Abstract
Neuregulins (NRGs) are protein ligands that act through ErbB receptor tyrosine kinases to regulate tissue morphogenesis, plasticity, and adaptive responses to physiologic needs in multiple tissues, including the heart and circulatory system. The role of NRG/ErbB signaling in cardiovascular biology, and how it responds to physiologic and pathologic stresses is a rapidly evolving field. While initial concepts focused on the role that NRG may play in regulating cardiac myocyte responses, including cell survival, growth, adaptation to stress, and proliferation, emerging data support a broader role for NRGs in the regulation of metabolism, inflammation, and fibrosis in response to injury. The constellation of effects modulated by NRGs may account for the findings that two distinct forms of recombinant NRG-1 have beneficial effects on cardiac function in humans with systolic heart failure. NRG-4 has recently emerged as an adipokine with similar potential to regulate cardiovascular responses to inflammation and injury. Beyond systolic heart failure, NRGs appear to have beneficial effects in diastolic heart failure, prevention of atherosclerosis, preventing adverse effects on diabetes on the heart and vasculature, including atherosclerosis, as well as the cardiac dysfunction associated with sepsis. Collectively, this literature supports the further examination of how this developmentally critical signaling system functions and how it might be leveraged to treat cardiovascular disease.
Keywords: epidermal growth factor receptor, growth factors, heart failure, myocardial remodeling
Introduction
Neuregulins (NRG) are polypeptide growth factors belonging to the epidermal growth factor family that signal through receptor tyrosine kinases encoded by the erythroblastic leukemia viral oncogene homolog receptor (ErbB) family [1]. ErbB receptor dimerization by NRG activates tyrosine kinases that induce vital pathways in embryogenesis, including cardiac development, neural development, and myogenesis [2–6]. Four NRG genes have been identified, each producing isoforms with specific post-translation modifications that have been demonstrated to regulate tissue morphogenesis and repair via context-specific cellular processes including proliferation, differentiation, survival, apoptosis, and migration.
The neuregulin/ErbB pathway has been a source of interest in cardiovascular biology since the disrupted expression of neuregulin 1 (NRG-1), ErbB2, and ErbB4 all produce an identical lethal phenotype from myocardial trabeculation failure at 10.5 days gestation [2–4]. The clinical cardiovascular relevance of this biology was awakened when Herceptin (Trastuzumab), an ErbB2 (HER-2, c-neu) monoclonal antibody used in breast cancer, was found to be associated with an unacceptably high incidence of left ventricular dysfunction when used with anthracycline therapy [7]. Since those initial reports, NRG-1 has been found to have cardioprotective effects, leading to work examining recombinant NRG-1 as a heart failure therapy with regenerative capabilities. This review aims to discuss the most recent discoveries regarding NRG/ErbB cardiovascular biology, clinical applications in cardiovascular repair, and areas of proposed future investigation.
Neuregulins
NRG-1 is the most extensively studied NRG and is one of four separate genes (Nrg1–Nrg4), each producing unique proteins able to signal through ErbB receptors. The Nrg1 gene can produce multiple different isoforms via alternative splicing [8–10]. The three structural components that differentiate these isoforms include either an α- or β-splice variant of the EGF-like domain, the N-terminal sequence, and a transmembrane domain. The EGF-like domain is the peptide sequence that provides binding specificity to ErbB3 and ErbB4 [11]. The immense diversity of isoforms has been predominantly studied in neuronal development and neuronal plasticity [12,13]. With regards to the adult cardiovascular system, NRG-1 type 1 isoforms are expressed in the microvascular endothelium of the heart, with NRG-1α expressed at higher levels than NRG-1β [14]. The NRG-1 isoforms 1 and 2 with β EGF domains are of critical importance as loss of function of these subunits results in a lethal phenotype from myocardial trabecular failure at 10.5 days of life [4].
NRG-1 subtypes expressed in the heart are pro-NRG transmembrane proteins that require protease processing for activation [14]. Early studies implicated the ADAM family of matrix metalloproteinases (MMPs), specifically ADAM17 and ADAM19, in the release of endothelial membrane pro-NRG-1 [15–18]. Further studies had shown ADAM17 to be the primary protease involved in the release of pro-NRG-1, while ADAM19 has no role [19]. There are likely other proteases that are able to activate pro-NRG-1, as ADAM17 knockout mice do not resemble NRG-1 deficient mice with respect to embryonic heart defects [19]. There remains much that is unknown about what regulates the activity of ADAM17 in relation to NRG-1 shedding from the cardiac endothelium and how these are altered in pathologic states such as heart failure. For example, in a canine tachycardia-induced heart failure model, NRG-1 expression increased without a change in the mRNA expression of ADAM17 [20]. More studies are required to fully decipher how NRG-1 is released from the endothelium during physiologic conditions and cardiovascular stress.
As will be discussed in more detail later, NRGs can be found in the circulation, although whether circulating NRGs are active has been questioned. Endocardial and endothelial cells of the cardiovascular system are the source of NRG-1, which are responsible for cardiac development and cardiac protection from stress, respectively [21]. Of the remaining NRGs, NRG-2, and NRG-4 are believed to have cardiovascular functions, while NRG-3 appears to be expressed predominantly in the central nervous system.
The Nrg2 gene produces the NRG-2 protein that has homology to NRG-1 but has a different activity and different selectivity for ErbB receptors. NRG-2 is expressed in the endocardial lining of the heart with higher expression in the atrium and lower expression in the ventricular outflow tracts [22]. Carraway et al. [22] demonstrated that NRG-1 favored heterodimerization of ErbB3 or ErbB4 with ErbB2 while NRG-2 favored ErbB3 heterodimerization with ErbB1 resulting in different cellular responses. In neuronal cell lines, ADAM10 and BACE2 are the predominant proteases required for proteolytic membrane release [23]. Further investigation is required to assess the role of NRG-2 in the heart.
The Nrg4 gene produces the NRG-4 protein, which was initially thought to have little influence on the cardiovascular system. However, recently NRG-4 serum levels have been associated with insulin resistance [24], metabolic syndrome [25], nonalcoholic fatty liver disease [26], and the severity of atherosclerosis [27]. The Nrg4 gene is capable of producing five splice variants with a wide tissue distribution [28]. The A1 and A2 variants of NRG-4 contain a transmembrane domain and are likely activated by proteolysis while the three B-isoforms lack a transmembrane domain and are likely secreted [29]. NRG-4 selectively binds ErbB4 producing homodimerization or heterodimerization with ErbB1 and ErbB2. NRG-4 was found to have approximately 8-fold lower binding affinity then NRG-1 [30].
ErbB receptors
The four ErbB receptor tyrosine kinases are ErbB1, ErbB2, ErbB3, and ErbB4. Of the four receptors, NRG-1 is known to directly interact with ErbB3 and ErbB4, causing a conformational change allowing homodimerization or heterodimerization with other ErbB receptors. Dimerization allows transphosphorylation catalyzed by the intracellular kinase domains of the receptor complex. ErbB2 has no natural ligand but gains kinase activity through heterodimerization. ErbB3 can bind NRG-1 but has low kinase activity; thus, homodimerization leads to minimal downstream signaling. When NRG binds ErbB4, a conformational change allows homodimerization with another ErbB4 or heterodimerization with ErbB1, ErbB2, or ErbB3 [31–33]. Expression levels of ErbB receptors vary in space (subcellular location and tissue distribution) and in time (e.g., developmental stage, physiologic, and pathologic stress). In embryonic mouse cardiac myocytes, the expression levels of ErbB2 and ErB4 are highest, with a progressive decline in myocyte expression starting early after birth [34]. ErbB3 is expressed in the myocardium at stable levels throughout postnatal development [35,36].
The NRG/ErbB signaling system regulates diverse cellular responses that are context specific. The molecular regulators of this specificity are not fully understood but include several factors such as specific isoforms of NRG-1, ErbB receptor expression, subcellular localization, and coupling to intracellular signaling cascades (ERK 1/2, PI3K/Akt, STAT, and FAK) that ultimately regulate cellular responses based on the cell type (Figures 1 and 2). ErbB2 and ErbB4 in adult mouse ventricular cardiomyocytes are enriched in a specific membrane compartment, the T-tubule system [37]. The T-tubules are invaginations of the sarcolemma that are exposed to the extracellular space and function to link depolarization to intracellular calcium release and subsequent myocyte contraction. ErbB2 and ErbB4 are present at intercalated discs and have a T-tubule like striated pattern [38]. In that context, ErbB2 interacts with FAK, which is also located at the intercalated disc, and NRG activation induces focal adhesion complex formation in adult rat cardiac myocytes [39]. The localization in both sarcolemma T-tubules and intercalated discs suggests that ErbB2 and ErbB4 support the hypothesis that NRG/ErbB signaling in the adult heart regulates myocyte-to-myocyte connectivity. Further investigation is needed to fully understand this and other functions of the NRG/ErbB system in the adult heart.
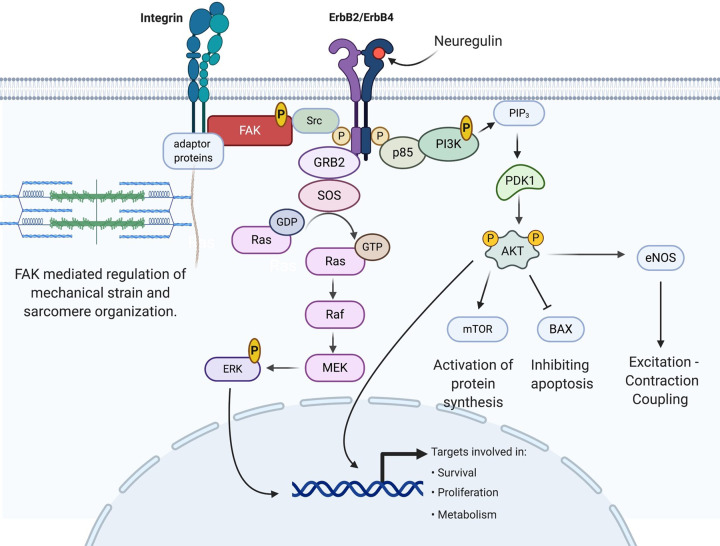
Figure 1. The NRG-1/ErbB signaling pathway in cardiac myocyte.
NRG-1 binds ErbB4 and induces dimerization with ErbB2, allowing receptor tyrosine kinase transphosphorylation. Activated ErbB receptors interact with and activate several pathways including RAS/ERK, PI3K/Akt, and Src/FAK [171–174]. Abbreviations: AKT, serine/threonine-specific protein kinase B; BAX, bcl-2-associated x protein; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GDP, guanosine diphosphate; GTP, guanosine triphosphate; GRB2, growth factor receptor-bound protein 2; MEK, mitogen-activated ERK kinase; mTOR, mammalian target of rapamycin; PDK1, phosphoinositide-dependent kinase 1; PIP3, phosphotidyl inositol (4,5,6)-triphosphate; PI3K, phosphatidyl inositol-3 kinase; p85, regulatory subunit of PI3K; Raf, proto-oncogene serine/threonine-protein kinase; Ras, Ras proteins, members of a large superfamily of small GTPases; SOS, son of sevenless; Src, proto-oncogene tyrosine-protein kinase.
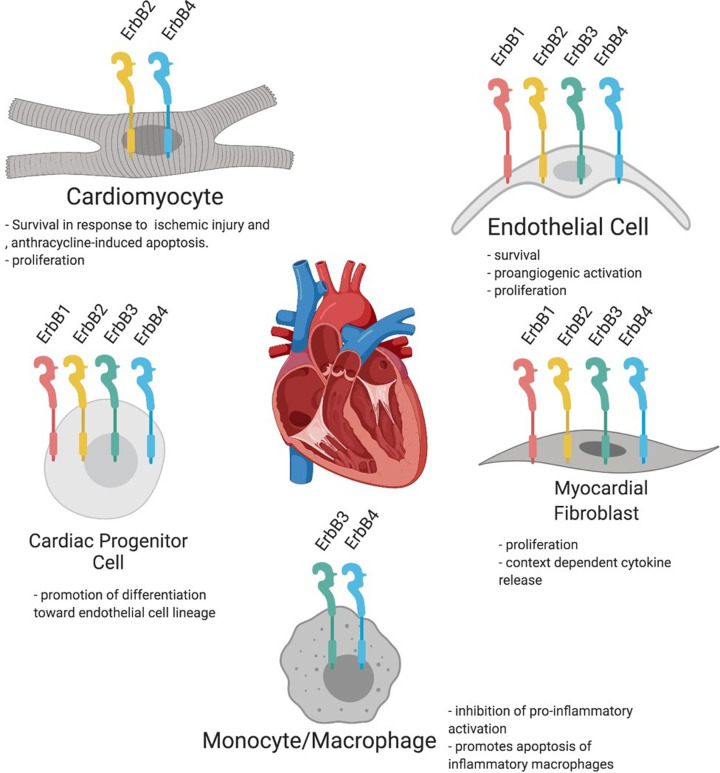
Figure 2. ErbB receptor expression in different subpopulations of cardiac cells.
Adult cardiomyocytes express ErbB2 and ErbB4. Expression of all ErbB receptors, including ErbB1, ErbB2, ErbB3, and ErbB4, has been demonstrated on cardiac endothelial cells, fibroblasts, and highly proliferative cells. ErbB3 and ErbB4 are expressed on monocytes and cardiac macrophages. See the text for references.
Myocardial NRG/ErbB levels in relation to physiologic and pathologic cardiac stress
NRG-1, ErbB2, and ErbB4 are essential for cardiac development as discussed above, as well as the preservation of heart function and adaptation to physiologic and pathologic stresses (Figure 3 and Table 1). NRG-1 appears to be constituvely active at some level, as it can be found in the coronary effluent of isolated hearts [40]. In pregnancy, the heart adapts to physiologic stress induced by an increase in effective circulating volume, leading to left ventricular (LV) growth and eccentric remodeling. Left ventricular tissue samples from pregnant rats have both elevated NRG-1 and activated ErbB2/ErbB4, implicating NRG/ErbB signaling in cardiac adaptation to physiologic stress [41]. Exercise, another physiologic cardiovascular stressor, activates NRG-1, in skeletal muscle [42], and circulating NRG-1 is positively correlated with cardio- respiratory fitness [43]. In a post-myocardial infarction rat model, exercise increases left ventricular tissue levels of NRG-1 as well as ErbB2/ErbB4 activation [44]. These studies all suggest that in adulthood, endogenous NRG/ErbB acts to preserve myocardial function in the setting of physiologic stress. Interestingly, sustained high level of NRG-1 expression in the cerebella throughout life is associated with an increased life span when compared across seven rodent species [45]. In correlation, diminished myocardial NRG-1/ErbB expression has been proposed to be related to age-associated myocardial senescence and loss of cardiomyocytes through apoptosis [46,47] suggesting NRG-1 has a role in the prevention of cardiovascular aging.
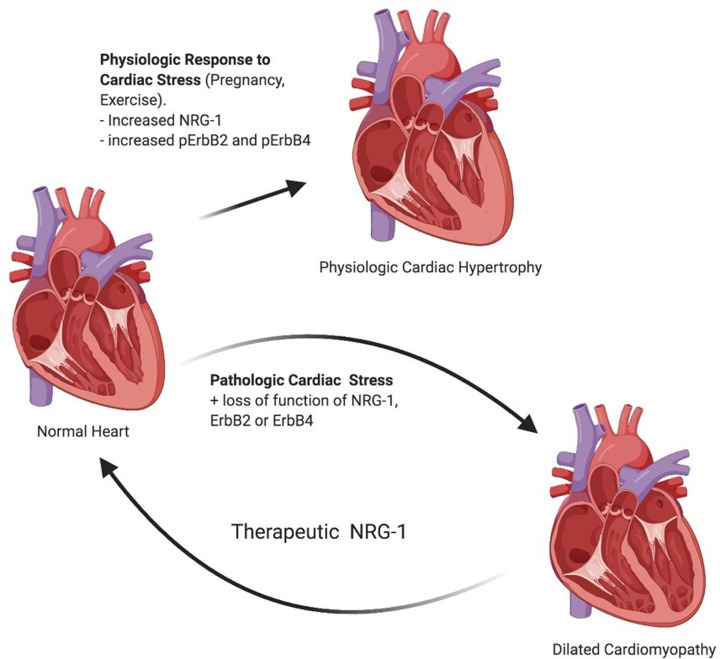
Figure 3. NRG-1 signaling through ErbB2 and ErbB4 regulate growth and survival pathways leading to physiological hypertrophy.
The loss of NRG-1, ErbB2, or ErbB4 results in dilated cardiomyopathy under cardiac stress. In vivo studies have suggested that NRG-1 can improve left ventricular ejection fraction in subjects with heart failure with a reduced ejection fraction. *pErbB2 and pErbB4 represent phosphorylated (activated) forms of ErbB receptors.
Table 1. NRG-1 and myocardial ErbB receptors expression in different types of physiologic and pathologic stress.
| Cardiac stress | NRG expression | Myocardial ERBB expression and activity |
|---|---|---|
| Physiologic: | ||
| Exercise | ↑ NRG1 [42–44,175] | ↑ p-ErbB2 and p- ErB4 [44] |
| Pregnancy | ↑ NRG1 [41] | ↑ p-ErbB2 |
| Early aortic stenosis | No change | No change in ErbB2 and ErbB4 |
| Early pacing induced cardiomyopathy | ↑ NRG1 | ↑ p-ErbB2 and p- ErbB4 with increased PI3K/pAkt and p ERK 1/2. |
| Pathologic: | ||
| Late aortic stenosis | No change [47] | ↓ ErbB2 and ErbB4 [47] |
| Late pacing induced cardiomyopathy | ↑ NRG1 [20] | ↑ p-ErbB2 and ↑ p- ErbB4 but decreased PI3K, pAkt, and p- ERK1/2 [20] |
| Decompensate systolic heart failure | ↑ NRG1 [50] | ↓ ErbB2, p-ErbB2 and ↓ ErbB4,p-ErbB4 [50] |
| Post LVAD implantation | ↓ NRG1 [50] | ↑ErbB2 and ErbB4 [50] |
The activation of ErbB receptors by NRG in vivo has consistently been shown to be protective against pathological stress. Trastuzumab, a monoclonal antibody that disrupts ErbB2 function, is associated with systolic dysfunction and heart failure in patients treated for Her2/Neu positive breast cancer. Cardiomyopathy seen with Trastuzumab treatment was most prevalent when given in combination with anthracyclines [48], suggesting NRG/ErbB regulates the myocardial response to pathologic stress of anthracycline-induced injury. Signaling through the NRG/ErbB pathway diminishes in advanced LV dysfunction, potentially contributing to disease acceleration. Diminished NRG/ErbB signaling occurs in rats with aortic banding. Initially, aortic banding increased expression of NRG, but after persistent stress, LV expression of ErbB2 and ErbB4 decreases as decompensated heart failure develops [47,49]. The correlation between diminished ErbB2/ErbB4 expression and decompensated heart failure was also found when comparing myocardial samples before left ventricular assist device placement (LVAD) with normal unused donor hearts. Patients undergoing LVAD had lower expression of activated ErbB2/ErbB4 despite increased NRG-1 [50]. There is a clear correlation between diminished ErbB2/ErbB4 function and progression to decompensated heart failure, but it is unclear what is affecting ErbB expression. It has been postulated by others that down-regulation of NRG-1/ErbB signaling associated with pump failure is related to increased levels of angiotensin II, and epinephrine [51] as in vitro both angiotensin II and epinephrine have been found to suppress NRG-1 mRNA synthesis in cardiac endothelium [49].
Others have postulated that an inhibitory cross-talk between ErbB receptor tyrosine kinases and G-protein–coupled receptors (GPCR) is responsible for shifting the balance between pathologic and physiologic cardiac hypertrophy. This inhibitory cross-talk in the myocardium has been described by Chung et al. [52] after they found that activation of endothelin type A receptors (ETA) inhibited ErbB2/ErbB4 at multiple levels, including NRG-1 induced ErbB2/ErbB4 autophosphorylation and downstream phosphorylation of Akt. However, studies there are also reports of positive interactions between GPCR and ErbB receptors. GPCR receptors can transactivate ErbB receptors via at least two mechanisms: phosphorylation of ErbB receptors via other tyrosine kinases such as Src [53] and through activation of the metalloproteinases leading to NRG activation [54–57]. Further studies are necessary to understand the role of cross-talk between GPCR and ErbB receptors in the progression of heart failure.
ErbB receptor expression is also regulated by micro-RNAs (miR) in many tissues, and this may also play a role in cardiac pathology. Cardiotoxic anthracycline treatment induces expression of miR-146a in mouse cardiac myocytes, which suppresses ErbB4 expression thereby increasing myocyte damage [58]. Pressure overload increases expression of miR-199a [59], which is suppressed by STAT3 (signal transducer and activator of transcription 3) [60]. Cardiac myocyte STAT3 deficienty in mice leads to cardiomyopathy [61]. Cardiac myocytes from STAT3-deficient mice have reduced ErbB4 as a result of increased miR-199a, resulting in impaired energy utilization and cardiac myocyte death [62]. Further studies are required to assess miRNA regulation of other ErbBs and NRGs.
Circulating neuregulin levels
The levels of circulating NRG-1 and NRG-4 in steady-state conditions have been examined in several studies (Table 2). We and others have found that the level of NRG-1 is characterized by large inter-individual variability with the range of absolute values covering two orders of magnitude, from hundreds to tens of thousands of picograms per milliliter of blood in healthy donors. The analysis of variability revealed the presence of high values of quartile coefficient of dispersion ranging between 0.8 and 0.9 in the blood plasma or serum [43,63]. This large inter-individual variability can explain the significant differences between levels of circulating NRG-1 found in different studies [43,63,64]. In contrast with large inter-individual variability, the individual level of circulating NRG-1 is stable, not changing even after exersice [43] in healthy volunteers. Similar to NRG-1, the levels of circulating NRG-4 also vary significantly with values of quartile coefficient of dispersion between 0.5 and 1.0 [24,65].
Table 2. The levels of circulating NRG-1 and NRG-4 in human cohorts.
| Neuregulins | Mean ± SD | Median [IQR] | Plasma or serum | N | Reference | Major finding |
|---|---|---|---|---|---|---|
| NRG-1, ng/ml | 217 ± 170 | Serum | n=9 | [43] | NRG-1 correlates with exercise capacity | |
| 0.77 ± 0.37 | Serum | n=36 | [64] | NRG-1 correlates with proangiogenic factors, VEGF and Angiopoietin-1 | ||
| 5.3 ± 8.8 | 0.9 [0.6, 7.0] | Platelet-free plasma | n=10 | [63] | Circulating NRG-1 is functionally inactive | |
| 9.0 ± 11.4 | 0.4 | Plasma | n=62 | [176] | NRG levels are reduced after exposure to cardiotoxic chemotherapy | |
| 2.6 [0.2, 19.1]/4.1 [2.0, 12.9] | Serum/plasma | n=21 | [70] | NRG-1 inversely correlates with CAD severity | ||
| 1.4 [0.2, 14.2] | Serum | n=319 | [177] | No changes during acute coronary syndrome | ||
| 4.4 [2.8, 8.7] | Serum | n=899 | [71] | Circulating NRG-1 is associated with heart failure severity and risk of death or cardiac transplantation | ||
| NRG-4, ng/ml | 2.3 [1.1, 3.7] | Serum | n=129 | [65] | The level of circulating NRG-4 is inversely associated with the risk of Type 2 diabetes mellitus | |
| 1.1 ± 0.9 | Serum | n=57 | [78] | Circulating NRG-4 is inversely associated with the risk of acute coronary syndrome | ||
| 0.08 [0, 0.55] | Serum | n=83 | [24] | The circulating NRG-4 is an independent risk factor associated with diabetes | ||
| 4.1 ± 2.0 | Plasma | n=41 | [178] | Circulating level of NRG-4 is reduced in diabetic peripheral neuropathy | ||
| 1.4 ± 0.2 | Plasma | n=32 | [27] | NRG-4 inversely correlates with the severity of CAD | ||
| 1.4 ± 0.1 | Serum | n=24 | [179] | NRG-4 is increased in patients with Type 2 diabetes mellitus |
Several clinical studies have demonstrated that the level of circulating NRG-1 and NRG-4 changes during the development and progression of cardiovascular disease. For example, the level of circulating NRG-1 is increased in patients with paroxysmal atrial fibrillation [66]. While the underlying mechanisms remain to be investigated, the increased level of NRG-1 may be associated with the induction of compensatory mechanisms in response to cardiac stress associated with increased inflammation, pressure overload, and generation of reactive oxygen species [67–69]. Increased level of NRG-1 has also been found in patients with stress-induced ischemia [70], as well as severe heart failure, where it associates with the risk of death or cardiac transplantation [71]. A recent study demonstrated the positive correlation between circulating NRG-1 and left ventricular ejection fraction [72], supporting the idea that NRG-1 is contributing to compensatory cardiac responses.
The levels of both NRG-1 and NRG-4 correlate inversely with the severity of coronary artery disease (CAD) [27,70] and coronary collateral circulation [73]. The potential explanation may include the reduction in the synthesis or secretion of NRG-1 and NRG-4 by endothelial cells [74,75] associated with endothelial dysfunction in CAD [76,77]. In addition, an association between higher NRG-4 level and lower risk of acute coronary syndrome has been described in patients with coronary artery disease [78].
These and other changes in the levels of circulating NRG-1 and NRG-4 indicate regulation of activation and/or expression, but not necessarily changes in circulating activity. Nitration of NRG-1’s EGF-like domain, found in circulating NRG-1, results in the inactivation of the ligand [79]. This may explain our recent finding that circulating NRG-1 is functionally not active in blood obtained from healthy donors [63]. In addition to NRG-1 inactivation, there are circulating NRG-binding proteins such as the endogenous soluble form of the human ErbB3 receptor that may negatively regulate circulating NRG activity [80]. The inactivation of NRGs and the presence of soluble binding partners, which limit the functional activity of NRGs, are adding to the complexity in interpreting the study results and emphasize the importance of functional testing of NRGs in healthy donors and patients with cardiovascular disease, using cell-based assays [63].
Neuregulin in repair of systolic heart failure
The clinical experience with Trastuzumab-associated cardiac dysfunction led to studies designed to understand the role of this signaling system in the adult myocardium [7]. Mice were created with inducible suppression of ErbB2 in cardiac myocytes using Cre-recombinase technology resulting in a dilated cardiomyopathy phenotype exacerbated by anthracyclines [37,81]. An identical dilated cardiomyopathy phenotype was also seen in a Cre-recombinase myocardial specific ErbB4 mouse model [82]. Recombinant NRG-1 was thus examined as a potential therapy for systolic heart failure [83]. The mechanisms of NRG-1’s beneficial effects in cardiac repair in these studies are still being investigated, as the biology of this system in the heart and other tissues is complex and incompletely understood (Figure 4).
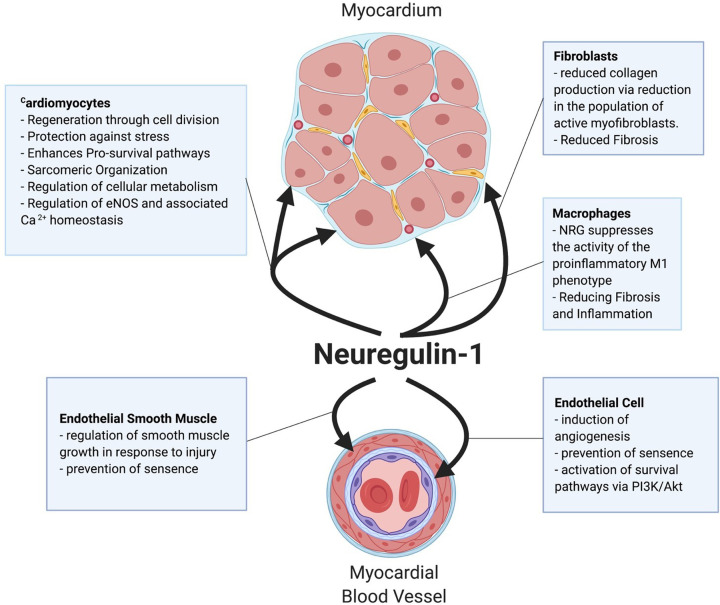
Figure 4. NRG-1 regulates functional responses in different cell types within the myocardium.
Heart injury is associated with the proteolytic activation and release of NRG-1 from the surface of cardiac endothelial cells. Active NRG-1 protects cardiomyocytes, reduces pro-fibrotic activation of fibroblasts, promotes survival of endothelial cells, and angiogenesis, and prevents pro-inflammatory activation of immune cells.
Cardiac myocyte growth, survival, and proliferation
The first evidence of direct effects of NRG-1 in cardiac myocytes was obtained from in vitro experiments using cells isolated from rat hearts [84]. The recombinant human glial growth factor 2 (GGF2), a recombinant protein with the active EGF-binding domain of NRG-1 (see Table 3 for further details), can improve embryonic cardiac myocytes survival in conditions of serum deprivation. Further studies demonstrated pro-survival effects of NRG-1, including protection from norepinephrine cytotoxicity [85]. NRG-1 also protects cardiac myocytes from anthracycline-induced apoptosis via ErbB4-dependent activation of the PI3K/Akt signaling pathway [86]. In addition, the mitogen-activated protein kinase signaling pathway is involved in NRG-1 mediated reprogramming toward enhanced cardiac myocyte growth and survival [87]. Endothelial cell-derived NRG-1 protects cardiomyocytes against hypoxia and reperfusion injury, both in vitro and in vivo [88]. The ability of NRG-1 to promote the survival of cardiomyocytes in response to a wide variety of cardiovascular stresses appears to be a critical role for NRG-1 in the postnatal heart.
Table 3. Neuregulins studied as therapies for systolic heart failure.
| Recombinant forms of NRG | Commercial name | Domains | Examples of in vivo studies | Human studies | Results of human studies |
|---|---|---|---|---|---|
| Human recombinant Neuregulin 1 (rhNRG1β) | Neucardin | β-Isoform of the growth factor domain (EGF- domain) of NRG-1 | Liu et al. [83], Gu et al. [180], Guo et al. [181], Fang et al. [182] | Jabbour et al. [116], Gao et al. [117] | Both phase 1 and 2 trials have been completed finding sustained improvement in LVEF in systolic heart failure patients at 90-day follow-up. A phase 3 trial is currently on going. |
| Human recombinant Neuregulin 1 (rdNRG1β) | None | β-Isoform of the growth factor domain (EGF- domain) of NRG-1 with Ig domain | Bersell et al. [89] | None. | None |
| Human recombinant glial growth factor 2 isoform of NRG1β (GGF2) | Cimaglermin Alfa | β-isoform of the growth factor domain (EGF- domain), type 2 isoform of NRG-1 gene, containing IgG and kringle domains | Bian et al [183], Hill et al. [184], Galindo et al. [95] | Lenihan et al. [118] | A phase 1 trial was completed finding sustained improvement in LVEF in systolic heart failure patients. There was a dose limiting side effect at the highest planned dose. See the text for details. |
| Engineered bivalent NRG1β (bivNRG) | None | 2 β-Isoform of the growth factor domain (EGF- domain) of NRG-1 connected by a linking peptide | Jay et al. [145] | None | None |
Neuregulins, via activation of ErbB receptors, can also stimulate the proliferation of cardiac myocytes in the adult heart. Bersell et al. [89] demonstrated that NRG-1, signaling through ErbB2/ErbB4, can induce mononuclear cardiomyocytes to disassemble their myofibrils, enter the cell cycle, divide and regenerate injured myocardial tissue. Less than 10% of cardiomyocytes are mononuclear, and in vivo, only 0.3% of mononuclear cardiomyocytes under the influence of exogenous NRG-1 underwent division [89] suggesting that pharmacologic NRG-1 produces limited regeneration. D’Uva et al. [36] demonstrated that the manipulation of ErbB2 signaling augments cardiac regenerative properties. Mice with constitutively active cardiomyocyte ErbB2 show significant cardiac hypertrophy, with the cellular division of both mononuclear and binuclear myocardial cells. Mice with constitutively active ErbB2 that underwent myocardial ischemia showed a robust dedifferentiated population of cardiac myocytes in the infarcted tissue associated with improved cardiac function. Polizzotti et al. [90] demonstrated, in mice subjected to cryoinjury, NRG induces cardiac myocyte regeneration in newborn mice, but this response was diminished 5 days post-birth. Similarly, they showed that cardiac myocytes obtained from human infant myocardium respond to NRG in an age-dependent manner, with cell division only seen in cells obtained from younger subjects (<6 months) [90]. Based upon a series of experiments in zebrafish and mice, it appears that NRG-1-dependent cardiac regeneration in early postnatal life is dependent on cardiac innervation [91].
Anti-fibrotic and anti-inflammatory effects of neuregulins
NRG/ErbB signaling appears to regulate inflammation and fibrotic responses to injury in multiple tissues, including the heart. NRG-1’s anti-inflammatory effects were first identified in CNS microglial cells after ischemic insult, where NRG-1 treatment reduced NF-kB mediated expression of TNFα and IL-6 [92]. NRGs appear to regulate the inflammatory response of the heart to injury, though the mechanisms are complex and incompletely understood. NRG-1 has direct anti- inflammatory effects in nonclassical monocytes in humans, acting via ErbB3, reducing TNFα production [93]. NRG-1 regulates macrophage phenotype, increasing the CD206 positive ‘regenerative phenotype’ when administered into a rat heart after myocardial infarction. This may relate to observations in other inflammatory diseases, including inflammatory bowel disease, where colonic macrophages exposed to inflammatory stimuli increased ErbB4 expression. NRG-4 administration reduced the number of colonic macrophages via induction of macrophage apoptosis and attenuated inflammation [94].
The effects of NRG-1 on the inflammatory response to injury are likely linked to the reduced fibrosis observed in several experimental systems. In left ventricular samples of post-MI swine treated with GGF2, there was more organized myofibril structure, reduced fibrosis, attenuated expression of pro-fibrotic genes, and a reduced number of myofibroblasts [95]. Similar anti-fibrotic effects were reported in a mouse model where treatment with recombinant human NRG1β (rhNRG-1β) attenuated the adverse effects of angiotensin II on cardiac hypertrophy by inhibiting fibrosis and activation of myocardial fibroblasts [96]. In mice with ErbB4-deficient macrophages, angiotensin exposure resulted in worse myocardial fibrosis, suggesting that active NRG/ErbB4 attenuates the fibrosis effects of fibroblasts [96]. An additional study found NRG-1 to have anti-fibrostic effects by suppressing macrophage activation and preventing bleomycin-induced fibrosis in the heart, lung, and skin tissue in vivo [96].
All of these data together provide strong support for the role of NRG-1 signaling through ErbB4 and ErbB3 in the control of myeloid cell activation, reduction of the number of pro-inflammatory macrophages via targeted apoptosis and suppressing the release of pro-inflammatory cytokines in multiple tissues. Because myocardial fibrosis and remodeling are leading molecular mechanisms in the progression of systolic heart failure, NRGs ability to prevent inflammation and pro-fibrotic transformation has substantial translational implications, providing a rationale for the use of NRGs as a therapy in systolic heart failure.
Regulation of remodeling via neuregulin's activation of focal adhesion kinase
Focal adhesion kinase (FAK) is a nonreceptor tyrosine kinase that is critical for the transmission of mechanical strain into biochemical signals that regulate cardiac myocyte growth and survival [97,98]. GGF2 treatment of adult rat ventricular myocytes induced formation of a multiprotein complex containing ErbB2, FAK p130CAS, and paxillin, which induces lamellipodia formation and myocyte elongation that ultimately restored myocyte cell-to-cell contact and synchronous beating [39]. Increased mechanical load on the heart results in increased tension and stretch on cardiomyocytes, in association with activation of FAK [99] and NRG/ErbB [20]. Complete knockout of the FAK protein results in embryonic lethality, and myocardial specific Cre-recombinase deletion results in a dilated cardiomyopathy after exposure to angiotensin II [100]. An important part of normal physiologic cardiac remodeling is maintaining optimal sarcomere length despite cardiac myocyte growth and lengthening [97,101]. The physiologic growth of the heart during pre- and postnatal maturation [34] or during pregnancy is regulated by NRG/ErbB signaling [41]. An attractive hypothesis emerges from this work is that load-dependent NRG/ErbB regulation of FAK orchestrates the normalization of sarcomere load via cardiac myocyte growth, and perhaps cell division, as discussed previously. Similarly, the reparative effects of NRGs in systolic heart failure may in part be mediated by ErbB2 activation of FAK. These results support the hypothesis that NRG/ErbB signaling through FAK regulates mechanical strain-induced pathologic eccentric hypertrophy [102].
NRG/ErbB regulation of angiogenesis
Angiogenesis plays a critical role in myocardial development, growth, and recovery from injury. ErbB1, ErbB2, ErbB3, and ErbB4 are expressed on endothelial cells (Figure 2), and NRG-1 can regulate their proliferation, function, and participation in angiogenesis. NRG-1 induces the growth of vascular endothelial cells in vitro in a manner independent of VEGF [103]. NRG-1 treatment reverses diabetic vascular injury by increasing myocardial capillary density and increasing myocardial blood flow in mice [104]. In ischemic injury, NRG-1 treatment increases capillary density within the peri-infarct region [83]. These and other studies highlight the ability of NRG-1/ErbB signaling to augment myocardial recovery by increasing angiogenetic potential.
The downstream signaling mechanisms by which NRG-1 induces angiogenesis are not completely clear. Recent literature demonstrates that ErbB2 heterodimerizes with neuropilin (Nrp1) on cardiac endothelium to form a functional receptor for a vascular guidance molecule semaphorin 3d, leading to the development of coronary veins [105]. Nrp1 is a transmembrane protein that interacts with co-receptors and has been shown to regulate angiogenesis and endothelial sprouting [106]. While Nrp1 is required for the embryonic vascular morphogenesis, it also has a role in the regulation of angiogenesis in adults [107]. The recent discovery of Nrp1’s dimerization of ErbB2 did not test NRGs ability to induce angiogenesis but further supports ongoing investigation into the ErbB-dependent regulation of angiogenesis. NRG-1 induces increased production and release of VEGF by endothelial cells in vivo [104]. However, in an in vivo model of corneal angiogenesis, NRG-1 induces angiogenesis independent of VEGF, suggesting that NRG is functionally angiogenic. Hedhli et al. [21] developed an endothelial-specific inducible NRG-1 knockout mouse. Mice lacking endothelial NRG-1 had decreased blood flow in response to femoral artery ischemic injury due to reduced angiogenesis that recovered with exogenous NRG-1. NRG-1 has therapeutic potential in ischemic cardiomyopathy as it can stimulate angiogenesis in post-infarction myocardium and could be studied as a therapeutic in stable coronary artery disease to induce collateralization [21,103,108].
NRG-1 can also regulate angiogenesis via the prevention of apoptosis of endothelial progenitor cells [109]. We have recently demonstrated that human cardiac highly proliferative cells are characterized by the expression of all four ErbB receptors [110]. These cells are characterized by high proliferative activity in vitro, and have been shown to possess progenitor properties. NRG-1-dependent stimulation of ErbB receptors of these cells induces their differentiation toward endothelial cell lineage, providing an additional mechanism by which NRG-1 regulates cardiac microvasculature formation.
Regulation of cardiac myocyte metabolism
NRG-1 regulates glucose uptake into skeletal muscle via GLUT4 [111] and cardiac myocytes in a manner independent of insulin [112]. NRG-1β induces glucose uptake in cardiac myoctyes [14] via activation of the PI3K/Akt pathway [113]. In animal models of heart failure, recombinant forms of NRG-1 induce glucose uptake by induction of glycolytic enzyme expression and suppression of oxidative phosphorylation in rhesus monkeys [114], mice [115], and in swine; this leads to symptomatic hypoglycemia [95]. In human phase I and II trials, NRG-1 did not show hypoglycemic effects [116–118]. The effects of NRG-1 on glucose uptake, as well as hepatocyte gluconeogenesis, have led to the concept that NRG-1 may have therapeutic potential in the treatment of Type 2 diabetes mellitus [119–121].
NRG’s effects on cardiac myocyte metabolism appear to be essential for its regenerative properties. Honkoop et al. [122] used a zebrafish model of cardiac regeneration to demonstrate that proliferating border zone cardiac myocytes have a very distinct transcriptome compared with nonproliferative cardiac myocytes in remote areas, with a definite shift toward anaerobic respiration. NRG-1/ErbB2 signaling induced this metabolic reprogramming, and the change toward anaerobic respiration was essential for myocardial regeneration [122].
The ability of the myocardium to utilize both fats and carbohydrates is well established, with oxidative phosphorylation being dominant in the adult myocardium [123–125]. The progression to decompensated heart failure is associated with a shift to anaerobic respiration, which is thought to be pathogenic [126–130]. Thus, the NRG-1 associated shift to anaerobic metabolism in the zebrafish model would seem to be maladaptive. This may be context or species-specific, as NRG-1 stimulation of adult rat cardiomyocytes increases the expression of many genes involved in mitochondrial β-oxidation [131]. Similarly, NRG-1 induces oxidative phosphorylation in cardiomyocytes generated from human embryonic stem cells [132]. Treatment of post-MI rats reverses the pathologic changes in metabolism transcriptome [115]. In post-MI swine, similar effects were seen along with normalization of mitochondrial structure as seen by transmission electron microscopy [95]. Although the exact mechanisms of improved mitochondrial function in cardiomyocytes requires further investigation, NRG-1 acts on complex 2-mediated mitochondrial respiration in skeletal muscles [133]. Incorporating noninvasive metabolic imaging [134] into future clinical trials with recombinant NRG-1 in heart failure may help to sort out to what extent changes in myocardial metabolism are mechanistic in humans.
Clinical trials of recombinant neuregulins in heart failure
Two forms of recombinant NRG-1β have been studied in clinical trials to examine the potential for a therapeutic effect in systolic heart failure (Table 3). Jabbour et al. [116] administered the EGF domain-only form of rhNRG-1β (Neucardin) to patients with chronic systolic heart failure and observed a sustained improvement in ejection fraction 84 days after therapy was completed. A nonsustained acute increase in cardiac output with rhNRG-1β infusion [135] was observed, likely due to a vasodilatory response, as rhNRG-1β has negative inotropic effects on isolated myocytes [136–138]. A phase II trial was completed by Gao et al. [117] where chronic systolic heart failure (EF<40%) patients were administered increasing doses of rhNRG-1β again producing sustained improvement in ejection fraction at the 90 days follow up. A phase 3 study with the same form of NRG-1 has been presented in abstract form reporting a mortality benefit in systolic heart failure patients [139]. An ongoing phase 3 trial will provide further insight into the potential mortality benefit with NRG-1 in systolic heart failure (Clinical Trials ID#: NCT03388593).
The larger rhNRG-1 GGF2 (Cimaglermin Alfa) has been studied in a phase 1 trial, where a single dose was associated with a sustained improvement in LVEF after 90 days in patients with chronic systolic heart failure. The most common side effect with both forms of rhNRG-1β was nausea. The GGF2 study reached its safety endpoint after one patient at the highest planned dose of GGF2 developed transient hyperbilirubinemia and elevated aminotransferases [118]. The mechanism for nausea, as well as the transient transaminitis most likely is due to on-target effects of the recombinant NRG, given the wide tissue distribution of ErbB receptors. Mosedale et al. [140] examined the impacts of GGF2 on the liver and found no evidence of hepatocyte necrosis but did find ´marked’ hyperplasia and hypertrophy of bile duct epithelium. Studies in cultured hepatocytes showed that GGF2 treatment changes the expression of genes regulating bilirubin metabolism and transport. While a major concern in the development of rhNRG-1β as a cardiac therapy has been the promotion of tumor growth given the well-established role of ErbB2 in oncogenesis [141], such an effect has not been reported in any trials to date.
Motivated by the potential oncogenic effects, a recombinant form of NRG that circumvents ErbB2 has been developed and studied in preclinical models. A bivalent neuregulin (bivNRG) containing two identical amino acid sequences, corresponding to the ErbB receptor binding domain, was created by linking them through a hydrophilic, protease-resistant spacer [142]. BivNRG promotes the dimerization (homo- and hetero-) of ErbB3 and ErbB4 receptors, inducing their transphosphorylation and activation. In cancer tissues, ErbB3 receptors often interact with ErbB2 [143] to form the most biologically active heterodimer activating the PI-3K/Akt signaling pathway [144]. Through the induction of minimally active ErbB3 homodimer formation at the expense of the more potently active ErbB2/ErbB3, bivNRG has a cytostatic effect in cancers. Through the induction of highly active ErbB4 homodimers, bivNRG retains its cardioprotective effects [145]. This unique property makes bivNRG a promising candidate for the treatment of anthracycline-induced cardiomyopathy. BivNRG and NRG-1 have similar cardioprotective effects during cardiac myocyte exposure to doxorubicin, while bivNRG, but not NRG-1, showed a growth-suppressive effect in a neoplastic cell line expressing ErbB2 and ErbB3 receptors [145]. Whether bivNRG will induce cardiac repair in other circumstances, where ErbB3/ErbB2 activation may be necessary for antifibrotic, angiogenic, and anti-inflammatory effects discussed previously will require further investigation.
Neuregulins in atherosclerosis
There is growing evidence that NRG maintains normal vascular function via signaling in endothelial cells, smooth muscle cells, and macrophages, and disruptions in this signaling system may be involved in atherosclerosis. Circumstantial evidence comes from human studies, with cell and animal studies providing more mechanistic information. Immunohistochemistry analysis of human coronary atherosclerotic lesions revealed an increased expression of NRG-1 in macrophages [146]. In a cohort of subjects with angiographic evidence of coronary disease, reduced levels of circulating NRG-1 was associated with the severity of disease [70]. Xu et al. [147] reported that NRG-1 reduced cholesterol ester accumulation in monocyte-derived macrophages in a dose-dependent manner in vitro, and administration of rhNRG-1 in ApoE-/- mice produced significantly reduced aortic atheromatous plaque surface area. In addition to the lipid regulation, NRG has anti-inflammatory effects mediated by ErbB4 on macrophages. Macrophages exposed to inflammatory cytokines increase their expression of ErbB4 (but not ErbB1, ErbB2 or ErbB3) and subsequent exposure to NRG-4 induces apoptosis [94]. NRG-1 suppresses macrophage cytokine release and subsequent myocardial fibrosis induced by angiotensin II [96]. NRG-1 has protective effects against oxidative stress-induced senescence in aortic endothelial and smooth muscle cells [148]. This could be of particular importance as the underlying pathophysiology of acute coronary syndrome is related to the thinning and rupture of the endothelium-smooth muscle layer over fatty atheromatous plaques [149]. All these studies taken together provide strong evidence that NRG has anti-inflammatory properties attributed to modulated macrophage function, and it could be a potential therapy to prevent or treat atherosclerosis.
Neuregulin 4 and its potential role in linking metabolic syndrome, inflammation, and coronary artery disease
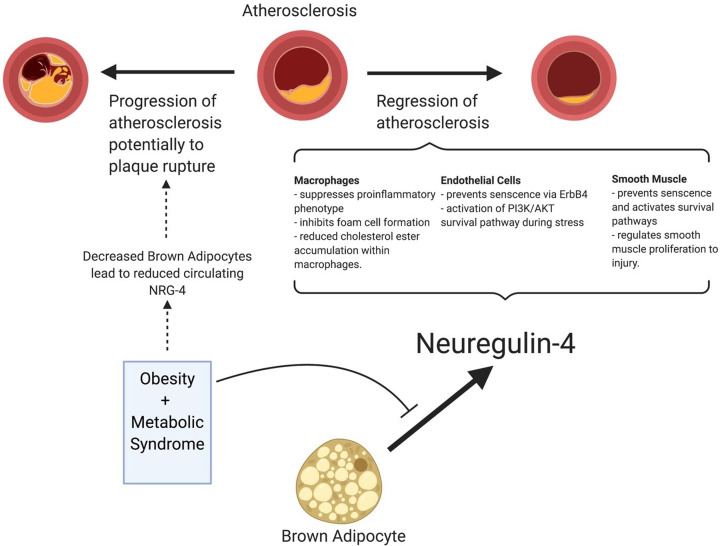
Recently, NRG-4 has become an area of interest within endocrinology because of its association with obesity and the progression to diabetes. NRG-4 was first identified in 1999 by Harari et al. [30] where they found it to be highly expressed in the pancreas, adipose tissue, and skeletal muscle. Interestingly, the EGF domain of NRG-4 is highly selective for ErbB4 as opposed to NRG-1, which interacts with both ErbB3 and ErbB4 [30]. NRG-4 is expressed in brown adipose tissue and acts in an endocrine-like function on hepatocyte regulation of gluconeogenesis and lipogenesis [150]. Several studies have found an inverse relationship between circulating NRG-4 and metabolic syndrome [25,26,151]. In addition, it has been shown that low levels of NRG-4 are an early marker of insulin resistance [24,152]. Reduced levels of NRG-4 have been associated with increased carotid intimal thickness [153], higher high-sensitivity c-reactive protein [154], increased angiographic severity of coronary artery disease [27], and acute coronary syndrome [155], suggesting that NRG-4 is an adipokine that mediates the link between metabolic syndrome and atherosclerosis (Figure 5).
Figure 5. NRG-4 protects against atherosclerosis progression.
NRG-4 is released from brown adipose tissue and reduces pro-inflammatory activation of tissue-resident macrophages and their differentiation into foam cells. NRG-4 promotes endothelial cell survival and prevents smooth muscle cell senescence, both of which suppress atherogenesis.
As discussed previously, there are many known risk factors of atherosclerosis but few known associations between the progression of atherosclerosis to plaque rupture events. NRG-4, the ErbB4 selective ligand, has been highlighted throughout this review to have beneficial effects on cardiac myocytes and the endothelium while having anti-inflammatory effects on macrophages leading to decreased tissue fibrosis and decreased atherosclerotic plaque burden. Further prospective studies are needed to investigate the role of NRG-4 in the progression of CAD.
Neuregulin in diastolic heart failure
There are very few clinical strategies that effectively treat diastolic heart failure despite numerous trials [156,157]. NRG-1 has been postulated to be a therapy for a subtype of diastolic heart failure—diabetic cardiomyopathy [158]. The Framingham Study established that men and women with diabetes are at higher risk for developing heart failure after adjusting for comorbid conditions [159]. Diabetic cardiomyopathy is often accompanied by comorbidities, including coronary artery disease, hypertension, autonomic neuropathy, and microvascular dysfunction. However, diabetes is independently associated with progressive left ventricular remodeling and dysfunction characteristic of diastolic heart failure [160]. There are a multitude of neurohormonal factors that influence the pathophysiology of diabetic cardiomyopathy, but decreased myocardial compliance is a hallmark [160]. NRG-1 has consistently been shown to increase myocardial compliance [96,161]. It has been suggested that mechanisms of NRG-1 mediated changes in compliance are attributed to calcium homeostasis [138], reduced inflammatory response and resulting fibrosis [96], and direct effects on sarcomeric tension [162].
Rats with diabetes, induced by streptozotocin, have impaired left ventricular function by echocardiography accompanied by significant fibrosis [163]. The level of NRG-1 in the hearts of diabetic rats is decreased along with reduced phosphorylation of ErbB2 and ErbB4 [163], suggesting that diminished levels of NRG-1 could be associated with the development of diastolic heart failure in diabetic cardiomyopathy. Animal models have suggested diabetes mellitus Type 2 (DM2) is associated with altered phosphorylation of titin, leading to reduced compliance [164,165]. The phosphorylation balance of a specific region of titin determines the compliance of the cardiac myocyte sarcomere. Hopf et al. [162] demonstrated rhNRG-1 modified the phosphorylation of titin in diabetic mice and found that rhNRG-1 could rapidly decrease the end-diastolic pressure–volume relationship (indicating improved diastolic relaxation) in Zucker diabetic obese rats [158]. The significance of this study is the suggestion that NRG-1 can revert the metabolic effects of insulin resistance on the sarcomere and supports the role of NRG-1 in the treatment of diabetic cardiomyopathy.
Activation of the pro-inflammatory immune cells, including macrophages, has been implicated in the pathogenesis of diabetic cardiomyopathy [160]. As previously mentioned in this review, NRG-1 and NRG-4, acting through ErbB3 and ErbB4 have been shown to induce apoptosis of the pro-inflammatory macrophages and reduce pro-inflammatory activation of myeloid cells. NRGs ability to modulate chronic inflammation in diabetic cardiomyopathy may also be an additional therapeutic mechanism.
Neuregulin in prevention of septic cardiomyopathy
Sepsis is a condition with life-threatening organ dysfunction caused by a dysregulated host response to infection [166]. Sepsis is characterized early on by an increase in cardiac output, but in some cases of sepsis, there is transient cardiomyopathy with reduced ejection fraction. [167]. Based on the observation of reduced NRG-1 in neuromuscular junctions during sepsis, Zhou et al. [168] suggested that exogenous NRG-1 may be beneficial during sepsis. They found that treatment of rats with rhNRG-1 improved survival. Hemodynamic measurements revealed improved mean arterial pressure, isovolumetric relaxation, and decreased left ventricular end-diastolic pressure accompanied by reduced levels of troponin, TNF-ɑ, IL-1β, and IL-6 in the NRG-1 treatment group [168]. The reduction of inflammatory cytokines and improved mortality suggest that NRG-1 has protective effects in septic cardiomyopathy via down-regulation of immune response-related cardiac injury. Kang et al. [169] further investigated the effects of NRG-1 in sepsis by examining the impact in endothelial cells where they demonstrated an inhibitory effect of NRG-1 on ICAM-1, VEGF, and nitric oxide, factors which increase in sepsis. The investigators also found that NRG-1 inhibited RhoA/ROCK signaling, which has previously been shown to promote endothelial cell shedding and increase vascular permeability [169]. Along with improvements in cardiac dysfunction in sepsis, NRG-1 appears to protect endothelial cell injury and the resulting loss of barrier function that contributes to multi-organ failure [170].
Conclusions
NRGs have therapeutic effects in multiple forms of cardiac disease through direct actions on cardiac myocytes, endothelial cells, inflammatory cells, and fibroblasts. Recent literature has expanded our knowledge of NRGs regulation of metabolism in multiple tissues, implicating NRGs as potential links between metabolic syndrome, diabetes, and coronary artery disease. The mechanisms by which NRG/ErbB signaling is cardioprotective have been elucidated, but the mechanisms by which these cardioprotective pathways are regulated in vivo remain unanswered. Promising clinical trial results with two distinct forms of recombinant NRG-1 in systolic heart failure support further investigation of this biologic therapy. Future NRG research is warranted that focuses on NRG-4/ErbB4 due to new literature suggesting the connection between metabolic syndrome, diabetes, and coronary artery disease. Acute coronary syndrome remains a plague on the cardiology community as we have excellent risk stratification tools for the development of CAD but no means to predict who is at risk for plaque rupture events and the deadly consequences. NRG-4 could be a biomarker for the risk of ACS as well as a potential therapy. NRG is protective of cardiovascular systolic dysfunction, and the literature supports a protective role of NRG in CAD, diastolic heart failure, diabetes, and inflammatory conditions affecting the heart. There remain many questions regarding the differential response between different NRGs and ErbB combinations across different tissue types and understanding these pathways in greater detail has the potential to greatly expand the clinical utility of NRG (Figure 6).
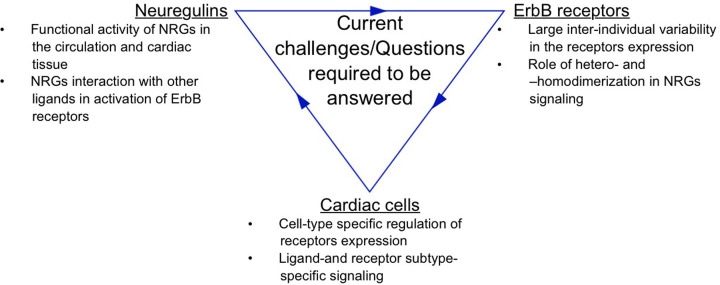
Figure 6. Current challenges and questions.
Large inter-individual variability in both NRG’s and ErbB receptors expression, the presence of active and inactive forms of NRG's, and their interaction with different ErbB receptors expressed on a variety of cardiac cells form a complex NRG/Erbb signaling network. A better understanding of cell-type- specific effects mediated by NRG’s in the heart is necessary for the development of cardioprotective therapies.
Abbreviations
- CAD
coronary artery disease
- ETA
endothelin type A receptors
- FAK
focal adhesion kinase
- NRG
neuregulin
- Nrp1
neuropilin
- STAT3
signal transducer and activator of transcription 3
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Riese D.J. II, van Raaij T.M., Plowman G.D., Andrews G.C. and Stern D.F. (1995) The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol. Cell. Biol. 15, 5770–5776 10.1128/MCB.15.10.5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gassmann M., Casagranda F., Orioli D. et al. (1995) Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378, 390–394 10.1038/378390a0 [DOI] [PubMed] [Google Scholar]
- 3.Lee K.F., Simon H., Chen H., Bates B., Hung M.C. and Hauser C. (1995) Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378, 394–398 10.1038/378394a0 [DOI] [PubMed] [Google Scholar]
- 4.Meyer D. and Birchmeier C. (1995) Multiple essential functions of neuregulin in development. Nature 378, 386–390 10.1038/378386a0 [DOI] [PubMed] [Google Scholar]
- 5.Burden S. and Yarden Y. (1997) Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18, 847–855 10.1016/S0896-6273(00)80324-4 [DOI] [PubMed] [Google Scholar]
- 6.Fischbach G.D. and Rosen K.M. (1997) ARIA: a neuromuscular junction neuregulin. Annu. Rev. Neurosci. 20, 429–458 10.1146/annurev.neuro.20.1.429 [DOI] [PubMed] [Google Scholar]
- 7.Seidman A., Hudis C., Pierri M.K. et al. (2002) Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 20, 1215–1221 10.1200/JCO.2002.20.5.1215 [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Bates R., Yin D.M. et al. (2011) Specific regulation of NRG1 isoform expression by neuronal activity. J. Neurosci. 31, 8491–8501 10.1523/JNEUROSCI.5317-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ledonne A. and Mercuri N.B. (2019) On the Modulatory Roles of Neuregulins/ErbB Signaling on Synaptic Plasticity. Int. J. Mol. Sci. 21, 10.3390/ijms21010275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falls D.L. (2003) Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 284, 14–30 10.1016/S0014-4827(02)00102-7 [DOI] [PubMed] [Google Scholar]
- 11.Jones J.T., Ballinger M.D., Pisacane P.I. et al. (1998) Binding Interaction of the Heregulinβ egf Domain with ErbB3 and ErbB4 Receptors Assessed by Alanine Scanning Mutagenesis. J. Biol. Chem. 273, 11667–11674 [DOI] [PubMed] [Google Scholar]
- 12.Mei L. and Xiong W-C. (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 9, 437–452 10.1038/nrn2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer D., Yamaai T., Garratt A. et al. (1997) Isoform-specific expression and function of neuregulin. Development 124, 3575–3586 [DOI] [PubMed] [Google Scholar]
- 14.Cote G.M., Miller T.A., Lebrasseur N.K., Kuramochi Y. and Sawyer D.B. (2005) Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp. Cell Res. 311, 135–146 10.1016/j.yexcr.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 15.Montero J.C., Yuste L., Díaz-Rodríguez E., Esparís-Ogando A. and Pandiella A. (2000) Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol. Cell. Neurosci. 16, 631–648 10.1006/mcne.2000.0896 [DOI] [PubMed] [Google Scholar]
- 16.Shi W., Chen H., Sun J. et al. (2003) TACE is required for fetal murine cardiac development and modeling. Dev. Biol. 261, 371–380 10.1016/S0012-1606(03)00315-4 [DOI] [PubMed] [Google Scholar]
- 17.Shirakabe K., Wakatsuki S., Kurisaki T. and Fujisawa-Sehara A. (2001) Roles of Meltrin beta /ADAM19 in the processing of neuregulin. J. Biol. Chem. 276, 9352–9358 10.1074/jbc.M007913200 [DOI] [PubMed] [Google Scholar]
- 18.Kurohara K., Komatsu K., Kurisaki T. et al. (2004) Essential roles of Meltrin beta (ADAM19) in heart development. Dev. Biol. 267, 14–28 10.1016/j.ydbio.2003.10.021 [DOI] [PubMed] [Google Scholar]
- 19.Horiuchi K., Zhou H.M., Kelly K., Manova K. and Blobel C.P. (2005) Evaluation of the contributions of ADAMs 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins beta1 and beta2. Dev. Biol. 283, 459–471 10.1016/j.ydbio.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Doggen K., Ray L., Mathieu M., Mc Entee K., Lemmens K. and De Keulenaer G.W. (2009) Ventricular ErbB2/ErbB4 activation and downstream signaling in pacing-induced heart failure. J. Mol. Cell Cardiol. 46, 33–38 10.1016/j.yjmcc.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 21.Hedhli N., Huang Q., Kalinowski A. et al. (2011) Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation 123, 2254–2262 10.1161/CIRCULATIONAHA.110.991125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carraway K.L. III, Weber J.L., Unger M.J. et al. (1997) Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature 387, 512–516 10.1038/387512a0 [DOI] [PubMed] [Google Scholar]
- 23.Czarnek M. and Bereta J. (2020) Proteolytic Processing of Neuregulin 2. Mol. Neurobiol. 57, 1799–1813 10.1007/s12035-019-01846-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L.L., Peng M.M., Zhang J.Y. et al. (2017) Elevated circulating Neuregulin4 level in patients with diabetes. Diabetes Metab. Res. Rev. 33, 10.1002/dmrr.2870 [DOI] [PubMed] [Google Scholar]
- 25.Yan P., Xu Y., Wan Q. et al. (2018) Plasma Neuregulin 4 Levels Are Associated with Metabolic Syndrome in Patients Newly Diagnosed with Type 2 Diabetes Mellitus. Dis. Markers 2018, 6974191 10.1155/2018/6974191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Y.N., Zhu J.Z., Fang Z.Y. et al. (2015) A case-control study: Association between serum neuregulin 4 level and non-alcoholic fatty liver disease. Metabolism 64, 1667–1673 10.1016/j.metabol.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 27.Tian Q.P., Liu M.L., Tang C.S., Xue L., Pang Y.Z. and Qi Y.F. (2019) Association of Circulating Neuregulin-4 with Presence and Severity of Coronary Artery Disease. Int. Heart J. 60, 45–49 10.1536/ihj.18-130 [DOI] [PubMed] [Google Scholar]
- 28.Hayes N.V., Blackburn E., Boyle M.M. et al. (2011) Expression of neuregulin 4 splice variants in normal human tissues and prostate cancer and their effects on cell motility. Endocr. Relat. Cancer 18, 39–49 10.1677/ERC-10-0112 [DOI] [PubMed] [Google Scholar]
- 29.Hayes N.V. and Gullick W.J. (2008) The neuregulin family of genes and their multiple splice variants in breast cancer. J. Mammary Gland Biol. Neoplasia 13, 205–214 10.1007/s10911-008-9078-4 [DOI] [PubMed] [Google Scholar]
- 30.Harari D., Tzahar E., Romano J. et al. (1999) Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene 18, 2681–2689 10.1038/sj.onc.1202631 [DOI] [PubMed] [Google Scholar]
- 31.Yarden Y. and Sliwkowski M.X. (2001) Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 32.Carraway K.L. 3rd and Cantley L.C. (1994) A neu acquaintance for erbB3 and erbB4: a role for receptor heterodimerization in growth signaling. Cell 78, 5–8 10.1016/0092-8674(94)90564-9 [DOI] [PubMed] [Google Scholar]
- 33.Sweeney C., Fambrough D., Huard C. et al. (2001) Growth factor-specific signaling pathway stimulation and gene expression mediated by ErbB receptors. J. Biol. Chem. 276, 22685–22698 10.1074/jbc.M100602200 [DOI] [PubMed] [Google Scholar]
- 34.Ma H., Yin C., Zhang Y., Qian L. and Liu J. (2016) ErbB2 is required for cardiomyocyte proliferation in murine neonatal hearts. Gene 592, 325–330 10.1016/j.gene.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campreciós G., Lorita J., Pardina E., Peinado-Onsurbe J., Soley M. and Ramírez I. (2011) Expression, localization, and regulation of the neuregulin receptor ErbB3 in mouse heart. J. Cell. Physiol. 226, 450–455 10.1002/jcp.22354 [DOI] [PubMed] [Google Scholar]
- 36.D'Uva G., Aharonov A., Lauriola M. et al. (2015) ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol. 17, 627–638 10.1038/ncb3149 [DOI] [PubMed] [Google Scholar]
- 37.Ozcelik C., Erdmann B., Pilz B. et al. (2002) Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Natl. Acad. Sci. USA 99, 8880–8885 10.1073/pnas.122249299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pentassuglia L., Arteaga C. and Sawyer D. (2008) Neuregulin 1β/erbB signalling in cardiac myocytes enhances integrin based myocyte-matrix interactions via src. FASEB J. 22, 820.2–820.2 [Google Scholar]
- 39.Kuramochi Y., Guo X. and Sawyer D.B. (2006) Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J. Mol. Cell Cardiol. 41, 228–235 10.1016/j.yjmcc.2006.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuramochi Y., Cote G.M., Guo X. et al. (2004) Cardiac endothelial cells regulate reactive oxygen species- induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J. Biol. Chem. 279, 51141–51147 10.1074/jbc.M408662200 [DOI] [PubMed] [Google Scholar]
- 41.Lemmens K., Doggen K. and De Keulenaer G.W. (2011) Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am. J. Physiol. Heart Circ. Physiol. 300, H931–H942 10.1152/ajpheart.00385.2010 [DOI] [PubMed] [Google Scholar]
- 42.Lebrasseur N.K., Cote G.M., Miller T.A., Fielding R.A. and Sawyer D.B. (2003) Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am. J. Physiol. Cell Physiol. 284, C1149–C1155 10.1152/ajpcell.00487.2002 [DOI] [PubMed] [Google Scholar]
- 43.Moondra V., Sarma S., Buxton T. et al. (2009) Serum Neuregulin-1beta as a Biomarker of Cardiovascular Fitness. Open Biomark J. 2, 1–5 10.2174/1875318300902010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai M.X., Shi X.C., Chen T. et al. (2016) Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 149, 1–9 10.1016/j.lfs.2016.02.055 [DOI] [PubMed] [Google Scholar]
- 45.Edrey Y.H., Casper D., Huchon D. et al. (2012) Sustained high levels of neuregulin-1 in the longest-lived rodents; a key determinant of rodent longevity. Aging Cell 11, 213–222 10.1111/j.1474-9726.2011.00772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohrbach S., Niemann B., Abushouk A.M. and Holtz J. (2006) Caloric restriction and mitochondrial function in the ageing myocardium. Exp. Gerontol. 41, 525–531 10.1016/j.exger.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 47.Rohrbach S., Yan X., Weinberg E.O. et al. (1999) Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation 100, 407–412 10.1161/01.CIR.100.4.407 [DOI] [PubMed] [Google Scholar]
- 48.Rayson D., Richel D., Chia S., Jackisch C., van der Vegt S. and Suter T. (2008) Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: current experience and future strategies. Ann. Oncol. 19, 1530–1539 10.1093/annonc/mdn292 [DOI] [PubMed] [Google Scholar]
- 49.Lemmens K., Segers V.F., Demolder M. and De Keulenaer G.W. (2006) Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J. Biol. Chem. 281, 19469–19477 10.1074/jbc.M600399200 [DOI] [PubMed] [Google Scholar]
- 50.Rohrbach S., Niemann B., Silber R.E. and Holtz J. (2005) Neuregulin receptors erbB2 and erbB4 in failing human myocardium – depressed expression and attenuated activation. Basic Res. Cardiol. 100, 240–249 10.1007/s00395-005-0514-4 [DOI] [PubMed] [Google Scholar]
- 51.Lemmens K., Doggen K. and De Keulenaer G.W. (2007) Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 116, 954–960 10.1161/CIRCULATIONAHA.107.690487 [DOI] [PubMed] [Google Scholar]
- 52.Chung K.Y. and Walker J.W. (2007) Interaction and inhibitory cross-talk between endothelin and ErbB receptors in the adult heart. Mol. Pharmacol. 71, 1494–1502 10.1124/mol.106.027599 [DOI] [PubMed] [Google Scholar]
- 53.Zwick E., Hackel P.O., Prenzel N. and Ullrich A. (1999) The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol. Sci. 20, 408–412 10.1016/S0165-6147(99)01373-5 [DOI] [PubMed] [Google Scholar]
- 54.Thomas S.M., Bhola N.E., Zhang Q. et al. (2006) Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma. Cancer Res. 66, 11831–11839 10.1158/0008-5472.CAN-06-2876 [DOI] [PubMed] [Google Scholar]
- 55.Eguchi S., Dempsey P.J., Frank G.D., Motley E.D. and Inagami T. (2001) Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J. Biol. Chem. 276, 7957–7962 10.1074/jbc.M008570200 [DOI] [PubMed] [Google Scholar]
- 56.Ohtsu H., Dempsey P.J. and Eguchi S. (2006) ADAMs as mediators of EGF receptor transactivation by G protein- coupled receptors. Am. J. Physiol. Cell Physiol. 291, C1–C10 10.1152/ajpcell.00620.2005 [DOI] [PubMed] [Google Scholar]
- 57.Asakura M., Kitakaze M., Takashima S. et al. (2002) Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: Metalloproteinase inhibitors as a new therapy. Nat. Med. 8, 35–40 10.1038/nm0102-35 [DOI] [PubMed] [Google Scholar]
- 58.Horie T., Ono K., Nishi H. et al. (2010) Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 87, 656–664 10.1093/cvr/cvq148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Rooij E., Sutherland L.B., Liu N. et al. (2006) A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. U.S.A. 103, 18255–18260 10.1073/pnas.0608791103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haghikia A., Missol-Kolka E., Tsikas D. et al. (2011) Signal transducer and activator of transcription 3-mediated regulation of miR-199a-5p links cardiomyocyte and endothelial cell function in the heart: a key role for ubiquitin-conjugating enzymes. Eur. Heart J. 32, 1287–1297 10.1093/eurheartj/ehq369 [DOI] [PubMed] [Google Scholar]
- 61.Hilfiker-Kleiner D., Kaminski K., Podewski E. et al. (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128, 589–600 10.1016/j.cell.2006.12.036 [DOI] [PubMed] [Google Scholar]
- 62.Stapel B., Kohlhaas M., Ricke-Hoch M. et al. (2017) Low STAT3 expression sensitizes to toxic effects of beta- adrenergic receptor stimulation in peripartum cardiomyopathy. Eur. Heart J. 38, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boateng E., deKay J.T., Peterson S.M. et al. (2020) High ErbB3 activating activity in human blood is not due to circulating neuregulin-1 beta. Life Sci. 251, 117634 10.1016/j.lfs.2020.117634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng Z., Gui C., Nong Q., Du F. and Zhu L. (2013) Serum neuregulin-1β levels are positively correlated with VEGF and angiopoietin-1 levels in patients with diabetes and unstable angina pectoris. Int. J. Cardiol. 168, 3077–3079 10.1016/j.ijcard.2013.04.088 [DOI] [PubMed] [Google Scholar]
- 65.Zhang L., Fu Y., Zhou N., Cheng X. and Chen C. (2017) Circulating neuregulin 4 concentrations in patients with newly diagnosed type 2 diabetes: a cross-sectional study. Endocrine 57, 535–538 10.1007/s12020-017-1324-3 [DOI] [PubMed] [Google Scholar]
- 66.Shao Q., Liu H., Ng C.Y. et al. (2014) Circulating serum levels of growth differentiation factor-15 and neuregulin- 1 in patients with paroxysmal non-valvular atrial fibrillation. Int. J. Cardiol. 172, e311–e313 10.1016/j.ijcard.2013.12.173 [DOI] [PubMed] [Google Scholar]
- 67.De Jong A.M., Maass A.H., Oberdorf-Maass S.U., Van Veldhuisen D.J., Van Gilst W.H. and Van Gelder I.C. (2010) Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc. Res. 89, 754–765 10.1093/cvr/cvq357 [DOI] [PubMed] [Google Scholar]
- 68.Yang K.-C. and Dudley S.C. (2013) Oxidative Stress and Atrial Fibrillation. Circulation 128, 1724–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Y., Lip G.Y.H. and Apostolakis S. (2012) Inflammation in Atrial Fibrillation. J. Am. Coll. Cardiol. 60, 2263 10.1016/j.jacc.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 70.Geisberg C.A., Wang G., Safa R.N. et al. (2011) Circulating neuregulin-1β levels vary according to the angiographic severity of coronary artery disease and ischemia. Coron. Artery Dis. 22, 577–582 10.1097/MCA.0b013e32834d3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ky B., Kimmel S.E., Safa R.N. et al. (2009) Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation 120, 310–317 10.1161/CIRCULATIONAHA.109.856310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miao J., Huang S., Su Y.R. et al. (2018) Effects of endogenous serum neuregulin-1β on morbidity and mortality in patients with heart failure and left ventricular systolic dysfunction. Biomarkers 23, 704–708 10.1080/1354750X.2018.1485054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang M., Zheng J., Chen Z., You C. and Huang Q. (2020) The Relationship Between Circulating Neuregulin-1 and Coronary Collateral Circulation in Patients with Coronary Artery Disease. Int. Heart J. 61, 115–120 10.1536/ihj.19-277 [DOI] [PubMed] [Google Scholar]
- 74.Lim S.L., Lam C.S.P., Segers V.F.M., Brutsaert D.L. and De Keulenaer G.W. (2015) Cardiac endothelium–myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur. Heart J. 36, 2050–2060 10.1093/eurheartj/ehv132 [DOI] [PubMed] [Google Scholar]
- 75.Kivelä R., Hemanthakumar Karthik A., Vaparanta K. et al. (2019) Endothelial Cells Regulate Physiological Cardiomyocyte Growth via VEGFR2-Mediated Paracrine Signaling. Circulation 139, 2570–2584 10.1161/CIRCULATIONAHA.118.036099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gutiérrez E., Flammer A.J., Lerman L.O., Elízaga J., Lerman A. and Fernández-Avilés F. (2013) Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 34, 3175–3181 10.1093/eurheartj/eht351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davignon J. and Ganz P. (2004) Role of Endothelial Dysfunction in Atherosclerosis. Circulation 109, III–27-III-32 [DOI] [PubMed] [Google Scholar]
- 78.Rahimzadeh M., Farshidi N., Naderi N., Farshidi H. and Montazerghaem H. (2020) Clinical significance of serum concentrations of neuregulin-4, in acute coronary syndrome. Sci. Rep. 10, 5797– 10.1038/s41598-020-62680-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nethery D.E., Ghosh S., Erzurum S.C. and Kern J.A. (2007) Inactivation of neuregulin-1 by nitration. 292, L287–L293 [DOI] [PubMed] [Google Scholar]
- 80.Lee H., Akita R.W., Sliwkowski M.X. and Maihle N.J. (2001) A naturally occurring secreted human ErbB3 receptor isoform inhibits heregulin-stimulated activation of ErbB2, ErbB3, and ErbB4. Cancer Res. 61, 4467–4473 [PubMed] [Google Scholar]
- 81.Crone S.A., Zhao Y.Y., Fan L. et al. (2002) ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 8, 459–465 10.1038/nm0502-459 [DOI] [PubMed] [Google Scholar]
- 82.García-Rivello H., Taranda J., Said M. et al. (2005) Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am. J. Physiol. Heart Circ. Physiol. 289, H1153–H1160 10.1152/ajpheart.00048.2005 [DOI] [PubMed] [Google Scholar]
- 83.Liu X., Gu X., Li Z. et al. (2006) Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J. Am. Coll. Cardiol. 48, 1438–1447 10.1016/j.jacc.2006.05.057 [DOI] [PubMed] [Google Scholar]
- 84.Zhao Y.Y., Sawyer D.R., Baliga R.R. et al. (1998) Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J. Biol. Chem. 273, 10261–10269 10.1074/jbc.273.17.10261 [DOI] [PubMed] [Google Scholar]
- 85.Kuramochi Y., Lim C.C., Guo X., Colucci W.S., Liao R. and Sawyer D.B. (2004) Myocyte contractile activity modulates norepinephrine cytotoxicity and survival effects of neuregulin-1β. Am. J. Physiol.-Cell Physiol. 286, C222–C229 10.1152/ajpcell.00312.2003 [DOI] [PubMed] [Google Scholar]
- 86.Fukazawa R., Miller T.A., Kuramochi Y. et al. (2003) Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J. Mol. Cell Cardiol. 35, 1473–1479 10.1016/j.yjmcc.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 87.Giraud M.N., Fluck M., Zuppinger C. and Suter T.M. (2005) Expressional reprogramming of survival pathways in rat cardiocytes by neuregulin-1beta. J. Appl. Physiol. (1985) 99, 313–322 10.1152/japplphysiol.00609.2004 [DOI] [PubMed] [Google Scholar]
- 88.Hedhli N., Huang Q., Kalinowski A. et al. (2011) Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation 123, 2254–2262 10.1161/CIRCULATIONAHA.110.991125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bersell K., Arab S., Haring B. and Kuhn B. (2009) Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 10.1016/j.cell.2009.04.060 [DOI] [PubMed] [Google Scholar]
- 90.Polizzotti B.D., Ganapathy B., Walsh S. et al. (2015) Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci. Transl. Med. 7, 281ra45 10.1126/scitranslmed.aaa5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahmoud A.I., O'Meara C.C., Gemberling M. et al. (2015) Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Dev. Cell 34, 387–399 10.1016/j.devcel.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simmons L.J., Surles-Zeigler M.C., Li Y., Ford G.D., Newman G.D. and Ford B.D. (2016) Regulation of inflammatory responses by neuregulin-1 in brain ischemia and microglial cells in vitro involves the NF-kappa B pathway. J. Neuroinflamm. 13, 237 10.1186/s12974-016-0703-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ryzhov S., Matafonov A., Galindo C.L. et al. (2017) ERBB signaling attenuates proinflammatory activation of nonclassical monocytes. Am. J. Physiol. Heart Circ. Physiol. 312, H907–H918 10.1152/ajpheart.00486.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schumacher M.A., Hedl M., Abraham C. et al. (2017) ErbB4 signaling stimulates pro-inflammatory macrophage apoptosis and limits colonic inflammation. Cell Death Dis. 8, e2622 10.1038/cddis.2017.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galindo C.L., Kasasbeh E., Murphy A. et al. (2014) Anti-remodeling and anti-fibrotic effects of the neuregulin- 1beta glial growth factor 2 in a large animal model of heart failure. J. Am. Heart Assoc. 3, e000773 10.1161/JAHA.113.000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vermeulen Z., Hervent A.S., Dugaucquier L. et al. (2017) Inhibitory actions of the NRG-1/ErbB4 pathway in macrophages during tissue fibrosis in the heart, skin, and lung. Am. J. Physiol. Heart Circ. Physiol. 313, H934–H945 10.1152/ajpheart.00206.2017 [DOI] [PubMed] [Google Scholar]
- 97.Sadoshima J. and Izumo S. (1997) The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 59, 551–571 10.1146/annurev.physiol.59.1.551 [DOI] [PubMed] [Google Scholar]
- 98.Kovacic-Milivojević B., Roediger F., Almeida E.A., Damsky C.H., Gardner D.G. and Ilić D. (2001) Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy. Mol. Biol. Cell 12, 2290–2307 10.1091/mbc.12.8.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torsoni A.S., Constancio S.S., Nadruz W. Jr, Hanks S.K. and Franchini K.G. (2003) Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ. Res. 93, 140–147 10.1161/01.RES.0000081595.25297.1B [DOI] [PubMed] [Google Scholar]
- 100.Peng X., Kraus M.S., Wei H. et al. (2006) Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J. Clin. Invest. 116, 217–227 10.1172/JCI24497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Konhilas J.P., Irving T.C. and de Tombe P.P. (2002) Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch. 445, 305–310 10.1007/s00424-002-0902-1 [DOI] [PubMed] [Google Scholar]
- 102.Pentassuglia L. and Sawyer D.B. (2013) ErbB/integrin signaling interactions in regulation of myocardial cell-cell and cell-matrix interactions. Biochim. Biophys. Acta 1833, 909–916 10.1016/j.bbamcr.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hedhli N., Kalinowski A. and Russell K.S. (2014) Cardiovascular effects of neuregulin-1/ErbB signaling: role in vascular signaling and angiogenesis. Curr. Pharm. Des. 20, 4899–4905 10.2174/1381612819666131125151058 [DOI] [PubMed] [Google Scholar]
- 104.Gui C., Zeng Z.Y., Chen Q., Luo Y.W., Li L. and Chen L.L. (2018) Neuregulin-1 Promotes Myocardial Angiogenesis in the Rat Model of Diabetic Cardiomyopathy. Cell. Physiol. Biochem. 46, 2325–2334 10.1159/000489622 [DOI] [PubMed] [Google Scholar]
- 105.Aghajanian H., Cho Y.K., Manderfield L.J. et al. (2016) Coronary vasculature patterning requires a novel endothelial ErbB2 holoreceptor. Nat. Commun. 7, 12038 10.1038/ncomms12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lampropoulou A. and Ruhrberg C. (2014) Neuropilin regulation of angiogenesis. Biochem. Soc. Trans. 42, 1623–1628 10.1042/BST20140244 [DOI] [PubMed] [Google Scholar]
- 107.Gelfand M.V., Hagan N., Tata A. et al. (2014) Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. eLife 3, e03720 10.7554/eLife.03720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Russell K.S., Stern D.F., Polverini P.J. and Bender J.R. (1999) Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am. J. Physiol. 277, H2205–H2211 [DOI] [PubMed] [Google Scholar]
- 109.Safa R.N., Peng X.Y., Pentassuglia L. et al. (2011) Neuregulin-1beta regulation of embryonic endothelial progenitor cell survival. Am. J. Physiol. Heart Circ. Physiol. 300, H1311–H1319 10.1152/ajpheart.01104.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ryzhov S., Robich M.P., Roberts D.J. et al. (2018) ErbB2 promotes endothelial phenotype of human left ventricular epicardial highly proliferative cells (eHiPC). J. Mol. Cell Cardiol. 115, 39–50 10.1016/j.yjmcc.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suárez E., Bach D., Cadefau J., Palacin M., Zorzano A. and Gumá A. (2001) A novel role of neuregulin in skeletal muscle. Neuregulin stimulates glucose uptake, glucose transporter translocation, and transporter expression in muscle cells. J. Biol. Chem. 276, 18257–18264 10.1074/jbc.M008100200 [DOI] [PubMed] [Google Scholar]
- 112.Cantó C., Suárez E., Lizcano J.M. et al. (2004) Neuregulin signaling on glucose transport in muscle cells. J. Biol. Chem. 279, 12260–12268 10.1074/jbc.M308554200 [DOI] [PubMed] [Google Scholar]
- 113.Heim P., Morandi C., Brouwer G.R., Xu L., Montessuit C. and Brink M. (2020) Neuregulin-1 triggers GLUT4 translocation and enhances glucose uptake independently of insulin receptor substrate and ErbB3 in neonatal rat cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Res. 1867, 118562 10.1016/j.bbamcr.2019.118562 [DOI] [PubMed] [Google Scholar]
- 114.Liu X.B., Li J., Xiao Y., Pang L.Y., Jiang X. and Wang L. (2009) Effects of neregulin on rhesus monkey heart failure induced by rapid pacing. Sichuan Da Xue Xue Bao Yi Xue Ban 40, 93–96 [PubMed] [Google Scholar]
- 115.Hill M.F., Patel A.V., Murphy A. et al. (2013) Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1β improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS ONE 8, e55741 10.1371/journal.pone.0055741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jabbour A., Gao L., Kwan J. et al. (2011) A recombinant human neuregulin-1 peptide improves preservation of the rodent heart after prolonged hypothermic storage. Transplantation 91, 961–967 10.1097/TP.0b013e3182115b4b [DOI] [PubMed] [Google Scholar]
- 117.Gao R., Zhang J., Cheng L. et al. (2010) A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J. Am. Coll. Cardiol. 55, 1907–1914 10.1016/j.jacc.2009.12.044 [DOI] [PubMed] [Google Scholar]
- 118.Lenihan D.J., Anderson S.A., Lenneman C.G. et al. (2016) A Phase I, Single Ascending Dose Study of Cimaglermin Alfa (Neuregulin 1β3) in Patients With Systolic Dysfunction and Heart Failure. JACC Basic Transl. Sci. 1, 576–586 10.1016/j.jacbts.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ennequin G., Boisseau N., Caillaud K. et al. (2015) Neuregulin 1 Improves Glucose Tolerance in db/db Mice. PLoS ONE 10, e0130568 10.1371/journal.pone.0130568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caillaud K., Boisseau N., Ennequin G. et al. (2016) Neuregulin 1 improves glucose tolerance in adult and old rats. Diabetes Metab. 42, 96–104 10.1016/j.diabet.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 121.López-Soldado I., Niisuke K., Veiga C. et al. (2016) Neuregulin improves response to glucose tolerance test in control and diabetic rats. Am. J. Physiol. Endocrinol. Metab. 310, E440–E451 10.1152/ajpendo.00226.2015 [DOI] [PubMed] [Google Scholar]
- 122.Honkoop H., de Bakker D.E., Aharonov A. et al. (2019) Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. Elife 8, 10.7554/eLife.50163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Neely J.R., Rovetto M.J. and Oram J.F. (1972) Myocardial utilization of carbohydrate and lipids. Prog. Cardiovasc. Dis. 15, 289–329 10.1016/0033-0620(72)90029-1 [DOI] [PubMed] [Google Scholar]
- 124.Lehman J.J. and Kelly D.P. (2002) Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin. Exp. Pharmacol. Physiol. 29, 339–345 10.1046/j.1440-1681.2002.03655.x [DOI] [PubMed] [Google Scholar]
- 125.Ferreira R., Nogueira-Ferreira R., Trindade F., Vitorino R., Powers S.K. and Moreira-Gonçalves D. (2018) Sugar or fat: The metabolic choice of the trained heart. Metabolism 87, 98–104 10.1016/j.metabol.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 126.Sack M.N., Rader T.A., Park S., Bastin J., McCune S.A. and Kelly D.P. (1996) Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94, 2837–2842 10.1161/01.CIR.94.11.2837 [DOI] [PubMed] [Google Scholar]
- 127.Barger P.M., Brandt J.M., Leone T.C., Weinheimer C.J. and Kelly D.P. (2000) Deactivation of peroxisome proliferator- activated receptor-alpha during cardiac hypertrophic growth. J. Clin. Invest. 105, 1723–1730 10.1172/JCI9056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kanda H., Nohara R., Hasegawa K., Kishimoto C. and Sasayama S. (2000) A nuclear complex containing PPARalpha/RXRalpha is markedly downregulated in the hypertrophied rat left ventricular myocardium with normal systolic function. Heart Vessels 15, 191–196 10.1007/s003800070022 [DOI] [PubMed] [Google Scholar]
- 129.Sihag S., Cresci S., Li A.Y., Sucharov C.C. and Lehman J.J. (2009) PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J. Mol. Cell Cardiol. 46, 201–212 10.1016/j.yjmcc.2008.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S. and Stanley W.C. (2010) Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 10.1152/physrev.00015.2009 [DOI] [PubMed] [Google Scholar]
- 131.Gumà A., Martínez-Redondo V., López-Soldado I., Cantó C. and Zorzano A. (2009) Emerging role of neuregulin as a modulator of muscle metabolism. Am. J. Physiol.-Endocrinol. Metab. 298, E742–E750 10.1152/ajpendo.00541.2009 [DOI] [PubMed] [Google Scholar]
- 132.Rupert C.E. and Coulombe K.L.K. (2017) IGF1 and NRG1 Enhance Proliferation, Metabolic Maturity, and the Force- Frequency Response in hESC-Derived Engineered Cardiac Tissues. Stem Cells Int. 2017, 7648409 10.1155/2017/7648409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ennequin G., Capel F., Caillaud K. et al. (2017) Neuregulin 1 improves complex 2-mediated mitochondrial respiration in skeletal muscle of healthy and diabetic mice. Sci. Rep. 7, 1742 10.1038/s41598-017-02029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bell S.P., Adkisson D.W., Lawson M.A. et al. (2014) Antifailure therapy including spironolactone improves left ventricular energy supply-demand relations in nonischemic dilated cardiomyopathy. J. Am. Heart Assoc. 3, 10.1161/JAHA.114.000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jabbour A., Hayward C.S., Keogh A.M. et al. (2011) Parenteral administration of recombinant human neuregulin- 1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur. J. Heart Fail. 13, 83–92 10.1093/eurjhf/hfq152 [DOI] [PubMed] [Google Scholar]
- 136.Lemmens K., Fransen P., Sys S.U., Brutsaert D.L. and De Keulenaer G.W. (2004) Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation 109, 324–326 10.1161/01.CIR.0000114521.88547.5E [DOI] [PubMed] [Google Scholar]
- 137.Okoshi K., Nakayama M., Yan X. et al. (2004) Neuregulins regulate cardiac parasympathetic activity: muscarinic modulation of beta-adrenergic activity in myocytes from mice with neuregulin-1 gene deletion. Circulation 110, 713–717 10.1161/01.CIR.0000138109.32748.80 [DOI] [PubMed] [Google Scholar]
- 138.Brero A., Ramella R., Fitou A. et al. (2010) Neuregulin-1beta1 rapidly modulates nitric oxide synthesis and calcium handling in rat cardiomyocytes. Cardiovasc. Res. 88, 443–452 10.1093/cvr/cvq238 [DOI] [PubMed] [Google Scholar]
- 139.Gao R., Zhang J., Liu H. et al. (2018) A Phase III, Randomized, Double-Blind, Multicenter, Placebo-Controlled Study of the Efficacy and Safety of Neucardin in Patients with Chronic Heart Failure. J. Am. College Cardiol. 7111, Supplement 10.1016/S0735-1097(18)31209-9 [DOI] [Google Scholar]
- 140.Mosedale M., Button D., Jackson J.P. et al. (2018) Transient Changes in Hepatic Physiology That Alter Bilirubin and Bile Acid Transport May Explain Elevations in Liver Chemistries Observed in Clinical Trials of GGF2 (Cimaglermin Alfa). Toxicol. Sci. 161, 401–411 10.1093/toxsci/kfx222 [DOI] [PubMed] [Google Scholar]
- 141.Kiguchi K., Carbajal S., Chan K. et al. (2001) Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 61, 6971–6976 [PubMed] [Google Scholar]
- 142.Jay S.M., Kurtagic E., Alvarez L.M. et al. (2011) Engineered bivalent ligands to bias ErbB receptor-mediated signaling and phenotypes. J. Biol. Chem. 286, 27729–27740 10.1074/jbc.M111.221093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stove C. and Bracke M. (2004) Roles for neuregulins in human cancer. Clin. Exp. Metastasis 21, 665–684 10.1007/s10585-004-6917-6 [DOI] [PubMed] [Google Scholar]
- 144.Tzahar E., Waterman H., Chen X. et al. (1996) A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 16, 5276–5287 10.1128/MCB.16.10.5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jay S.M., Murthy A.C., Hawkins J.F. et al. (2013) An engineered bivalent neuregulin protects against doxorubicin- induced cardiotoxicity with reduced proneoplastic potential. Circulation 128, 152–161 10.1161/CIRCULATIONAHA.113.002203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Panutsopulos D., Arvanitis D.L., Tsatsanis C., Papalambros E., Sigala F. and Spandidos D.A. (2005) Expression of heregulin in human coronary atherosclerotic lesions. J. Vasc. Res. 42, 463–474 10.1159/000088100 [DOI] [PubMed] [Google Scholar]
- 147.Xu G., Watanabe T., Iso Y. et al. (2009) Preventive Effects of Heregulin-β1 on Macrophage Foam Cell Formation and Atherosclerosis. Circ. Res. 105, 500–510 10.1161/CIRCRESAHA.109.193870 [DOI] [PubMed] [Google Scholar]
- 148.Shakeri H., Gevaert A.B., Schrijvers D.M. et al. (2018) Neuregulin-1 attenuates stress-induced vascular senescence. Cardiovasc. Res. 114, 1041–1051 10.1093/cvr/cvy059 [DOI] [PubMed] [Google Scholar]
- 149.Bentzon J.F., Otsuka F., Virmani R. and Falk E. (2014) Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 10.1161/CIRCRESAHA.114.302721 [DOI] [PubMed] [Google Scholar]
- 150.Wang G.X., Zhao X.Y., Meng Z.X. et al. (2014) The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20, 1436–1443 10.1038/nm.3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cai C., Lin M., Xu Y., Li X., Yang S. and Zhang H. (2016) Association of circulating neuregulin 4 with metabolic syndrome in obese adults: a cross-sectional study. BMC Med. 14, 165 10.1186/s12916-016-0703-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang Y.E., Kim J.M., Choung S. et al. (2016) Comparison of serum Neuregulin 4 (Nrg4) levels in adults with newly diagnosed type 2 diabetes mellitus and controls without diabetes. Diabetes Res. Clin. Pract. 117, 1–3 10.1016/j.diabres.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 153.Jiang J., Lin M., Xu Y. et al. (2016) Circulating neuregulin 4 levels are inversely associated with subclinical cardiovascular disease in obese adults. Sci. Rep. 6, 36710 10.1038/srep36710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan P.J., Xu Y., Wan Q. et al. (2017) Decreased plasma neuregulin 4 concentration is associated with increased high-sensitivity C-reactive protein in newly diagnosed type 2 diabetes mellitus patients: a cross-sectional study. Acta Diabetol. 54, 1091–1099 10.1007/s00592-017-1044-4 [DOI] [PubMed] [Google Scholar]
- 155.Rahimzadeh M., Farshidi N., Naderi N., Farshidi H. and Montazerghaem H. (2020) Clinical significance of serum concentrations of neuregulin-4, in acute coronary syndrome. Sci. Rep. 10, 5797 10.1038/s41598-020-62680-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Massie B.M. and Fabi M.R. (2005) Clinical trials in diastolic heart failure. Prog. Cardiovasc. Dis. 47, 389–395 10.1016/j.pcad.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 157.Thohan V. and Patel S. (2009) The challenges associated with current clinical trials for diastolic heart failure. Curr. Opin. Cardiol. 24, 230–238 10.1097/HCO.0b013e328329f8fd [DOI] [PubMed] [Google Scholar]
- 158.Hopf A.E., Andresen C., Kötter S. et al. (2018) Diabetes-Induced Cardiomyocyte Passive Stiffening Is Caused by Impaired Insulin-Dependent Titin Modification and Can Be Modulated by Neuregulin-1. Circ. Res. 123, 342–355 10.1161/CIRCRESAHA.117.312166 [DOI] [PubMed] [Google Scholar]
- 159.Fein F.S. and Sonnenblick E.H. (1994) Diabetic cardiomyopathy. Cardiovasc. Drugs Ther. 8, 65–73 10.1007/BF00877091 [DOI] [PubMed] [Google Scholar]
- 160.Jia G., Whaley-Connell A. and Sowers J.R. (2018) Diabetic cardiomyopathy: a hyperglycaemia- and insulin- resistance-induced heart disease. Diabetologia 61, 21–28 10.1007/s00125-017-4390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li B., Zheng Z., Wei Y. et al. (2011) Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc. Diabetol. 10, 69 10.1186/1475-2840-10-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hopf A.E., Andresen C., Kotter S. et al. (2018) Diabetes-Induced Cardiomyocyte Passive Stiffening Is Caused by Impaired Insulin-Dependent Titin Modification and Can Be Modulated by Neuregulin-1. Circ. Res. 123, 342–355 10.1161/CIRCRESAHA.117.312166 [DOI] [PubMed] [Google Scholar]
- 163.Gui C., Zhu L., Hu M., Lei L. and Long Q. (2012) Neuregulin-1/ErbB signaling is impaired in the rat model of diabetic cardiomyopathy. Cardiovasc. Pathol. 21, 414–420 10.1016/j.carpath.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 164.Kass D.A., Bronzwaer J.G. and Paulus W.J. (2004) What mechanisms underlie diastolic dysfunction in heart failure? Circ. Res. 94, 1533–1542 10.1161/01.RES.0000129254.25507.d6 [DOI] [PubMed] [Google Scholar]
- 165.Methawasin M. and Granzier H., Softening the Stressed Giant Titin in Diabetes Mellitus. Circ. Res. 2018, 315–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rhodes A., Evans L.E., Alhazzani W. et al. (2017) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 43, 304–377 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 167.Beesley S.J., Weber G., Sarge T. et al. (2018) Septic Cardiomyopathy. Crit. Care Med. 46, 625–634 10.1097/CCM.0000000000002851 [DOI] [PubMed] [Google Scholar]
- 168.Zhou Q., Pan X., Wang L., Wang X. and Xiong D. (2016) The protective role of neuregulin-1: A potential therapy for sepsis-induced cardiomyopathy. Eur. J. Pharmacol. 788, 234–240 10.1016/j.ejphar.2016.06.042 [DOI] [PubMed] [Google Scholar]
- 169.Kang W., Cheng Y., Zhou F. et al. (2019) Neuregulin-1 protects cardiac function in septic rats through multiple targets based on endothelial cells. Int. J. Mol. Med. 44, 1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kang W., Cheng Y., Zhou F. et al. (2019) Neuregulin1 protects cardiac function in septic rats through multiple targets based on endothelial cells. Int. J. Mol. Med. 44, 1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vermeulen Z., Segers V.F. and De Keulenaer G.W. (2016) ErbB2 signaling at the crossing between heart failure and cancer. Basic Res. Cardiol. 111, 60 10.1007/s00395-016-0576-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vasti C. and Hertig C.M. (2014) Neuregulin-1/erbB activities with focus on the susceptibility of the heart to anthracyclines. World J. Cardiol. 6, 653–662 10.4330/wjc.v6.i7.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pentassuglia L. and Sawyer D.B. (2013) ErbB/integrin signaling interactions in regulation of myocardial cell–cell and cell–matrix interactions. Biochim. Biophys. Acta - Mol. Cell Res. 1833, 909–916 10.1016/j.bbamcr.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mendes-Ferreira P., De Keulenaer G.W., Leite-Moreira A.F. and Bras-Silva C. (2013) Therapeutic potential of neuregulin-1 in cardiovascular disease. Drug Discov. Today 18, 836–842 10.1016/j.drudis.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 175.LeBrasseur N.K., Mizer K.C., Parkington J.D., Sawyer D.B. and Fielding R.A. (2005) The expression of neuregulin and erbB receptors in human skeletal muscle: effects of progressive resistance training. Eur. J. Appl. Physiol. 94, 371–375 10.1007/s00421-005-1333-4 [DOI] [PubMed] [Google Scholar]
- 176.Geisberg C.A., Abdallah W.M., da Silva M. et al. (2013) Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J. Card. Fail. 19, 10–15 10.1016/j.cardfail.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yiadom M.Y.A.B., Greenberg J., Smith H.M. et al. (2016) Diagnostic Utility of Neuregulin for Acute Coronary Syndrome. Dis. Markers 2016, 8025271 10.1155/2016/8025271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yan P., Xu Y., Zhang Z. et al. (2019) Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Cytokine 113, 356–364 10.1016/j.cyto.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 179.Kocak M.Z., Aktas G., Erkus E. et al. (2019) Neuregulin-4 is associated with plasma glucose and increased risk of type 2 diabetes mellitus. Swiss Med. Wkly. 149, w20139. [DOI] [PubMed] [Google Scholar]
- 180.Gu X., Liu X., Xu D. et al. (2010) Cardiac functional improvement in rats with myocardial infarction by up- regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc. Res. 88, 334–343 10.1093/cvr/cvq223 [DOI] [PubMed] [Google Scholar]
- 181.Guo Y.F., Zhang X.X., Liu Y., Duan H.Y., Jie B.Z. and Wu X.S. (2012) Neuregulin-1 attenuates mitochondrial dysfunction in a rat model of heart failure. Chin. Med. J. (Engl.) 125, 807–814 [PubMed] [Google Scholar]
- 182.Fang S.J., Wu X.S., Han Z.H. et al. (2010) Neuregulin-1 preconditioning protects the heart against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Chin. Med. J. (Engl.) 123, 3597–3604 [PubMed] [Google Scholar]
- 183.Bian Y., Sun M., Silver M. et al. (2009) Neuregulin-1 attenuated doxorubicin-induced decrease in cardiac troponins. Am. J. Physiol. Heart Circul. Physiol. 297, H1974–H1983 10.1152/ajpheart.01010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hill M.F., Patel A.V., Murphy A. et al. (2013) Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1beta improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS ONE 8, e55741 10.1371/journal.pone.0055741 [DOI] [PMC free article] [PubMed] [Google Scholar]