK). Grouping according to genotype (AA, n = 16; AG, n = 14; GG, n = 14) and vasodilator responses to ACh (ACh Emax) were similar (Table 3). Similarly, vessels had similar potency for ET-1 (pEC50 AA 9.34, AG 9.45, and GG 9.32; P = 0.533) and maximum vasoconstriction to ET-1 (Emax AA 122.3%, AG 115.5%, GG 129.7%; P = 0.533; Figure 4A; Table 3).
Table 3.
Pathophysiology: vascular biology of ET-1
| SNP (rs9349379) genotype (n = 44) |
P-valuea | |||
|---|---|---|---|---|
| AA (N = 16) | AG (N = 14) | GG (N = 14) | ||
| Vessel diameter (um) | 344 (±88) | 342 (±89) | 347 (±125) | 0.851 |
| Vessel length (mm) | 1.85 (±0.12) | 1.87 (±0.10) | 1.82 (±0.11) | 0.276 |
| ACh Emax (%) | 77.7 (52.9–97.8) | 80.2 (59.9–97.6) | 92.5 (57.8–99.1) | 0.696 |
| ACh pEC50 | 7.28 (6.88–7.82) | 7.26 (6.82–8.00) | 6.96 (6.84–7.44) | 0.308 |
| ET-1 Emax (%) | 122.3 (115.7–134.7) | 115.5 (107.5–125.2) | 129.7 (115.8–151.2) | 0.533 |
| ET-1 pEC50 | 9.34 (9.15–9.52) | 9.45 (9.24–9.67) | 9.32 (8.96–9.69) | 0.533 |
| BQ123 pKB (±SEM) | 7.07 (±0.23) | 7.79 (±0.35) | 7.41 (±0.26) | 0.209 |
Forty-four (65%) of 68 patients who underwent invasive biopsies had a sufficient number of small arteries to undergo paired cumulative concentration response curves (CCRCs) to ET-1 in the presence and absence of an ETA receptor antagonist. Data are expressed as mean (±SD) or mean (95% CI for pooled best fit CCRC). CCRC, cumulative concentration response curves were drawn with best-fit derived values. pKB data involved paired vessels undergoing ET-1 CCRC in the presence or absence of BQ123 ETA receptor antagonist (available in 37 out of the 44 subjects: AA N = 14; AG N = 10; GG N = 13).
Significance determined using ANOVA for normally distributed means, Kruskal–Wallis test used for between group comparison of non-parametric variables and extra-sum of squares F test (for CCRC pooled best fit ET-1 data). There were no differences in between group baseline demographics in this vascular sub-study.
Figure 4.
Endothelin-1 ex vivo vascular biology by genotype. (A) cumulative concentration response curve to endothelin-1 in the three groups in the presence and absence of ETA antagonist BQ123 (n = 44). Similar antagonist potency (rightward curve shift) for each group suggesting firstly that the ETA receptors are the dominant effectors of the endothelin-1 vasoconstrictor response and secondly that the ETA receptor pathway is not down-regulated in spite of the elevated endothelin-1 gene expression and known increase in endothelin-1 activity in the G allele single-nucleotide polymorphism patients. (B) Antagonist potency of novel therapeutic oral ETA receptor antagonist zibotentan [N = 8, mean 7.54 (95% confidence interval 7.27–7.82)] is similar to peptide antagonist BQ123 [N = 27, mean 7.53 (95% confidence interval 7.37–7.69)]. Higher pKB represents a higher antagonist potency. (C) Zibotentan: reversal of established endothelin-1 vasoconstriction. Proof of concept dose-dependent reversal of potent and established endothelin-1-mediated peripheral arteriolar vasoconstriction. Crucially, the highest concentration tested which is also the plasma concentration achieved by a clinically relevant dose of 10 mg/day rapidly and fully reversed the established endothelin-1 constrictor response, indicative of efficacy in conditions of vasospasm. Comparison using ordinary two-way analysis of variance including time and dose both significant factors (P < 0.001 after adjustment for multiple testing).
Notably, the selective ETA receptor antagonist, BQ123, caused a parallel rightward shift of the CCRC with comparable pKB values between groups AA, AG, and GG [pKB values of 7.07 (±0.23), 7.79 (±0.35), and 7.41 (±0.26), respectively; P = 0.209; Figure 4B]. Zibotentan, a highly selective orally active ETA receptor antagonist, attenuated the constrictor response to ET-1 with pKB of 7.54 (95% CI 7.27–7.82), comparable to that of BQ123, pKB 7.53 (95% CI 7.37–7.69).
Crucially, these studies confirmed that zibotentan produced a concentration-dependent inhibition of an established constrictor response to ET-1 and was still efficacious in subjects with G allele (P < 0.001; Figure 4C). Figure 5 shows representative investigations from a female subject with few traditional cardiovascular risk factors but high-risk ET-1 enhancer genotype (GG).
Figure 5.
GG (high-risk endothelin-1 gene enhancer). Illustrative case from a patient with stable angina including representative images from invasive and non-invasive work up are shown in relation to clinical presentation and endothelin-1 enhancer genotype. Maximum ST represents the maximum planar or down sloping ST-segment depression during the exercise treadmill test. Invasive coronary angiography of both subjects is near identical showing only minimal luminal irregularities. White arrows represent subendocardial inducible ischaemic myocardium during adenosine stress magnetic resonance imaging in a patient with severe coronary microvascular dysfunction. Ex vivo vascular biology (bottom panel) shows typical endothelin-1-mediated vessel constriction during wire myography. Increasing vessel tension corresponds to the rising curve at each dose titration. A paired identical vessel experiment is performed after incubation with BQ123, an ETA receptor antagonist. This curve is marked in blue, the curve of endothelin-1 response is shifted to the right indicating that the ETA receptor mediates vasoconstriction. Despite the endothelin-1 gene enhancer, the GG subject does not appear to have ETA receptor down-regulation with similar levels of antagonist potency. This supports that ETA receptor antagonism in this group of patients may have therapeutic benefit. CFR, coronary flow reserve; ETA, endothelin A receptor; FFR, fractional flow reserve; IMR, index of microcirculatory resistance.
Discussion
We identify a novel genetic risk locus for CMD. Our study extends a report from the WISE investigators on genotype associations with arterial vasomotion.13 Our results support the hypothesis that dysregulation of the ET-1/ETA receptor system underpins abnormalities in the coronary microcirculation leading to myocardial ischaemia. Firstly, rs9349379-G allele status is associated with higher serum ET-1 and the presence and extent of CMD in patients with angina but without obstructive coronary disease. Secondly, the genetic polymorphism associates with ischaemia testing using distinct, non-invasive modalities including exercise stress electrocardiography and stress perfusion CMR. Thirdly, we demonstrate in ex vivo human small peripheral resistance vessels that the ETA vasoconstrictor response is not down-regulated in the presence of increases in endothelin gene expression and ET-1 activity in patients with the rs9349379-G allele. Finally, we provide proof-of-concept mechanistic data supporting a role for zibotentan, an orally active highly selective ETA receptor antagonist, in reversing established ET-1-mediated vasoconstriction. These findings have potential clinical relevance since zibotentan is available for repositioning as a novel, disease-modifying therapy in this patient population. The results of our study support the rationale for the ‘Precision Medicine with Zibotentan in Microvascular Angina (PRIZE)’ trial involving gene testing for the SNP rs9349379 and linked therapy (ClinicalTrials.gov Identifier: NCT04097314).
Endothelin dysregulation
Pre-clinical studies in experimental models of CMD implicate increased cardiac ET-1 production leading to endothelial dysfunction, enhanced vascular expression of rho-kinases, and reactive oxidant species such as superoxide and enhanced ET-1-mediated vasoconstriction.38 In patients with angina but no obstructive CAD, microvascular dysfunction is a systemic phenomenon characterized by peripheral endothelial dysfunction and enhanced peripheral small-vessel vasoconstriction.31,39 Further, impaired coronary microvascular function and the propensity to myocardial ischaemia may increase longer-term risk of major adverse cardiac events.40,41 Our study is distinct and builds on our prior vascular studies of ET-1 in microvascular angina as we used zibotentan which has more potential for clinical translation requiring future phase II studies.31 In addition, subjects were analysed by ET-1 rs9349379-G allele status rather than presence or absence of CMD. We observed that chronic exposure to increased circulating concentrations of ET-1, as reflected by rs9349379-G allele status, did not lead to down-regulation to ETA-mediated ET-1 vasoconstriction in patients with microvascular angina. The converse SNP (rs9349379-A) was recently found to be associated with spontaneous coronary artery dissection (SCAD) which typically occurs in patients without atherosclerosis.21 This finding is consistent with our work, particularly given that microvascular function is typically normal in SCAD.42
We showed that rs9349379-G allele was associated with higher serum ET-1 levels which is consistent with previous studies whereby the SNP associates with higher levels of ET-1 and its precursor (Big ET-1) in healthy subjects. Interestingly, the ET-1 plasma concentration in our INOCA population is comparable to ET-1 plasma concentrations in other conditions including pulmonary artery hypertension43 but lower than in other INOCA cohorts.44 We acknowledge that abluminal secretion of ET-1 away from endothelial cells towards underlying vascular smooth muscle means circulating concentrations of ET-1 are an imperfect measure of ET-1 activity in vascular tissues.45 Chronic elevation of circulating ET-1 may lead to adaptive down-regulation of its endogenous G-protein coupled receptors. This phenomenon has been described for ETA receptors in mice in which the clearing ETB receptor has been knocked out.46 Cardiovascular risk factors, including blood pressure, were not associated with rs9349379-G allele in our population, whereas an inverse associations have been observed in much larger populations.17 This is particularly interesting given its association with atherogenesis and CAD. It is thought that excess ET-1 effects healthy populations mediate hypotension via hypotension via ETB-induced nitric oxide and prostacyclin production, resultant vasodilation, diuresis, and natriuresis.47 Our study was underpowered to determine significant differences between baseline blood pressures which may also be confounded by treatment for hypertension.
Microvascular angina is a chronic, debilitating condition of unmet therapeutic need. Our vascular pharmacology findings indicate that despite a genetic predisposition to enhanced endothelin gene expression based on the rs9349379-G allele status, potentially leading to lifelong enhanced exposure to circulating concentrations of ET-1, the net effect on ET-1 response or sensitivity to ETA antagonists was similar between the groups by rs9349379 allele status. The ETA receptor may not be down-regulated in affected patients raising the potential for health gain by treatment with a selective ETA receptor antagonist, such as zibotentan. Importantly, BQ123 fully blocked the constrictor responses in all of the groups. Our vascular pharmacology study was specifically focused on the relationships between the rs9349379-G allele status, ET-1 vasoactive responses, and ETA receptor blockade. Patients with microvascular angina may have similar tissue responses to oral ETA receptor blocker therapy—this important possibility merits further (NCT04097314).
In a mechanistic, randomized, controlled trial in patients with microvascular angina, Johnson and Gould48 reported that ETA receptor antagonism increased (improved) the homogeneity of resting myocardial perfusion. Their study used cardiac positron emission tomography (PET) to quantify the homogeneity index (a visual notion of homogeneity derived from PET).49 Kaski et al.50 showed that patients with microvascular angina were exposed to increased circulating concentrations of ET-1 which in turn was associated with increased coronary vascular resistance and impaired coronary blood flow. Recently, Theuerle et al.51 have shown that plasma ET-1 is associated with invasive CMD in a 32 INOCA patients, however, the relationship was driven by elevated microvascular resistance and not impaired CFR.
Limitations
We describe compelling mechanistic evidence for a functional SNP being linked to CMD. We have followed accepted guidelines for CMD classifications, but it is recognized there are caveats with any classification system and acknowledge these are also relevant to this study. Firstly, we adopted binary cut-offs for the IDP test. It is possible that indeterminate (grey-zone or borderline) test results may have misclassified some patients. Furthermore, patients with CMD were heterogeneous and we aggregated patients with different types of microvascular dysfunction, e.g. impaired flow reserve, increased microvascular resistance, abnormal ACh response. Nonetheless, the vascular phenotype of affected patients was of coronary vascular dysfunction based on consensus guidelines for abnormal coronary microvascular response during systemic adenosine, an abnormal vasomotor response to intracoronary ACh, or both.6 In support of this approach, we observed a strong linear relationship between CMD and non-invasive ischaemia testing on the exercise treadmill (Figure 3F). In addition, heterogeneity is the rule rather than exception when considering many similar cardiovascular disorders, for example heart failure with preserved ejection fraction.52 Our stratified sensitivity analysis by CMD type, i.e. structural microvascular disease (i.e. raised IMR) and impaired vasodilator reserve (reduced CFR) (Table 2), lend further support to the design of our translational study. Secondly, not all patients underwent treadmill exercise testing. The tests were indicated as part of standard care and clinical, rather than core laboratory, reports were available for analysis. Nevertheless, they were performed according to the Bruce protocol and the results were determined in a standardized manner, blinded to rs9349379 allele status. Treadmill exercise testing is an imperfect measure of ischaemia and hence it is plausible that the known association of the rs9349379-G allele with epicardial CAD is a confounding factor. Gould and Johnson53 recently highlighted how flush ostial branch vessel occlusion may account for ischaemia despite a visual ‘normal’ angiogram without stenosis. On the other hand, the DTS has a mature associated literature with proven utility in CMD patients.54,55 The relatively small sample size and possibility of unmeasured baseline differences increases the possibility of Type I error. Thirdly, we administered intra-arterial doses of short acting GTN (100–200 μg) to facilitate procedure safety relating to transradial access, coronary arteriography, and invasive coronary vasoreactivity testing. Theoretically, GTN may affect the vascular responses to ACh; however, the half-life of GTN is around 2 min. Hence, after 10 min, only 3% of the GTN dose is bioavailable and we think the potential for confounding and a false negative test for microvascular vasospasm is unlikely. Conversely, a positive ACh test confounds assessment of true resting flow and may lead to falsely lowered CFR and hence we support ACh testing after adenosine assessment. Finally, we compared the allele prevalence within our cohort from Scotland with a pooled multicentre contemporary medical genome reference group of controls. Our study would have been strengthened by a control comparator group from the same area and ethnic background as our subjects. Further, although the SNP did not fulfil the Hardy–Weinberg equilibrium for the population as a whole, the control group from this study without CMD was consistent with the equilibrium (χ2 2.99, P = 0.084). It is plausible that HW was not met in the CMD group due to its association with the rs9349379-G allele of interest. This study is a cross-sectional analysis of a single genetic locus and provides associative findings of clinical interest but may overlook other important genetic risk determinants.
Clinical translation
These observations hypothesis generating particularly given the small sample size and heterogeneous patient population. The findings require external validation in other CMD cohorts whilst future work in populations from different regions would provide helpful context.
Overall, our study supports the case for selective ETA blockade distinct from ETB modulation in patients with microvascular disease in the heart. Oral ETA-selective blockade has therapeutic potential by attenuating the propensity to microvascular vasospasm, increasing coronary blood flow, and further improving coronary endothelial function through NO-mediated release.56 Zibotentan is one compound that holds promise as the most ETA selective of all orally active ETA receptor antagonists, which makes it particularly suited to use in microvascular angina. A targeted approach using selective ETA receptor antagonist therapy in patients based on genotype is being assessed in the PRIZE trial (NCT04097314).
Conclusion
We identified a genetic risk locus for CMD. The common genetic polymorphism (SNP rs9349379-G allele) was associated with higher ET-1 and both invasive CMD and non-invasive tests for ischaemia in subjects with angina but no obstructive CAD. Mechanistic ex vivo studies confirmed subjects with this functional allele have preserved response to ETA receptor blockade. Zibotentan, an orally active ETA receptor antagonist, reversed an established ET-1-mediated vasoconstriction. This study offers hope for angina patients although future trials are needed to determine whether CMD represents a potential new disease subtype for ETA antagonist therapy.
Supplementary Material
Acknowledgements
We sincerely thank the patients and staff who supported this study.
Funding
This work was supported by the British Heart Foundation (PG/17/2532884, RE/13/5/30177, and RE/18/6134217) and the Wellcome Trust (to A.P.D., J.J.M., 107715/Z/15/Z). This work was funded in part by the intramural program of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Conflict of interest: C.B. is employed by the University of Glasgow which holds consultancy and research agreements with companies that have commercial interests in the diagnosis and treatment of angina. The companies include Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Coroventis, GSK, HeartFlow, Novartis, Opsens, Philips, and Siemens Healthcare. K.G.O. has received consultant and speaker fees from Abbott Vascular, Biosensors and Boston Scientific. P.R. has received consultant and speaker fees from AstraZeneca. None of these companies have had any involvement with this study except AstraZeneca who provided zibotentan for the laboratory studies. None of the other authors have any potential conflicts of interest.
See page 3252 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz954)
References
- 1. Likoff W, Segal BL, Kasparian H.. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med 1967;276:1063–1066. [DOI] [PubMed] [Google Scholar]
- 2. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, Beussink-Nelson L, Ljung Faxén U, Fermer ML, Broberg MA, Gan L-M, Lund LH.. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohandas R, Segal MS, Huo T, Handberg EM, Petersen JW, Johnson BD, Sopko G, Bairey Merz CN, Pepine CJ.. Renal function and coronary microvascular dysfunction in women with symptoms/signs of ischemia. PLoS One 2015;10:e0125374.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh A, Greenwood JP, Berry C, Dawson DK, Hogrefe K, Kelly DJ, Dhakshinamurthy V, Lang CC, Khoo JP, Sprigings D, Steeds RP, Jerosch-Herold M, Neubauer S, Prendergast B, Williams B, Zhang R, Hudson I, Squire IB, Ford I, Samani NJ, McCann GP.. Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) Study. Eur Heart J 2017;38:1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, Bhatt DL, Di Carli MF, Taqueti VR.. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol 2018;72:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group (COVADIS) . International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 7. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS.. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C.. Stratified medical therapy using invasive coronary function testing in angina: the CorMicA trial. J Am Coll Cardiol 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 9. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A.. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 10. Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schaufele T, Mahrholdt H, Kaski JC, Sechtem U.. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 11. Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF.. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G, Investigators W.. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47(3 Suppl):S21–S29. [DOI] [PubMed] [Google Scholar]
- 13. Pacheco Claudio C, Quesada O, Pepine CJ, Noel Bairey Merz C.. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol 2018;41:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halcox JP, Nour KR, Zalos G, Quyyumi AA.. Endogenous endothelin in human coronary vascular function: differential contribution of endothelin receptor types A and B. Hypertension 2007;49:1134–1141. [DOI] [PubMed] [Google Scholar]
- 15. Lanza GA, Crea F.. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 2010;121:2317–2325. [DOI] [PubMed] [Google Scholar]
- 16. Pekdemir H, Polat G, Cin VG, Camsari A, Cicek D, Akkus MN, Doven O, Katircibasi MT, Muslu N.. Elevated plasma endothelin-1 levels in coronary sinus during rapid right atrial pacing in patients with slow coronary flow. Int J Cardiol 2004;97:35–41. [DOI] [PubMed] [Google Scholar]
- 17. Gupta RM, Hadaya J, Trehan A, Zekavat SM, Roselli C, Klarin D, Emdin CA, Hilvering CRE, Bianchi V, Mueller C, Khera AV, Ryan RJH, Engreitz JM, Issner R, Shoresh N, Epstein CB, de Laat W, Brown JD, Schnabel RB, Bernstein BE, Kathiresan S.. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell 2017;170:522–533.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The CDC, Nikpay M, Goel A, Won H-H, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang S-J, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikäinen L-P, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han B-G, Huang J, Jalilzadeh S, Kessler T, König IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki M-L, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon F-U-R, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim B-J, Kooner JS, Kullo IJ, Lehtimäki T, Loos RJF, Melander O, Metspalu A, März W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O'Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M.. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ford TJ, Corcoran D, Oldroyd KG, McEntegart M, Rocchiccioli P, Watkins S, Brooksbank K, Padmanabhan S, Sattar N, Briggs A, McConnachie A, Touyz R, Berry C.. Rationale and design of the British Heart Foundation (BHF) Coronary Microvascular Angina (CorMicA) stratified medicine clinical trial. Am Heart J 2018;201:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rose G, McCartney P, Reid DD.. Self-administration of a questionnaire on chest pain and intermittent claudication. Br J Prev Soc Med 1977;31:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adlam D, Olson TM, Combaret N, Kovacic JC, Iismaa SE, Al-Hussaini A, O'Byrne MM, Bouajila S, Georges A, Mishra K, Braund PS, d’Escamard V, Huang S, Margaritis M, Nelson CP, de Andrade M, Kadian-Dodov D, Welch CA, Mazurkiewicz S, Jeunemaitre X, Wong CMY, Giannoulatou E, Sweeting M, Muller D, Wood A, McGrath-Cadell L, Fatkin D, Dunwoodie SL, Harvey R, Holloway C, Empana J-P, Jouven XCARDIoGRAMPlusC4D Study GroupOlin JW, Gulati R, Tweet MS, Hayes SN, Samani NJ, Graham RM, Motreff P, Bouatia-Naji N.. Association of the PHACTR1/EDN1 genetic locus with spontaneous coronary artery dissection. J Am Coll Cardiol 2019;73:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa HB, Merz CN Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 23. Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C.. Stratified medical therapy using invasive coronary function testing in angina: CorMicA Trial. J Am Coll Cardiol 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 24. De Bruyne B, Baudhuin T, Melin JA, Pijls NH, Sys SU, Bol A, Paulus WJ, Heyndrickx GR, Wijns W.. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 1994;89:1013–1022. [DOI] [PubMed] [Google Scholar]
- 25. Pijls NHJ, De Bruyne B, Smith L, Aarnoudse W, Barbato E, Bartunek J, Bech GJW, Van De Vosse F.. Coronary thermodilution to assess flow reserve: validation in humans. Circulation 2002;105:2482–2486. [DOI] [PubMed] [Google Scholar]
- 26. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF.. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC.. Novel index for invasively assessing the coronary microcirculation. Circulation 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 28. Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA.. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015;131:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lerman A, Holmes DR, Bell MR, Garratt KN, Nishimura RA, Burnett JC.. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation 1995;92:2426–2431. [DOI] [PubMed] [Google Scholar]
- 30. Ohba K, Sugiyama S, Sumida H, Nozaki T, Matsubara J, Matsuzawa Y, Konishi M, Akiyama E, Kurokawa H, Maeda H, Sugamura K, Nagayoshi Y, Morihisa K, Sakamoto K, Tsujita K, Yamamoto E, Yamamuro M, Kojima S, Kaikita K, Tayama S, Hokimoto S, Matsui K, Sakamoto T, Ogawa H.. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease. J Am Heart Assoc 2012;1:e002485.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, Yii E, Sidik N, Harvey A, Montezano AC, Beattie E, Haddow L, Oldroyd KG, Touyz RM, Berry C.. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J 2018;39:4086–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS;American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 33. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E.. Standardized image interpretation and post processing in cardiovascular magnetic resonance: society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsu L-Y, Jacobs M, Benovoy M, Ta AD, Conn HM, Winkler S, Greve AM, Chen MY, Shanbhag SM, Bandettini WP, Arai AE.. Diagnostic performance of fully automated pixel-wise quantitative myocardial perfusion imaging by cardiovascular magnetic resonance. JACC Cardiovasc Imaging 2018;11:697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw LJ, Peterson ED, Shaw LK, Kesler KL, DeLong ER, Harrell FE Jr, Muhlbaier LH, Mark DB.. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation 1998;98:1622–1630. [DOI] [PubMed] [Google Scholar]
- 36. Mark DB, Shaw L, Harrell FE Jr, Hlatky MA, Lee KL, Bengtson JR, McCants CB, Califf RM, Pryor DB.. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med 1991;325:849–853. [DOI] [PubMed] [Google Scholar]
- 37. Woodward M, Brindle P, Tunstall-Pedoe H.. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart 2005;93:172–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L.. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc Res 2017;113:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jaarsma C, Vink H, van Haare J, Bekkers S, van Rooijen BD, Backes WH, Wildberger JE, Crijns HJ, van Teeffelen J, Schalla S.. Non-invasive assessment of microvascular dysfunction in patients with microvascular angina. Int J Cardiol 2017;248:433–439. [DOI] [PubMed] [Google Scholar]
- 40. Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, Sakata Y, Shimokawa H.. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol 2019;74:2350–2360. [DOI] [PubMed] [Google Scholar]
- 41. Ford TJ, Berry C, De Bruyne B, Yong ASC, Barlis P, Fearon WF, Ng M.. Physiological predictors of acute coronary syndromes: emerging insights from the plaque to the vulnerable patient. JACC Cardiovasc Interv 2017;10:2539–2547. [DOI] [PubMed] [Google Scholar]
- 42. Waterbury TM, Tweet MS, Hayes SN, Prasad A, Lerman A, Gulati R.. Coronary endothelial function and spontaneous coronary artery dissection. Eur Heart J Acute Cardiovasc Care 2018;doi:10.1177/2048872618795255. [DOI] [PubMed] [Google Scholar]
- 43. Jankowich MD, Wu WC, Choudhary G.. Association of elevated plasma endothelin-1 levels with pulmonary hypertension, mortality, and heart failure in African American Individuals: the Jackson Heart Study. JAMA Cardiol 2016;1:461–469. [DOI] [PubMed] [Google Scholar]
- 44. Kaski JC, Elliott PM, Salomone O, Dickinson K, Gordon D, Hann C, Holt DW.. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Br Heart J 1995;74:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ.. Endothelin. Pharmacol Rev 2016;68:357–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuc RE, Maguire JJ, Davenport AP.. Quantification of endothelin receptor subtypes in peripheral tissues reveals downregulation of ET(A) receptors in ET(B)-deficient mice. Exp Biol Med (Maywood) 2006;231:741–745. [PubMed] [Google Scholar]
- 47. Miller E, Czopek A, Duthie KM, Kirkby NS, van de Putte EE, Christen S, Kimmitt RA, Moorhouse R, Castellan RF, Kotelevtsev YV, Kuc RE, Davenport AP, Dhaun N, Webb DJ, Hadoke PW.. Smooth muscle endothelin B receptors regulate blood pressure but not vascular function or neointimal remodeling. Hypertension 2017;69:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson NP, Gould KL.. Physiology of endothelin in producing myocardial perfusion heterogeneity: a mechanistic study using Darusentan and positron emission tomography. J Nucl Cardiol 2013;20:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson NP, Gould KL.. Clinical evaluation of a new concept: resting myocardial perfusion heterogeneity quantified by Markovian analysis of PET identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med 2005;46:1427–1437. [PubMed] [Google Scholar]
- 50. Cox ID, Bøtker HE, Bagger JP, Sonne HS, Kristensen BØ, Kaski JC.. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. J Am Coll Cardiol 1999;34:455–460. [DOI] [PubMed] [Google Scholar]
- 51. Theuerle J, Farouque O, Vasanthakumar S, Patel SK, Burrell LM, Clark DJ, Al-Fiadh AH.. Plasma endothelin-1 and adrenomedullin are associated with coronary artery function and cardiovascular outcomes in humans. Int J Cardiol 2019;291:168–172. [DOI] [PubMed] [Google Scholar]
- 52. Kitzman DW, Upadhya B.. Heart failure with preserved ejection fraction: a heterogenous disorder with multifactorial pathophysiology. J Am Coll Cardiol 2014;63:457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gould KL, Johnson NP.. Coronary physiology beyond coronary flow reserve in microvascular angina: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2642–2662. [DOI] [PubMed] [Google Scholar]
- 54. Alexander KP, Shaw LJ, DeLong ER, Mark DB, Peterson ED.. Value of exercise treadmill testing in women. J Am Coll Cardiol 1998;32:1657–1664. [DOI] [PubMed] [Google Scholar]
- 55. Youn HJ, Park CS, Moon KW, Oh YS, Chung WS, Kim JH, Choi KB, Hong SJ.. Relation between Duke treadmill score and coronary flow reserve using transesophageal Doppler echocardiography in patients with microvascular angina. Int J Cardiol 2005;98:403–408. [DOI] [PubMed] [Google Scholar]
- 56. Wenzel RR, Fleisch M, Shaw S, Noll G, Kaufmann U, Schmitt R, Jones CR, Clozel M, Meier B, Lüscher TF.. Hemodynamic and coronary effects of the endothelin antagonist bosentan in patients with coronary artery disease. Circulation 1998;98:2235–2240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.