Abstract
This study was designed to investigate the long non-coding RNA (lncRNA)-metastasis associated lung adenocarcinoma transcript 1 (MALAT1) expression in patients with coronary atherosclerosis and its predictive value for in-stent restenosis. Ninety-five patients with coronary heart disease who came to our hospital for treatment and underwent stent implantation were selected as a research group (RG), and 95 volunteers undergoing physical examination who did not suffer from coronary heart disease during the same period were selected as a control group (CG). MALAT1 of subjects in both groups before and after treatment were detected by RT-qPCR, and N-terminal pro-brain natriuretic peptide (NT-proBNP), high sensitivity C-reactive protein (hs-CRP), lactate dehydrogenase (LDH), and creatine kinase isoenzyme (CK-MB) of them in the RG before treatment were detected. The level was evaluated and detected, and its correlation with MALAT1 was analyzed. Then, the predictive value of MALAT1 for in-stent restenosis in patients with coronary heart disease was analyzed. MALAT1 expression in patients with coronary heart disease was higher than that of normal subjects (P<0.05); after treatment, the expression levels of MALAT1, NT-proBNP, hs-CRP, LDH, and CK-MB in the serum of patients were significantly lower than those before treatment (P<0.05); MALAT1 expression was positively correlated with the expression levels of NT-proBNP, hs-CRP, LDH, and CK-MB (P<0.05). Receiver operating characteristic of MALAT1 for predicting in-stent restenosis in patients with coronary heart disease was over 0.8; the number of lesions, MALAT1, diabetes, NT-proBNP and hs-CRP were independent risk factors for in-stent restenosis. MALAT1 is highly expressed in the serum of patients with coronary heart disease, and it has high value in its diagnosis and the prediction of in-stent restenosis. It is also an independent risk factor for in-stent restenosis in patients with coronary heart disease.
Keywords: MALAT1, coronary atherosclerosis, expression, in-stent restenosis, predictive value
Introduction
Coronary heart disease is one of the most common cardiovascular diseases at present. In recent years, with the changes of social environment and living habits, the incidence of coronary heart disease is also getting higher and higher. It has been called one of the main causes of human death in cardiovascular diseases, which poses a serious threat to human life and health (1,2). The gold standard for the diagnosis of coronary heart disease has been coronary angiography, which has high sensitivity and specificity. However, due to its complicated operation, high economic cost and invasive detection, many patients have low acceptance (3). At present, coronary stent implantation is a vital interventional method in treating coronary heart disease, which mainly improves myocardial blood supply by reconstructing narrow lumen, thus achieving the purpose of treatment (4). However, in-stent restenosis after stent therapy has always been a difficult point in coronary interventional therapy. Although the occurrence of drug-coated stents has reduced the occurrence of in-stent restenosis recently, it's still impossible to completely avoid its occurrence (5,6). Therefore, if the occurrence of in-stent restenosis can be predicted through detection of serum molecular markers, it can improve the basis for clinical intervention, thus improving the prognosis of patients.
In recent years, the role of long non-coding RNA (LncRNA) in cardiovascular diseases has also been paid more and more attention. For example, research (7) has found that LncRNA ANRIL has high diagnostic value for coronary heart disease. Some research (8) also has verified that LncRNA can be used as a new regulator of cardiac electrophysiology and arrhythmia. LncRNA-MALAT1 is a highly conserved LncRNA located on chromosome 11q13.1(9). In the past, it was found to act as an oncogene in tumors. For example, research (10) indicated that when MALAT1 expression was knocked down, the progression of thyroid cancer could be inhibited by regulating miR-200a-3p/FOXA1. Recently, there have also been many studies on the expression and function of MALAT1 in coronary heart disease. For example, some studies (11) have found that MALAT1 SNP rs619586 AG/GG genotype can prevent the occurrence of coronary heart disease. However, the predictive value of MALAT1 for in-stent restenosis in patients with coronary heart disease has not been studied and discussed. N-terminal pro-brain natriuretic peptide (NT-proBNP), high sensitivity C-reactive protein (hs-CRP), lactate dehydrogenase (LDH) and creatine kinase isoenzyme (CK-MB) are all vital evaluation indexes in cardiovascular diseases (12,13). We also analyzed the correlation between MALAT1 and these indexes.
In order to provide more biological indicators for the prediction of in-stent restenosis, we investigated the MALAT1 expression in the serum of patients with coronary heart disease, correlation with cardiovascular related indexes and the predictive value for in-stent restenosis.
Patients and methods
General information
Ninety-five patients with coronary heart disease who came to The Second Hospital of Shandong University (Jinan, China) for treatment and underwent stent implantation were selected as a research group (RG), including 51 male patients and 44 female patients, and their average age was (65.26±8.15) years. Ninety-five volunteers undergoing physical examination who did not suffer from coronary heart disease during the same period in our hospital were selected as a control group (CG). Inclusion criteria: Patients diagnosed as coronary heart disease by coronary angiography were included in the RG; patients aged 55-75 and those with tolerance to stent implantation were included. Exclusion criteria were as follows: Patients with malignant tumors; patients with severe liver and kidney dysfunction; patients with other infectious or immune diseases; patients with communication and cognitive dysfunction. All patients and their families agreed to participate in the experiment and sign an informed consent. This experiment was approved by the Ethics Committee of the The Second Hospital of Shandong University.
Treatment methods
After all patients were admitted to hospital, the department director performed the stent implantation operation. According to the specific situation of the patients, the appropriate stent, matching balloon and umbrella were selected, and the operation was performed according to the conventional stent implantation operation procedure. Besides, antiplatelet therapy was given after surgery.
Index detection
MALAT1 expression detected by RT-qPCR
Altogether 5 ml venous blood was collected from patients on an empty stomach before treatment and 1 month after treatment, and it was centrifuged for 5 min at 3,000 x g at 4˚C. After extraction, and the supernatant was taken for detection after centrifugation. TRIzol was added to the serum to extract total RNA, and the purity, concentration and integrity of total RNA were detected by ultraviolet spectrophotometer and agarose gel electrophoresis. Then cDNA reverse transcription and MALAT1 detection were performed according to the kit instructions (TransGen Biotech, Beijing, China). MALAT1 amplification system were as follows: cDNA 1 µl, upstream and downstream primers 0.4 µl each, 2X SYBR-Green mixture 10 µl, Passive Reference Dye (50X) 0.4 µl, ddH2O added to 20 µl. Amplification conditions were as follows: PCR reaction conditions: Pre-denaturation at 95˚C for 30 sec, denaturation at 95˚C for 10 sec, annealing extension at 60˚C for 20 sec, a total of 40 cycles. GAPDH was used as internal reference, and primer sequence was shown in Table I. The experiment was repeated 3 times and we took the average of three experiments.
Table I.
Primer sequences.
| Genes | Upstream primers | Downstream primers |
|---|---|---|
| MALAT1 | 5'-AAAGCAAGGTCTCCCCACAAG-3' | 5'-GGTCTGTGCTAGATCAAAAGGCA-3' |
| GAPDH | 5'-GAAGGTGAAGGTCGGAGTC-3' | 5'-GAAGATGGTGATGGGATTTC-3' |
Detection of other relevant indicators
The biochemical indexes related to other coronary heart diseases of the patients in the RG before and 1 month after treatment were detected. First, 3 ml venous blood was drawn on an empty stomach, sodium citrate was added to the test tube for anticoagulation, and all biochemical indexes were detected by Hitachi 7600 automatic biochemical analyzer. The biochemical indexes included N-terminal pro-brain natriuretic peptide (NT-proBNP), high sensitivity C-reactive protein (hs-CRP), lactate dehydrogenase (LDH) and creatine kinase isoenzyme (CK-MB).
Coronary angiography
One year after surgery, the patients were followed up by angiography. The right radial artery and femoral artery were punctured by Judikins method and arteriography was performed. The degree of in-stent restenosis was evaluated according to computer quantitative analysis. Evaluation criteria for in-stent restenosis (14): Coronary angiography was performed 12 months after surgery. The diameter of the vessel at the distal end of the stent was taken as the CG. In-stent restenosis was considered when the diameter of the lumen in the vessel was 5 mm or more in the stent and the distal and proximal edge of the stent was 50 mm or more.
Statistical methods
In this study, the collected data were statistically analyzed via SPSS19.0, and the required pictures were drawn via GraphPad 6. The counting data were expressed in the number of cases/percentage n (%), and their comparison between groups was expressed in chi-square test. The measurement data were expressed in the mean ± standard deviation (SD). Comparison between the two groups was under independent-samples t test, comparison before and after treatment was under paired t-test, and that among multiple groups was under one-way analysis of variance. LSD/t-test was used for back testing, Pearson was used for correlation analysis, and the risk factors of in-stent restenosis were assessed via Logistic single-factor and multi-factor regression analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
General information
There was no significant difference in gender, age and BMI between the two groups (P>0.05), which was comparable (Table II).
Table II.
General information [n (%), mean ± SD].
| Characteristics | Research group n=95 | Control group n=95 | χ2 | P-value |
|---|---|---|---|---|
| Sex | 0.022 | 0.884 | ||
| Male | 51 (53.68) | 50 (52.63) | ||
| Female | 44 (46.32) | 45 (47.37) | ||
| Age, years | 0.021 | 0.885 | ||
| ≥65 | 47 (49.47) | 46 (48.42) | ||
| <65 | 48 (50.53) | 49 (51.58) | ||
| BMI, kg/m2 | 0.085 | 0.771 | ||
| ≥22 | 53 (55.79) | 51 (53.68) | ||
| <22 | 42 (45.26) | 44 (46.32) | ||
| Family history of coronary heart disease | 0.024 | 0.877 | ||
| Yes | 31 (32.63) | 30 (31.58) | ||
| No | 64 (67.37) | 65 (68.42) | ||
| Diabetes | 0.202 | 0.653 | ||
| Yes | 61 (64.21) | 58 (61.05) | ||
| No | 34 (35.79) | 37 (38.95) | ||
| Hypertension | 0.198 | 0.656 | ||
| Yes | 59 (62.11) | 56 (58.95) | ||
| No | 36 (37.89) | 39 (41.05) | ||
| HbA1 (%) | 7.09±0.46 | 5.24±0.34 | 31.52 | <0.001 |
| TG (mmol/l) | 1.86±0.23 | 1.61±0.21 | 6.885 | <0.001 |
| TC (mmol/l) | 4.46±0.39 | 4.21±0.34 | 4.710 | <0.001 |
| HDL (mmol/l) | 1.16±0.31 | 1.59±0.23 | 10.86 | <0.001 |
BMI, body mass index; HbA1, hemoglobin A1c; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein.
Expression of serum MALAT1 before and after treatment and its diagnostic value for coronary heart disease
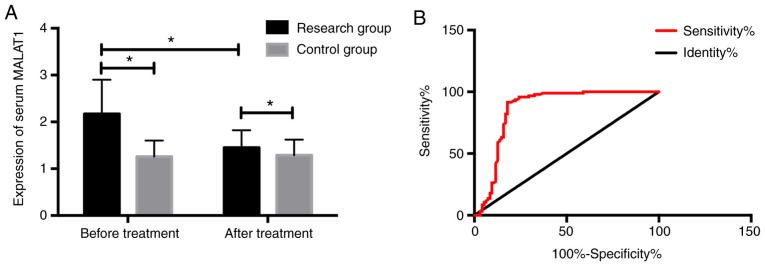
The MALAT1 expression in the RG was significantly higher than that in the CG before treatment (P<0.05); the expression in the RG after treatment was significantly higher than that before treatment, but still higher than that in normal people (P<0.05). After receiver operating characteristic (ROC) curve was drawn, it was found that the sensitivity, specificity and area under curve (AUC) of MALAT1 expression in serum for coronary heart disease diagnosis were 86.32%, 82.11% and 0.863, respectively, which had higher diagnostic value (Fig. 1).
Figure 1.
Expression of serum MALAT1 before and after treatment and its diagnostic value for coronary heart disease. (A) Expression of serum MALAT1 before and after treatment. (B) Diagnostic value analysis of serum MALAT1 for coronary heart disease. *P<0.05. MALAT1, metastasis associated lung adenocarcinoma transcript 1.
Comparison of other related indexes before and after treatment and correlation analysis with serum MALAT1 in patients from the RG
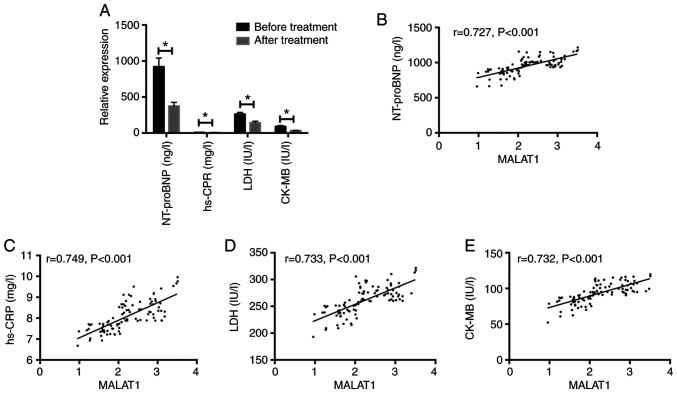
After testing other related indexes of patients before and after treatment, we found that their NT-proBNP, hs-CRP, LDH, and CK-MB after treatment were significantly lower than those before treatment (P<0.05). Serum MALAT1 and NT-proBNP, hs-CRP, LDH, CK-MB levels of coronary heart disease were positively correlated (r=0.727, P<0.001; r=0.749, P<0.001; r=0.733, P<0.001; r=0.732, P<0.001) (Fig. 2).
Figure 2.
Comparison of other related indexes before and after treatment and correlation analysis with serum MALAT1 of patients from the RG. (A) Comparison of other related indexes of patients before and after treatment in the RG. (B) Correlation analysis of serum MALAT1 and NT-proBNP in patients with coronary heart disease. (C) Correlation analysis of serum MALAT1 and hs-CRP in patients with coronary heart disease. (D) Correlation analysis of serum MALAT1 and LDH in patients with coronary heart disease. (E) Correlation analysis of serum MALAT1 and CK-MB in patients with coronary heart disease. *P<0.05. MALAT1, metastasis associated lung adenocarcinoma transcript 1; RG, research group; hs-CRP, high sensitivity C-reactive protein; LDH, lactate dehydrogenase; CK-MB, creatine kinase-MB.
Predictive value of MALAT1 for in-stent restenosis from patients
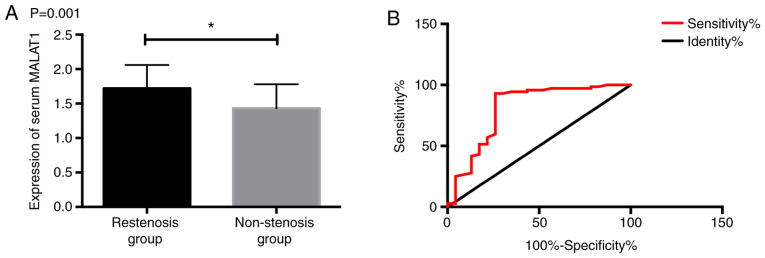
The patients were divided into restenosis group (RG) (23 patients) and non-stenosis group (NSG) (72 patients) according to their efficacy; the MALAT1 expression in the RG was significantly higher than that in the NG (P<0.05); after ROC curve was drawn, it was found that the sensitivity, specificity and AUC of MALAT1 expression in the serum of patients in January after surgery were 91.67%, 74.65% and 0.905, respectively for in-stent restenosis in patients with coronary heart disease, which had high predictive value (Fig. 3).
Figure 3.
Predictive value of MALAT1 for in-stent restenosis. (A) Serum MALAT1 expression in patients with different stenosis. (B) ROC of MALAT1 for predicting in-stent restenosis. *P<0.05. MALAT1, metastasis associated lung adenocarcinoma transcript 1; ROC, receiver operating characteristic.
Univariate analysis of in-stent restenosis of patients
After carrying out univariate analysis of patients in the restenosis group (RG) and non-stenotic group (NSG), we found that there were no significant differences in gender, age, hypertension, history of smoking, LDH, CK-MB and other aspects between the two groups (P>0.05), but there were significant differences in number of lesions, diabetes, MALAT1, NT-proBNP, and hs-CRP levels (P<0.05) (Table III).
Table III.
Univariate analysis of in-stent restenosis of patients [n (%), mean ± SD].
| Factor | Restenosis group n=23 | Non-stenosis group n=72 | t/χ2 | P-value |
|---|---|---|---|---|
| Sex | 0.098 | 0.754 | ||
| Male | 13 (56.52) | 38 (52.78) | ||
| Female | 10 (43.48) | 34 (47.22) | ||
| Age, years | 0.089 | 0.766 | ||
| ≥65 | 12 (52.17) | 35 (48.61) | ||
| <65 | 11 (47.83) | 37 (51.39) | ||
| Hypertension | 14 (60.87) | 45 (62.50) | 0.020 | 0.874 |
| Diabetes | 22 (95.65) | 39 (54.17) | 13.05 | <0.001 |
| History of smokinga | 12 (52.17) | 35 (48.61) | 0.125 | 0.724 |
| Number of lesions | 10.09 | 0.006 | ||
| Single-vessel disease | 3 (13.04) | 29 (40.28) | ||
| Double-vessel disease | 6 (26.09) | 24 (33.33) | ||
| Multi-vessel disease | 14 (60.87) | 19 (26.39) | ||
| MALAT1 | 1.41±0.29 | 1.12±0.26 | 4.528 | <0.001 |
| NT-proBNP (ng/l) | 854.21±119.75 | 746.29±104.33 | 4.165 | <0.001 |
| hs-CRP (mg/l) | 7.64±0.83 | 6.57±0.85 | 5.285 | <0.001 |
| LDH (IU/l) | 258.79±25.64 | 152.61±21.54 | 19.64 | <0.001 |
| CK-MB (IU/l) | 67.41±11.62 | 35.71±4.88 | 18.69 | 0.506 |
aHistory of smoking was defined as at least one cigarette per day for one consecutive year. MALAT1, metastasis associated lung adenocarcinoma transcript 1; NT-proBNP, N-terminal pro-brain natriuretic peptide; hs-CRP, high sensitivity C-reactive protein; LDH, lactate dehydrogenase; CK-MB, creatine kinase-MB.
Multivariate analysis of in-stent restenosis of patients
The number of lesions, MALAT1, diabetes, NT-proBNP, and hs-CRP were analysed, and listed as dependent variables for assignment (Table IV), considering whether in-stent restenosis occurred as dependent variables; Logistic regression model was used for multivariate analysis; we found that the number of lesions, MALAT1, diabetes, NT-proBNP, and hs-CRP were independent risk factors for in-stent restenosis (Table V).
Table IV.
Assignment.
| Factor | Assignment |
|---|---|
| No. of lesions | Single-vessel and double-vessel disease=1, multi-vessel disease=2 |
| Diabetes | No=1, yes=-2 |
| MALAT1 | The data belong to continuous variables and are analyzed with original data. |
| NT-proBNP (ng/l) | The data belong to continuous variables and are analyzed with original data. |
| hs-CRP (mg/l) | The data belong to continuous variables and are analyzed with original data. |
MALAT1, metastasis associated lung adenocarcinoma transcript 1; NT-proBNP, N-terminal pro-brain natriuretic peptide; hs-CRP, high sensitivity C-reactive protein.
Table V.
Multivariate analysis of poor prognosis in patients with heart failure.
| Factor | β | SE | Wald | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| No. of lesions | 0.063 | 0.015 | 3.172 | 1.231 | 1.085-1.404 | <0.05 |
| Diabetes | 0.017 | 0.055 | 3.689 | 1.424 | 1.043-1.716 | <0.05 |
| MALAT1 | 0.037 | 0.112 | 0.051 | 1.422 | 1.107-2.416 | <0.05 |
| NT-proBNP (ng/l) | 1.013 | 0.327 | 9.405 | 2.751 | 1.433-5.217 | <0.05 |
| hs-CRP (mg/l) | 0.204 | 0.415 | 7.171 | 1.219 | 1.185-4.405 | <0.05 |
OR, odds ratio; β, regression coefficient; SE, standard error; Wald, Chi-square value; OR, risk value; CI, confidence interval; MALAT1, metastasis associated lung adenocarcinoma transcript 1; NT-proBNP, N-terminal pro-brain natriuretic peptide; hs-CRP, high sensitivity C-reactive protein.
Discussion
Coronary heart disease is one of the most common atherosclerosis diseases. Its high morbidity and mortality have posed a serious threat to the life and health of modern people (15). At present, coronary stent implantation is the main treatment for coronary heart disease, but in-stent restenosis is one of the main causes of death of patients after stent therapy, and its specific pathogenesis has not been studied and elaborated in detail (16,17). Finding effective molecular indicators that can predict in-stent restenosis is of great clinical significance for patients undergoing stent implantation.
In our study, in order to provide molecular direction for the prediction of in-stent restenosis, we analyzed the MALAT1 expression in the serum of patients with coronary heart disease and the predictive value in-stent restenosis. First of all, we found that the MALAT1 expression in the serum of patients with coronary heart disease was significantly higher than that of subjects without coronary heart disease, and the serum MALAT1 of patients with coronary heart disease after treatment was significantly lower than that before treatment. In recent years, research on MALAT1 in cardiovascular diseases have been quite popular. For example, some studies (18) have found that MALAT1 is very sensitive in the peripheral matrix and can regulate the proliferation and migration of coronary artery vascular smooth muscle. Previous studies (19) have discovered that the MALAT1 expression in myocardial infarction has increased significantly, but few studies have been conducted on the expression and mechanism of MALAT1 in coronary heart disease. Then we further carried out ROC analysis and confirmed that the AUC of MALAT1 for coronary heart disease diagnosis was over 0.8, which suggested that MALAT1 had certain value for it. Then, in order to further analyze the correlation between MALAT1 and coronary heart disease, we detected other coronary heart disease related indexes like NT-proBNP, hs-CRP, LDH, and CK-MB before and after treatment and analyzed the correlation between them and MALAT1; we verified that NT-proBNP, hs-CRP, LDH, and CK-MB in the serum of coronary heart disease patients after treatment were significantly lower than those before treatment. Moreover, the expression levels of NT-proBNP, hs-CRP, LDH, CK-MB and MALAT1 in serum of patients with coronary heart disease were positively correlated. NT-proBNP is the product of BNP premise cleavage, which is mainly excluded by the kidney and has a high half-life, it often increases significantly in patients with myocardial ischemia and it's a vital clinical evaluation index for in-stent restenosis of coronary artery (20). Hs-CRP is an acute phase reaction protein synthesized and secreted by liver, which can evaluate the degree of inflammatory reaction (13). In the past, studies have proved that hs-CRP can activate endothelial cell function in atherosclerotic sites, thus leading to intimal damage and thrombosis and further leading to stenosis of the lumen (21). However, LDH and CK-MB are classic myocardial injury indicators, which can effectively reflect the myocardial injury of patients (22). The correlation analysis between MALAT1 and NT-proBNP, hs-CRP, LDH, CK-MB also proved that MALAT1 might be closely related to vascular stenosis.
Then we divided the patients into the RG and NSG according to the coronary angiography results one year later. Comparing the serum MALAT1 expression of patients in the two groups one month after stent implantation, we found that their serum MALAT1 expression in the RG was significantly higher than that in the NSG, and ROC analysis found that MALAT1 had higher predictive value for predicting AUC>0.8 of in-stent restenosis. This suggested that we could predict the discovery of in-stent restenosis by detecting the MALAT1 expression in the serum of patients after stent therapy, thus taking early intervention measures to effectively predict the occurrence of in-stent restenosis. Then we further analyzed the risk factors of in-stent restenosis, and discovered that the number of lesions, MALAT1, diabetes, NT-proBNP, and hs-CRP were independent risk factors of in-stent restenosis. Previous studies (23) have pointed out that the increase of hs-CRP has certain predictive value for the occurrence of in-stent restenosis, which is consistent with our research results. Some research (24) also have explained that NT-proBNP has certain value for poor prognosis of patients with coronary artery lesions after coronary intervention, which is also of certain reference significance for us.
To sum up, MALAT1 is highly expressed in the serum of patients with coronary heart disease, and it has high value in the diagnosis of coronary heart disease and the prediction of in-stent restenosis, and it is also an independent risk factor for poor prognosis of those with coronary heart disease. However, there are still some limitations in this study. For example, because the study of lncRNA in coronary heart disease is still at a preliminary stage, the relevant literature supports less, and a large number of experiments and data are still needed to support the research results in the later stage. Secondly, because this study is still an exploratory experiment, the sample size included is small, and we have not conducted in vitro cell experiments, the molecular mechanism of MALAT1 in coronary heart disease is still unclear, which requires us to further explore.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SQ wrote the manuscript, interpreted and analyzed the data. JS designed the study and performed the experiment. SQ was responsible for the analysis and discussion of the data. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of The Second Hospital of Shandong University (Jinan, China). Patients who participated in the study had complete clinical data. Signed written informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arslan M, Schaap J, Rood PP, Nieman K, Budde RP, Attrach M, Dubois EA, Dedic A. HEART score improves efficiency of coronary computed tomography angiography in patients suspected of acute coronary syndrome in the emergency department. Eur Heart J Acute Cardiovasc Care. 2020;9:23–29. doi: 10.1177/2048872619882424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaman MO, Mojadidi MK, Elgendy IY. Revascularization strategies for patients with myocardial infarction and multi-vessel disease: A critical appraisal of the current evidence. J Geriatr Cardiol. 2019;16:717–723. doi: 10.11909/j.issn.1671-5411.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alizadehsani R, Roshanzamir M, Abdar M, Beykikhoshk A, Khosravi A, Panahiazar M, Koohestani A, Khozeimeh F, Nahavandi S, Sarrafzadegan N. A database for using machine learning and data mining techniques for coronary artery disease diagnosis. Sci Data. 2019;6(227) doi: 10.1038/s41597-019-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verheye S, Vrolix M, Kumsars I, Erglis A, Sondore D, Agostoni P, Cornelis K, Janssens L, Maeng M, Slagboom T, et al. The SABRE trial (Sirolimus Angioplasty Balloon for Coronary In-Stent Restenosis): Angiographic results and 1-year clinical outcomes. JACC Cardiovasc Interv. 2017;10:2029–2037. doi: 10.1016/j.jcin.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Ohyama K, Matsumoto Y, Amamizu H, Uzuka H, Nishimiya K, Morosawa S, Hirano M, Watabe H, Funaki Y, Miyata S, et al. Association of coronary perivascular adipose tissue inflammation and drug-eluting stent-induced coronary hyperconstricting responses in pigs: 18F-fluorodeoxyglucose positron emission tomography imaging study. Arterioscler Thromb Vasc Biol. 2017;37:1757–1764. doi: 10.1161/ATVBAHA.117.309843. [DOI] [PubMed] [Google Scholar]
- 6.Cutlip DE, Garratt KN, Novack V, Barakat M, Meraj P, Maillard L, Erglis A, Jauhar R, Popma JJ, Stoler R, et al. 9-Month clinical and angiographic outcomes of the COBRA Polyzene-F NanoCoated coronary stent system. JACC Cardiovasc Interv. 2017;10:160–167. doi: 10.1016/j.jcin.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Hu J. Diagnostic value of circulating lncRNA ANRIL and its correlation with coronary artery disease parameters. Braz J Med Biol Res. 2019;52(e8309) doi: 10.1590/1414-431X20198309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Gao W, Long QQ, Zhang J, Li YF, Liu DC, Yan JJ, Yang ZJ, Wang LS. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep. 2017;7(7491) doi: 10.1038/s41598-017-07611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gou L, Zou H, Li B. Long noncoding RNA MALAT1 knockdown inhibits progression of anaplastic thyroid carcinoma by regulating miR-200a-3p/FOXA1. Cancer Biol Ther. 2019;20:1355–1365. doi: 10.1080/15384047.2019.1617567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu W, Ding H, Ouyang A, Zhang X, Xu Q, Han Y, Zhang X, Jin Y. LncRNA MALAT1 gene polymorphisms in coronary artery disease: A case-control study in a Chinese population. Biosci Rep. 2019;39(BSR20182213) doi: 10.1042/BSR20182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Untersteller K, Girerd N, Duarte K, Rogacev KS, Seiler-Mussler S, Fliser D, Rossignol P, Heine GH. NT-proBNP and echocardiographic parameters for prediction of cardiovascular outcomes in patients with CKD stages G2-G4. Clin J Am Soc Nephrol. 2016;11:1978–1988. doi: 10.2215/CJN.01660216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tayefi M, Tajfard M, Saffar S, Hanachi P, Amirabadizadeh AR, Esmaeily H, Taghipour A, Ferns GA, Moohebati M, Ghayour-Mobarhan M. hs-CRP is strongly associated with coronary heart disease (CHD): A data mining approach using decision tree algorithm. Comput Methods Programs Biomed. 2017;141:105–109. doi: 10.1016/j.cmpb.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Sun Z, Almutairi AM. Diagnostic accuracy of 64 multislice CT angiography in the assessment of coronary in-stent restenosis: A meta-analysis. Eur J Radiol. 2010;73:266–273. doi: 10.1016/j.ejrad.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Andreini D, Pontone G, Mushtaq S, Pepi M, Bartorelli AL. Multidetector computed tomography coronary angiography for the assessment of coronary in-stent restenosis. Am J Cardiol. 2010;105:645–655. doi: 10.1016/j.amjcard.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Amanuma M, Kondo T, Sano T, Takayanagi T, Matsutani H, Sekine T, Arai T, Morita H, Ishizaka K, Arakita K, et al. Assessment of coronary in-stent restenosis: value of subtraction coronary computed tomography angiography. Int J Cardiovasc Imaging. 2016;32:661–670. doi: 10.1007/s10554-015-0826-4. [DOI] [PubMed] [Google Scholar]
- 17.Seo DJ, Kim YK, Seo YH, Song IG, Kim KH, Kwon TG, Park HW, Bae JH. In-stent restenosis-prone coronary plaque composition: A retrospective virtual histology-intravascular ultrasound study. Cardiol J. 2018;25:7–13. doi: 10.5603/CJ.a2017.0124. [DOI] [PubMed] [Google Scholar]
- 18.Yang CH, Chuang LY, Lin YD. Multiobjective multifactor dimensionality reduction to detect SNP-SNP interactions. Bioinformatics. 2018;34:2228–2236. doi: 10.1093/bioinformatics/bty076. [DOI] [PubMed] [Google Scholar]
- 19.Zhao ZH, Wei H, Meng QT, Du XB, Lei SQ, Xia ZY. Long non-coding RNA MALAT1 functions as a mediator in cardioprotective effects of fentanyl in myocardial ischemia-reperfusion injury. Cell Biol Int. 2017;41:62–70. doi: 10.1002/cbin.10701. [DOI] [PubMed] [Google Scholar]
- 20.Grenning BA, Raymond I, Hildebrandt PR, Nilsson JC, Baumann M, Pedersen F. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma N-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population. Heart. 2004;90:297–303. doi: 10.1136/hrt.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Jiao Y, Wang H, Shang Q, Lu F, Huang L, Liu J, Xu H, Chen K. Sodium tanshinone IIA sulfate adjunct therapy reduces high-sensitivity C-reactive protein level in coronary artery disease patients: A randomized controlled trial. Sci Rep. 2017;7(17451) doi: 10.1038/s41598-017-16980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang WY, Zhang QL, Xu MJ. Effects of propofol on myocardial ischemia reperfusion injury through inhibiting the JAK/STAT pathway. Eur Rev Med Pharmacol Sci. 2019;23:6339–6345. doi: 10.26355/eurrev_201907_18457. [DOI] [PubMed] [Google Scholar]
- 23.Choi JH, Park HS, Kim DH, Cha JK, Huh JT, Kang M. Comparative analysis of endovascular stroke therapy using urokinase, penumbra system and retrievable (Solitare) stent. J Korean Neurosurg Soc. 2015;57:342–349. doi: 10.3340/jkns.2015.57.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao XY, Li JX, Tang XF, Xu JJ, Song Y, Jiang L, Chen J, Song L, Gao LJ, Gao Z, et al. Prognostic value of NT-proBNP in stable coronary artery disease in chinese patients after percutaneous coronary intervention in the drug-eluting stent Era. Biomed Environ Sci. 2018;31:859–866. doi: 10.3967/bes2018.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.