Abstract
The present study is a clinical trial analyzing follicular fluid. The current study aimed to assess whether a correlation exists among estradiol (E2), anti-Mullerian hormone (AMH) and prokineticin 1 (PROK1) levels in the follicular fluid. A total of 81 infertile patients (53 with primary infertility and 28 with secondary infertility) who received routine in vitro fertilization (IVF) and embryo transfer (ET) or intracytoplasmic sperm injection at Yuhuangding Hospital (Yantai, China) were included in the present study. On the day of egg retrieval, follicular puncture and follicular fluid extraction were performed on patients using double lumen needles under the guidance of a vaginal ultrasound. In 77 cases, follicular fluid was collected from the follicle with the largest diameter. A total of 53 cases underwent ET and subsequent pregnancy outcomes were traced. Concentrations of E2, AMH and PROK1 in the single follicular fluid specimens were determined. The concentration of E2 in follicular fluid from the largest follicles in absolute pregnancy group was significantly lower than that in absolute non-pregnancy group. The concentrations of PROK1 and AMH in follicular fluid from the largest follicles in absolute pregnancy group were not significantly different from those in absolute non-pregnancy group. The concentration of E2 was associated with the dosage of gonadotropin, but was not associated with age, AMH and PROK1 levels in follicular fluid, fertilization rate or number of usable blastocysts. The area under curve revealed that E2 level in the follicular fluid exhibited a low predictive value for pregnancy outcome. The present study demonstrated that E2 level is a better predictor for the outcome of IVF-ET than AMH or PROK1 levels in the follicular fluid.
Keywords: estradiol, anti-Mullerian hormone, prokineticin 1, follicular fluid, in vitro fertilization, embryo transfer
Introduction
The outcome of in vitro fertilization (IVF) is influenced by a number of factors. One of the most important factors is oocyte quality. The microenvironment of the follicular fluid is important for oocyte development (1). The analysis of oocyte-derived products, including follicular fluid and cumulus cells, has provided novel candidates that can be used to assess oocyte competence and embryo implantation ability (2-6). In the endometrium, prokineticin (PROK) 1 protein expression is highest at the midluteal phase of the menstrual cycle and this corresponds to the window of embryo implantation and has been reported to be a biomarker for human endometrial receptivity (7-9). PROKs promote the contractile function of ileal longitudinal muscles (10). PROK1 and PROK2 share 44% homology but exhibit different effects (10). PROK2 is mainly expressed in the central nervous system and in primary spermatocytes. PROK2 (-/-) mice display hypoplasia of olfactory bulb as well as an insufficient number and a reduced function of gonadotropin (Gn) releasing hormone (GnRH) neurons, which reduces GnRH secretion and affects the initiation of reproductive axis, which is related to the occurrence of Kallmann Syndrome (11). Additionally, PROK2 plays an important role in the final stage of spermatogenesis (12). The selection of dominant follicles often requires more complex capillary networks and angiogenesis is closely associated with follicular development (13). PROK1 has been revealed to promote angiogenesis and is also known as endocrine gland (EG)-derived vascular endothelial growth factor (VEGF) (10). The PROK1 precursor protein contains 19 signal peptides that are formed by amino acids (AA). Mature PROK1 is composed of 86 AA but does not belong to the VEGF family. However, mature PROK1 shares homology with mamba intestinal toxin and bombina variegate 8(14). Human PROK1 is expressed mainly in the glands that produce natural steroids (10,14-19). Kisliouk et al (18) demonstrated that the PROK mRNA was expressed in luteinizing granulosa cells. Treatment with denylate cyclase activator forskolin was revealed to upregulate PROK1 mRNA expression in SVOG cells in a dose-dependent manner (18). By contrast, classical angiogenic factors, including hypoxia and thrombin, inhibit PROK1 expression in SVOG cells (18). Studies have indicated that PROK1 expression in human, chimpanzee and bovine ovaries exhibited clear spatial and temporal specificity (8,14,18). It has also been revealed that PROK1 is dynamically distributed during folliculogenesis, meaning that the expression of PROK1 in primordial and primary follicles is significantly higher compared with expression in the antral follicles (19). This suggests that the differential regulation of PROK1 expression by hormones exists during follicular maturation (19). It has been revealed that human chorionic Gn (HCG) can increase PROK1 expression in luteinizing granulosa cells and the placenta by binding with luteinizing hormone/choriogonadotropin receptor (20). This indicates that the increase in PROK1 secretion and expression is a response to peaks in physiological luteinizing hormone (LH) and that exogenous HCG and is an important factor in the final maturation of oocytes (20). A previous study has demonstrated that the level of PROK1 in the follicular fluid of the implantation group was significantly higher compared with the non-implantation group. Implantation potential could also be predicted if PROK1 >5.7 ng/follicle (19). Furthermore, it has also been reported that no significant difference in PROK1 level in the follicular fluid was observed between the pregnant group with a normal ovarian response and the non-pregnant group with a normal ovarian response (21). Anti-Mullerian hormone (AMH) is a glycoprotein in the transforming growth factor-β superfamily and is secreted by granular cells in the sinusoidal and preantral follicles (22). After HCG injection, the granular cells are highly luteinized and the secretion of AMH may therefore be reduced (23). It remains undetermined whether changes in follicular fluid or peripheral blood PROK1 during an ovulation induction cycle are closely associated with vascularization and follicular development and if this can represent the degree of follicle maturation or predict pregnancy outcome. In the present study, PROK1, AMH and estradiol (E2) was assessed in follicular fluid and the present study aimed to assess if these can predict the outcome of clinical pregnancy. Assessments were performed using follicular fluid secreted by highly luteinizing granulosa cells during the induction of ovulation. The current study also aimed to assess whether there was a correlation among E2, AMH and PROK1 levels in in follicular fluid and whether E2, AMH and PROK1 levels were associated with fertilization rate, good embryo rate and the number of usable blastocysts, which indirectly reflected the potential success rate of embryo implantation.
Materials and methods
Patients
A total of 81 infertile female patients (53 with primary infertility and 28 with secondary infertility) who received routine IVF-ET or intracytoplasmic sperm injection (ICSI) between May and July 2016 at the Reproductive Medical Center of the Yuhuangding Hospital (Yantai, China) were included in the present study. The mean age of the patients was 32.30±3.67 years, mean body mass index (BMI) was 24.05±3.98 kg/m2, infertile period was 4.57±2.8 years, basal follicle stimulating hormone (FSH) level was 6.56±2.16 mIU/ml and basal AMH level prior to downregulation was 4.12±2.98 ng/ml. Among all patients included in the current study, 63 underwent routine IVF (including 1 patient who received a sperm donation), 15 underwent ICSI and 3 underwent IVF + rescue ICSI. Patients aged >45 years, patients who exhibited karyotype abnormalities, had a history of ovarian hyperstimulation syndrome (OHSS), endometriosis, repeated embryo implantation failures, ectopic pregnancy, ovarian surgery, ovarian benign or malignant tumors or smoking were excluded from the present study. No eligible follicular fluid was obtained from 4 of the patients and the oocytes were not mature after ovum retrieval in 2 patients. A total of 75 cases underwent IVF/ICSI and embryo culture. Among these 75 cases, 22 cases cancelled the embryo transfer. As a result, a total of 53 cases underwent embryo transfer and their pregnancy outcomes were traced. All procedures performed in the current study were approved by the Ethics Committee of Yuhuangding Hospital. Written informed consent was obtained from all patients or their families.
Downregulation, ovulation induction and follicular monitoring
Among the 81 patients, 62 received downregulation using a long regimen with luteal phase Gn releasing hormone (GnRH) agonist (24). A total of 7 days after ovulation, 0.05 mg triptorelin (Ipsen Pharma Biotech S.A.S.) was given to patients for a period of 16 days of downregulation. The starting dose and timing of Gn were subsequently determined according to the number of antral follicles, body mass index and size of follicles in patients (25,26). Transvaginal ultrasound scans were performed on day 6 of ovarian stimulation. Follicle diameters <10 mm and E2 levels <200 pg/ml on day 6 were defined as a slow response. If the slow response occurred, the dose of Gn was increased. Gn included recombinant human follicle stimulating hormone (Gonal-F®; Merck KGaA) and human menopausal Gn (Lizhu Pharmaceutical Trading Co., Ltd.). Transvaginal ultrasound scan was performed on day 6 of ovarian stimulation. If the low response occurred, the dose of Gn needed to be increased. The remaining 19 patients underwent GnRH-antagonist regimen (27). During the menstrual period, a transvaginal ultrasound was used to confirm that no follicle exceeded 10 mm in diameter. According to the ovarian reserve, body mass index and follicle size, the starting dose of Gn was determined (26). Patients with follicle diameters >14 mm or luteinizing hormone (LH) expression levels >10 mIU/ml, received injections of 0.25 mg ganirelix acetate (Orgalutran; Merck Sharp & Dohme-Hoddesdon) prior to the injection of human chorionic Gn (HCG). The time of HCG injection (8000 IU; Lizhu Pharmaceutical Trading Co., Ltd.) was determined by follicular size (the diameter of at least 2 dominance follicles ≥18 mm) and levels of E2 (at least 300 pg/ml per dominant follicle) (28).
Oocyte retrieval and fertilization
At 34-36 h after HCG injection, oocytes were retrieved under the guidance of a vaginal ultrasound. Oocytes were hatched 4 h prior to in vitro fertilization. Embryos were transferred on day 3 (cleavage stage) or day 5/day 6 (blastocyst stage). Only fertilized oocytes (2 pronuclei; 2PN) were further cultured to the blastocyst stage for 5 or 6 days, and unfertilized oocytes were discarded. Luteal support was provided after transplantation. Progesterone capsules (200 mg; UTROGESTAN; Besins Healthcare) were given via vaginal administration, q8 h, until week 10 of gestation. Transplantation was cancelled if (OHSS) occurred, the thickness of endometrium was <7 mm or no transplantable embryo was available. The total fertilization rate of IVF was calculated using the following formula: N=number of 2PN oocytes + number of 1PN oocytes + number of multi-PN oocytes + number of 0PN cleavage oocytes)/total number of sperm oocytes x100. Total fertilization rate of ICSI was calculated using the following formula: N=(number of 2PN oocytes + number of 1PN oocytes + number of multi-PN oocytes)/total number of oocytes injected with MetaphaseII x100. These results were combined together to get the overall fertilization rate, The formula for calculating the good embryos rate is as follows: Good embryo rate=the number of good embryos/the number of cleavage stage embryos. Cleavage embryos with seven or eight cells on day 3 after oocyte retrieval that contained <20% anucleate fragments and no apparent morphological abnormalities were classified as good embryo (29).
Sample collection
On the day of egg retrieval, follicular puncture and follicular fluid extraction were performed on patients using double lumen needles under the guidance of a vaginal ultrasound. Follicular fluid was collected from the follicle with the largest diameter (single follicular fluid) prior to centrifugation at 3,000 x g for 15 min at 4˚C to separate cells. Fasting elbow vein blood (2 ml) was collected in anticoagulant blood tubes on the morning of egg retrieval. The blood was centrifuged at 450 x g for 30 min in 25˚C prior to serum collection.
Grouping standard
A total of 2 weeks after transplantation, patients with blood β-HCG <10 mIU/ml were included in the absolute non-pregnancy group (n=27). A total of 34 days after transplantation, patients with blood β-HCG >10 mIU/ml received ultrasonography and those with a gestational sac were included in the absolute pregnancy group (n=20). If blood β-HCG was >10 mIU/ml but no gestational sac was observed, the patients were included in the possible pregnancy group (n=6), which was not included in the analysis of the current study.
Electrochemiluminescence
A Cobas e Immunoassay Analyzer (Roche Diagnostics) was used to determine E2 and β-HCG levels in the follicular fluid according to the manufacturer's protocol. The dilution ratio of follicular fluid ranged from 1:100 to 1:400. The lower and upper limits of E2 detection were 18.4 pmol/l (5 pg/ml) and 11,010 pmol/l (3,000 pg/ml), respectively.
ELISA
The levels of PROK1 in serum and follicular fluid were determined using a PROK1 ELISA kit (cat. no. 900-k244; PeproTech Inc.), and the detection concentration range was 6-2000 pg/ml. The sample was diluted if the concentration was out of the aforementioned range. AMH level in both serum and follicular fluid. was determined using an AMH ELISA kit (cat. no. KR-001; Guangzhou Kangrun Biotech Co., Ltd.) according to manufacturer's protocol. The detection range was 0.06-18 ng/ml.
Statistical analysis
All results were analyzed using SPSS software (version 21.0; IBM Corp.). Each measurement was repeated three times. The means of two groups of data with a normal distribution were compared using a Student's t-test. A Mann-Whitney U rank sum test was used to compare the difference between two groups of non-normal distribution. When the two variables were normally distributed, a Pearson correlation analysis was used. When the variables did not conform to the assumption of a normal distribution of bivariate variables, a Spearman's rank correlation analysis was performed. Linear regression was used to analyze the association between the dose of Gn and the levels of E2 and PROK1. A χ2 test was used for the comparison of frequencies. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the accuracy of follicular fluid E2, AMH and PROK1 levels in predicting pregnancy rate. P<0.05 was considered to indicate a statistically significant difference.
Results
General characteristics of participants
A total of 81 patients met the inclusion criteria and 77 follicular fluid specimens that met the requirements were obtained (Table I).
Table I.
Baseline characteristics of enrolled patients.
| Characteristic | Statistics, median (interquartile range)/mean ± SD/% rate |
|---|---|
| Gn dosage (IU) | 1,575 (350-2,025) |
| E2 on HCG day (pg/ml) | 2,425 (1,373.75-3,767.75) |
| Endometrium thickness on HCG day (mm) | 11 (6-15) |
| E2 concentration in peripheral blood on day of OR (pg/ml) | 1,415 (942.55-1,992) |
| PROK1 concentration in peripheral blood on day of OR (pg/ml) | 39.55 (28-63.34) |
| AMH concentration in peripheral blood on day of OR (ng/ml) | 0.866 (0.42-1.36) |
| Follicular fluid specimen volume (ml) | 3.4 (1.2-5.7) |
| E2 concentration in follicular fluid (pg/ml) | 64,0349±291,957 |
| AMH concentration in follicular fluid (ng/ml) | 1.52 (0.96-2.65) |
| PROK1 concentration in follicular fluid (pg/ml) | 833 (462-1,376) |
| No. of retrieved oocytes | 8.40±4.41 |
| No. of mature oocytes | 6.44±3.67 |
| No. of fertilized oocytes | 6 (3-9) |
| Good embryo rate | 57.03% |
FSH, follicle-stimulating hormone; AMH, anti-Mullerian hormone; Gn, gonadotropin; E2, estradiol; HCG, human chorionic Gn; PROK1, prokineticin 1; OR, oocyte retrieval; SD, standard deviation. Data with normal distribution are expressed as the mean ± SD. Data with non-normal distribution were expressed using the median (25-75%).
Comparison between the absolute pregnancy group and absolute non-pregnancy group
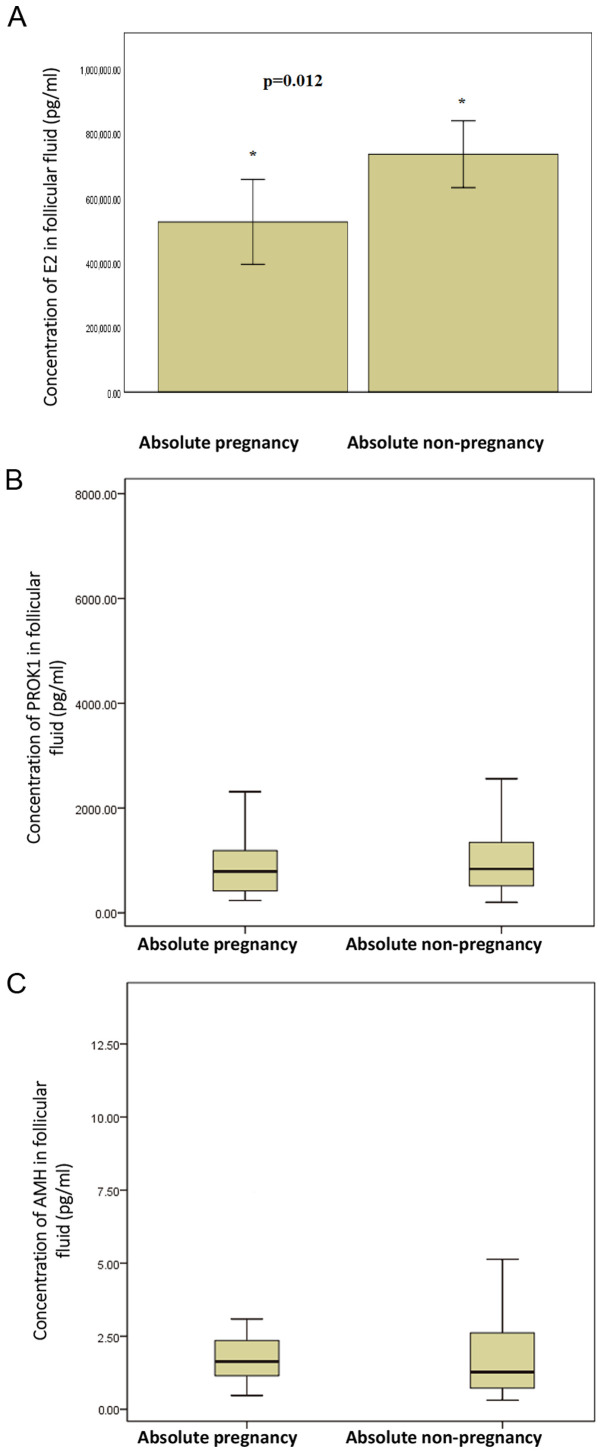
Among 53 patients with embryo transfer, 20 cases exhibited an absolute pregnancy, 27 cases exhibited an absolute non-pregnancy, and 6 cases exhibited a possible pregnancy. Among the 20 cases with absolute pregnancy, 13 had a single fetus and 7 had a double fetus. The concentration of E2 in the follicular fluid from the largest follicles in the absolute pregnancy group (526419±289944 pg/ml) was significantly lower compared with the absolute non-pregnancy group (736085±261885 pg/ml; t=-2.742; P=0.012; Fig. 1A; Table II). The concentrations of PROK1 and AMH in the follicular fluid from the largest follicles in absolute pregnancy group were not significantly different from those in the absolute non-pregnancy group (Fig. 1B and C; Table II). In addition, the concentrations of peripheral blood PROK1 and AMH in the absolute pregnancy group were not significantly different from those in the absolute non-pregnancy group on the day of egg retrieval (Table II). The fertilization rate of absolute pregnancy group was significantly higher than that of absolute non-pregnancy group (χ2=5.861; P=0.015; Table II).
Figure 1.
Concentrations of (A) E2, (B) PROK1 and (C) AMH in follicular fluid in absolute pregnancy group and absolute non-pregnancy group. The results in (A) are presented as the mean ± SD. The results in (B) and (C) are presented as box-plot diagrams (These are split up by the maximum-upper quartile-median-lower quartile-minimum). *P<0.05. E2, estradiol; PROK1, prokineticin 1; AMH, anti-Mullerian hormone; SD, standard deviation
Table II.
Comparison of variables between absolute pregnancy group and non-pregnancy group.
| Variable | Absolute pregnancy group group (n=21) | Absolute non-pregnancy (n=27) | Statistical value | P-value |
|---|---|---|---|---|
| Age (years) | 32.29±2.59 | 32.26±4.27 | 0.027a | 0.979 |
| Years of infertility | 4.95±3.09 | 4.87±2.98 | 0.093a | 0.926 |
| Constituent ratio of primary infertility | 47.62% (10/21) | 70.37% (19/27) | 2.557b | 0.110 |
| Body mass index (kg/m2) | 24.84±3.94 | 23.87±3.41 | 0.909a | 0.368 |
| Basal FSH (mIU/ml) | 6.54 (2.95-10.04) | 6.56 (3.25-12.45) | -0.753c | 0.451 |
| Basal AMH (ng/ml) | 4.17±2.69 | 3.67±2.25 | 0.707a | 0.483 |
| Gn dosage (IU) | 1,855±421 | 1,628±448 | 1.797a | 0.079 |
| E2 on HCG day (pg/ml) | 2,232±1,317 | 2,640±1,974 | 0.108a | 0.421 |
| Endometrium thickness on HCG day (mm) | 11 (9-12) | 7 (4.25-10.75) | -0.274c | 0.784 |
| No. of retrieved oocytes | 9.00±3.45 | 7.28±3.56 | 1.789a | 0.079 |
| E2 concentration on day of OR (pg/ml) | 1,415 (985-1,673) | 1,199 (718-2336) | 0.322c | 0.747 |
| PROK1 concentration on day of OR (pg/ml) | 44.33 (16-285) | 41.03 (13-314) | -1.091c | 0.275 |
| AMH concentration on day of OR (ng/ml) | 0.798 (0.15-4.14) | 0.835 (0.66-4.8) | -0.200c | 0.841 |
| PROK1 concentration in follicular fluid (pg/ml) | 797.91 (235.99-2,653.33) | 836.78 (201.61-7,468.00) | -0.785c | 0.433 |
| AMH concentration in follicular fluid (ng/ml) | 1.63 (0.47-6.96) | 1.27 (0.31-13.69) | -1.232c | 0.218 |
| E2 concentration in follicular fluid (pg/ml) | 526,419±289,944 | 736,085±261,885 | -2.626a | 0.012 |
| Fertilization rate | 88.4% (137/155) | 78.5% (146/186) | 5.861b | 0.015 |
| No. of usable blastocysts | 2.62±2.31 | 1.58±2.04 | 1.596a | 0.118 |
Data with normal distribution are expressed as the mean ± SD. Data with non-normal distribution were expressed using the median (± interquartile range).
aData analyzed using Student-t test;
bdata analyzed using χ2-square test;
cdata analyzed using Mann-Whitney U rank sum test. FSH, follicle-stimulating hormone; AMH, anti-Mullerian hormone; Gn, gonadotropin; E2, estradiol; OR, oocyte retrieval; HCG, human chorionic Gn; PROK1, prokineticin.
Concentration of E2 in follicular fluid is associated with the Gn dose
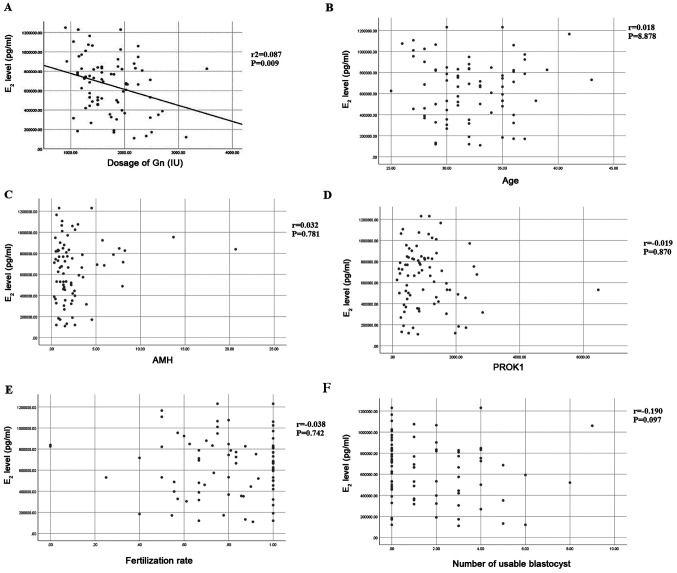
The relationship between Gn dose and E2 concentration in follicular fluid was analyzed by linear regression analysis. The regression coefficient is -164.865, constant 942432.470. T statistic value of regression coefficient t-test is -2.670, r2=0.087, P=0.009 (Fig. 2A). Therefore, regression coefficient can be considered to be significant, from which the linear regression equation can be obtained: Y=942432.470-164.865X. To examine if E2 was correlated with other variables, a correlation analysis was performed. The concentration of E2 was not associated with age (r=0.018, P=0.878), AMH level in follicular fluid (r=0.032, P=0.781), PROK1 level in follicular fluid (r=-0.019, P=0.870), fertilization rate (r=-0.038, P=0.742) or number of usable blastocysts (r=-0.109, P=0.097) (Fig. 2B-F).
Figure 2.
Association between the E2 concentration in follicular fluid and (A) Gn dosage, (B) age, (C) AMH in follicular fluid, (D) PROK1 in follicular fluid, (E) fertilization rate and (F) the number of usable blastocysts. E2, estradiol; Gn, gonadotropin; AMH, anti-Mullerian hormone; PROK1, prokineticin 1.
Follicular fluid PROK1 level is not correlated with Gn dosage but age
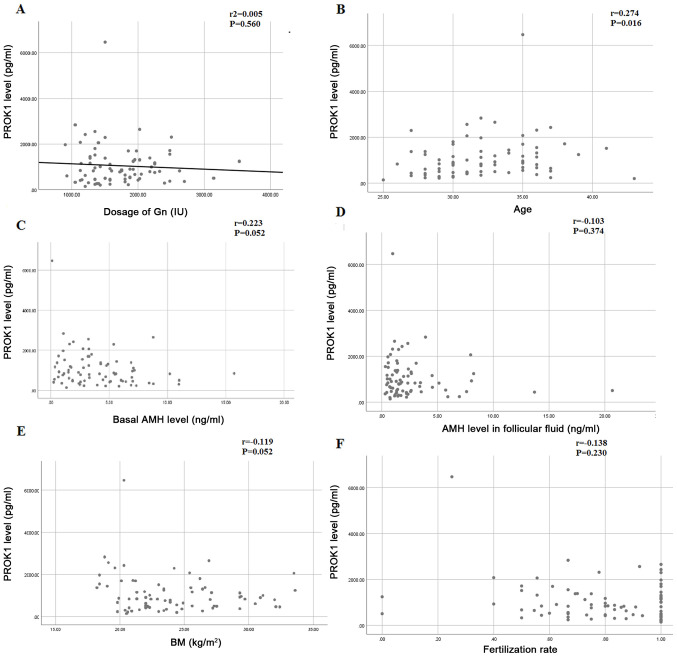
The relationship between the dose of Gn and the concentration of PROK1 in follicular fluid was analyzed using a linear regression analysis. The dose of Gn is the independent variable on the x-axis, the concentration of PROK1 in follicular fluid is the dependent variable on the y-axis, the determination coefficient, r2=0.005, the regression coefficient is -0.119, the constant is 1263, the observation value of T statistics of regression coefficient T test is -0.585, and the P value of t-test is 0.560. Therefore, the regression coefficient displayed no significance (Fig. 3A). To examine if PROK1 was correlated with other variables, a correlation analysis was performed. The levels of PROK1 in follicular fluid was correlated with age (r=0.274, P=0.016) (Fig. 3B). There was no significant correlation between PROK1 in follicular fluid and basal AMH (r=0.223, P=0.052), AMH in follicular fluid (r=-0.103, P=0.374), body mass index (BMI) (r=-0.119, P=0.301) and fertilization rate (r=-0.138, P=0.230) (Fig. 3C-F)
Figure 3.
Correlation between PROK1 in follicular fluid and (A) Gn dosage, (B) age, (C) basal AMH, (D) AMH in follicular fluid, (E) BMI and (F) fertilization rate. PROK1, prokineticin 1; AMH, anti-Mullerian hormone; BMI, body mass index.
E2 level does not affect the number of retrieved oocytes, fertilization rate and good embryo rate
Using the median of E2 levels in final fertilized follicular fluid (676610 pg/ml) from the 75 cases as the cut-off point, these cases were divided into the low E2 group (≤676610 pg/ml; 37 cases) and high E2 group (>676610 pg/ml; 38 cases). The number of retrieved oocytes, fertilization rate and good embryo rate were not significantly different between the two groups (Table III). The result suggests that E2 level did not affect the number of retrieved oocytes, fertilization rate and good t embryo rate.
Table III.
Number of retrieved eggs, fertilization rate and good embryo rate in the low and high E2 groups.
| Characteristic | Low E2 group (mean ± SD) | High E2 group (mean ± SD) | χ2/t-value | P-value |
|---|---|---|---|---|
| No. of retrieved eggs | 9.03±4.23 | 8.37±4.59 | 0.565a | 0.520 |
| Fertilization rate | 81.99% (264/322) | 80.74% (239/296) | 0.158b | 0.691 |
| Good embryo rate | 70.89% (168/237) | 65.78% (148/225) | 1.393b | 0.238 |
aData analyzed using Student's t-test;
bdata analyzed using χ2-square test. E2, estradiol.
E2 level in follicular fluid can predict the outcome of pregnancy
To evaluate the predictive value of E2 level in follicular fluid, a ROC curve was plotted and AUC was calculated. The AUC was 0.283 (95% CI, 0.144-0.423; P=0.007; Fig. 4). The AUC was significant which indicates that E2 levels can discriminate between pregnancy outcomes. The optimum cut off value of E2 level in follicular fluid is 689345 pg/ml, which was shown to predict pregnancy outcome with a sensitivity of 27.3%, and specificity of 37.5%.
Figure 4.
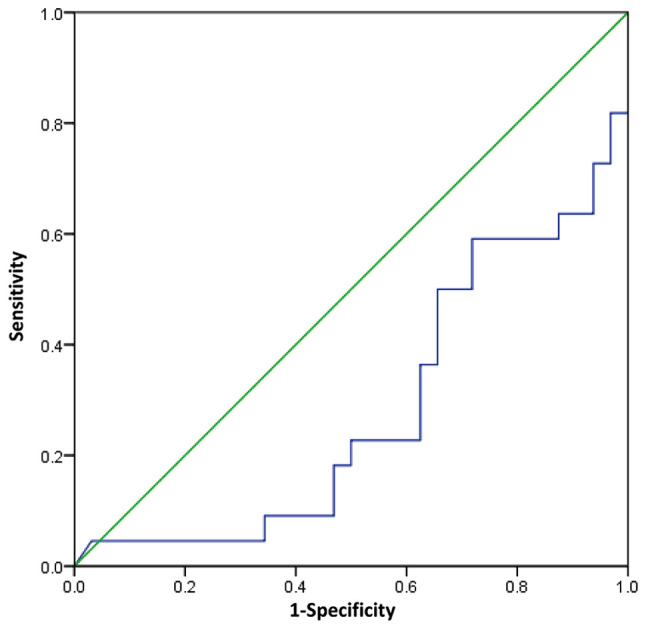
ROC curve of E2 level in follicular fluid and pregnancy outcome. The ROC and area under curve were calculated. The green line represents the reference line. The blue line represents the E2 results. ROC, receiver operating characteristic curve; E2, estradiol.
Discussion
The concentration of E2 in the largest single follicular fluid (657425±275979 pg/ml) of the present study was 10-50 times greater than has been reported previously (30,31). This may be due to the high number of granulosa cell layers in the largest follicle, which increases E2 secretion (32). Additionally, E2 concentration in peripheral blood on the day of egg retrieval was 1565±1001 pg/ml, 400 times lower than that in the follicular fluid. The median level of PROK1 in the follicular fluid on the day of egg retrieval was only 21 times greater than that in the peripheral blood, demonstrating that vascular endothelial cells underwent rapid proliferation from luteinized granular cells to well-functioning granular corpus luteum and membranous corpus luteum. PROK1 vasculogenic factor is associated with this aforementioned process, in which PROK1 aggregates in a short amount of time to exhibit an increased PROK1 level compared with the peripheral blood (29,33). By contrast, the median level of AMH in the follicular fluid on the day of egg retrieval was only slightly higher than levels in the peripheral blood. This result may be due to AMH secreted by small antral follicles being the main source of AMH in the peripheral blood, instead of follicular fluid AMH (34).
E2 level in the follicular fluid of the absolute pregnancy group was demonstrated to be significantly lower than that in the absolute non-pregnancy group, suggesting that high levels of estrogen had a negative effect on the pregnancy outcome. It has been previously reported that high estrogen levels in the peripheral blood can lead to poor pregnancy outcomes by affecting endometrial receptivity (35,36). In the present study, high E2 levels in the follicular fluid exhibited an adverse effect on pregnancy outcome, suggesting that abnormally high estrogen levels might affect the development potential of adjacent oocytes via a variety of autocrine paracrine signaling pathways. One previous study has revealed that E2 concentration in single follicular fluid was negatively correlated with good embryo rate (7). However, the current study failed to demonstrate this correlation (data not shown). The results indicated that a high E2 level in the follicular fluid was negatively associated with Gn dose. The ROC curve analyzed the diagnostic accuracy of E2 levels to discriminate between absolute pregnancy per transfer and absolute non pregnancy per transfer. In the ROC model, E2 levels were predictive of pregnancy outcome. However, the AUC was <0.5 and the sensitivity and specificity of the cut off value was low.
The selection of dominant follicles often requires complex capillary networks, and angiogenesis is closely associated with follicular development (9). In the ovaries, PROK1 not only promotes angiogenesis, but also affects ovarian function (19,29). However, its specific roles have not yet been clearly determined. A previous study has demonstrated that PROK1 secretion in the follicular fluid is significantly different between pregnant and non-pregnant patient groups (19); however, contradictory results have also been reported (21). In the present study, no significant differences were observed between PROK1 expression in the follicular fluid of the pregnant and non-pregnant group. The promoter of the PROK1 gene has a cAMP response element (CRE) binding site (20). It has been previously reported that AMH inhibited the binding of follicle-stimulating hormone to follicle-stimulating hormone receptor through autocrine paracrine and affects the transcription of estrogen synthetase (37). The results in the present study revealed that PROK1 expression and AMH in the follicular fluid exhibited no correlation. The level of PROK1 in follicular fluid was correlated with age but not correlated with basal AMH. The level of PROK1 in follicular fluid of older people showed an increasing trend However, this possible correlation between the two requires further study.
In the present study, no significant differences were exhibited in the baseline characteristics between the absolute pregnancy group and the absolute non-pregnancy group, suggesting that the two groups were comparable. However, the limitation of the present study is that a small number of cases was used for analysis, which may affect the results. Additionally, the upper limit of the electrochemiluminescence reagent for the determination of E2 concentration was 3,000 pg, and some samples required dilution 400 times, which may have also influenced the results.
In conclusion, the present study demonstrated that E2 level in follicular fluid is a better predictor of the outcome of IVF-ET compared with AMH or PROK1 level determination.
Acknowledgements
The authors wish to thank Dr Jiangfeng Lv from Clinical Laboratory of Jinan People's Hospital (Jinan, China) and Dr Xiumei Yu from Affiliated Yuhuangding Hospital of Qingdao University (Yantai, China) for their help in the hormone tests.
Funding
The current study was supported by the National Natural Science Foundation of China (grant. no. 81671416).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Each author believes that the manuscript represents honest work. YL and CH collaborated to design the study. YL and XH were responsible for performing experiments. YL and SD analyzed the data. All authors collaborated to interpret results and develop the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures performed in the current study were approved by the Ethics Committee of Affiliated Yuhuangding Hospital of Qingdao University. Written informed consent was obtained from all patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Durán Reyes G, Rosales AM, Hicks Gómez JJ. Participation of the follicular fluid in follicular development, oocyte maturation and spermatic function. Ginecol Obstet Mex. 1997;65:349–356. (In Spanish) [PubMed] [Google Scholar]
- 2.Hammadeh ME, Ertan AK, Zeppezauer M, Baltes S, Georg T, Rosenbaum P, Schmidt W. Immunoglobulins and cytokines level in follicular fluid in relation to etiology of infertility and their relevance to IVF outcome. Am J Reprod Immunol. 2002;47:82–90. doi: 10.1034/j.1600-0897.2002.1o024.x. [DOI] [PubMed] [Google Scholar]
- 3.Lédée N, Petitbarat M, Rahmati M, Dubanchet S, Chaouat G, Sandra O, Perrier-d'Hauterive S, Munaut C, Foidart JM. New pre-conception immune biomarkers for clinical practice: Interleukin-18, interleukin-15 and TWEAK on the endometrial side, G-CSF on the follicular side. J Reprod Immunol. 2011;88:118–123. doi: 10.1016/j.jri.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Vercammen M, Verloes A, Haentjens P, Van de Velde H. Can soluble human leucocyte antigen-G predict successful pregnancy in assisted reproductive technology? Curr Opin Obstet Gynecol. 2009;21:285–290. doi: 10.1097/gco.0b013e32832924cd. [DOI] [PubMed] [Google Scholar]
- 5.Hammadeh ME, Fischer-Hammadeh C, Amer AS, Rosenbaum P, Schmidt W. Relationship between cytokine concentration in serum and preovulatory follicular fluid and in vitro fertilization/intracytoplasmic sperm injection outcome. Chem Immunol Allergy. 2005;88:80–97. doi: 10.1159/000087822. [DOI] [PubMed] [Google Scholar]
- 6.Ovayolu A, Özdamar Ö, Gün İ, Arslanbuğa CY, Kutlu T, Tunalı G, Uluhan R. The assesment of follicular fluid presepsin levels in poor ovarian responder womenandits relationship with the reproductive outcomes. Int J Clin Exp Med. 2015;8:9961–9966. [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta BN, Chimote MN, Chimote NN, Nath NM, Chimote NM. Follicular-fluid anti-Mullerian hormone (FF AMH) is a plausible biochemical indicator of functional viability of oocyte in conventional in vitro fertilization (IVF) cycles. J Hum Reprod Sci. 2013;6:99–105. doi: 10.4103/0974-1208.117168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallam HN, Sallam NH, Sallam SH. Non-invasive methods for embryo selection. Facts Views Vis Obgyn. 2016;8:87–100. [PMC free article] [PubMed] [Google Scholar]
- 9.Koo YA, Lee B, Park HJ, Choi J, Lee E, Choi D. Altered vascular endothelial growth factor expression during GnRH antagonist protocol in women of reproductive age with normal baseline hormone profiles. Fertil Steril. 2009;91:744–748. doi: 10.1016/j.fertnstert.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: Recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu LH, Yu LL, Xiong CL, Zhang HP. Potential role of prokineticin 2 in experimental varicoceleinduced rat testes. Urology. 2012;80:952.e15–19. doi: 10.1016/j.urology.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Oh DS, Jeong JH, Shin BS, Joo BS, Lee KS. Follicular blood flow is a better predictor of the outcome of in vitro fertilization-embryo transfer than follicular fluid vascular endothelial growth factor and nitric oxide concentrations. Fertil Steril. 2004;82:586–592. doi: 10.1016/j.fertnstert.2004.02.120. [DOI] [PubMed] [Google Scholar]
- 14.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 15.Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724–8729. doi: 10.1074/jbc.M110594200. [DOI] [PubMed] [Google Scholar]
- 16.Kisliouk T, Friedman A, Klipper E, Zhou QY, Schams D, Alfaidy N, Meidan R. Expression pattern of prokineticin 1 and its receptors in bovine ovaries during the estrous cycle: Involvement in corpus luteum regression and follicular atresia. Biol Reprod. 2007;76:749–758. doi: 10.1095/biolreprod.106.054734. [DOI] [PubMed] [Google Scholar]
- 17.Lee KF, Lee YL, Chan RW, Cheong AW, Ng EH, Ho PC, Yeung WS. Up-regulation of endocrine gland-derived vascular endothelial growth factor but not vascular endothelial growth factor in human ectopic endometriotic tissue. Fertil Steril. 2010;93:1052–1060. doi: 10.1016/j.fertnstert.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Kisliouk T, Levy N, Hurwitz A, Meidan R. Presence and regulation of endocrine gland vascular endothelial growth factor/prokineticin-1 and its receptors in ovarian cells. J Clin Endocrinol Metab. 2003;88:3700–3707. doi: 10.1210/jc.2003-030492. [DOI] [PubMed] [Google Scholar]
- 19.Alfaidy N, Hoffmann P, Gillois P, Gueniffey A, Lebayle C, Garçin H, Thomas-Cadi C, Bessonnat J, Coutton C, Villaret L, et al. PROK1 level in the follicular microenvironment: A new noninvasive predictive biomarker of embryo implantation. J Clin Endocrinol Metab. 2016;101:435–444. doi: 10.1210/jc.2015-1988. [DOI] [PubMed] [Google Scholar]
- 20.Brouillet S, Hoffmann P, Chauvet S, Salomon A, Chamboredon S, Sergent F, Benharouga M, Feige JJ, Alfaidy N. Revisiting the role of hCG: New regulation of the angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci. 2012;69:1537–1550. doi: 10.1007/s00018-011-0889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao MZ, Zhao XM, Lin Y, Sun ZG, Zhang HQ. Effects of EG-VEGF, VEGF and TGF-β1 on pregnancy outcome in patients undergoing IVF-ET treatment. J Assist Reprod Genet. 2012;29:1091–1096. doi: 10.1007/s10815-012-9833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visser JA, Themmen AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Fanchin R, Louafi N, Méndez Lozano DH, Frydman N, Frydman R, Taieb J. Per-follicle measurements indicate that anti-müllerian hormone secretion is modulated by the extent of follicular development and luteinization and may reflect qualitatively the ovarian follicular status. Fertil Steril. 2005;84:167–173. doi: 10.1016/j.fertnstert.2005.01.115. [DOI] [PubMed] [Google Scholar]
- 24.Qin Y, Zhao Z, Sun M, Geng L, Che L, Chen ZJ. Association of basal serum testosterone levels with ovarian response and in vitro fertilization outcome. Reprod Biol Endocrinol. 2011;9(9) doi: 10.1186/1477-7827-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan AMA, Kotb MMM, AwadAllah AMA, Shehata NAA, Wahba A. Follicular sensitivity index (FSI): A novel tool to predict clinical pregnancy rate in IVF/ICSI cycles. J Assist Reprod Genet. 2017;34:1317–1324. doi: 10.1007/s10815-017-0984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Marca A, Grisendi V, Giulini S, Argento C, Tirelli A, Dondi G, Papaleo E, Volpe A. Individualization of the FSH starting dose in IVF/ICSI cycles using the antral follicle count. J Ovarian Res. 2013;6(11) doi: 10.1186/1757-2215-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolibianakis EM, Venetis CA, Kalogeropoulou L, Papanikolaou E, Tarlatzis BC. Fixed versus flexible gonadotropin-releasing hormone antagonist administration in in vitro fertilization: A randomized controlled trial. Fertil Steril. 2011;95:558–562. doi: 10.1016/j.fertnstert.2010.05.052. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Hao C, Shen X, Liu X, Shan Y, Zhang Y, Chen L. Differences in the transcriptional profiles of human cumulus cells isolated from MI and MII oocytes of patients with polycystic ovary syndrome. Reproduction. 2013;145:597–608. doi: 10.1530/REP-13-0005. [DOI] [PubMed] [Google Scholar]
- 29.Vural F, Vural B, Doğer E, Çakıroğlu Y, Çekmen M. Perifollicular blood flow and its relationship with endometrial vascularity, follicular fluid EG-VEGF, IGF-1, and inhibin-a levels and IVF outcomes. J Assist Reprod Genet. 2016;33:1355–1362. doi: 10.1007/s10815-016-0780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz de Assín R, Clavero A, Gonzalvo MC, Ramírez JP, Zamora S, Fernández A, Martínez L, Castilla JA. Comparison of methods to determine the assigned value in an external quality control programme for embryo evaluation. Reprod Biomed Online. 2009;19:824–829. doi: 10.1016/j.rbmo.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, Laing I. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–1593. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 33.Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427–434. doi: 10.1210/jc.2004-0843. [DOI] [PubMed] [Google Scholar]
- 34.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: The role of anti-Mullerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 35.Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: A systematic review. Hum Reprod. 2004;19:2446–2453. doi: 10.1093/humrep/deh473. [DOI] [PubMed] [Google Scholar]
- 36.Yu Ng EH, Yeung WS, Yee Lan Lau E, So WW, Ho PC. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen-thawed embryo transfer cycles. Hum Reprod. 2000;15:250–255. doi: 10.1093/humrep/15.2.250. [DOI] [PubMed] [Google Scholar]
- 37.Kanakkaparambil R, Singh R, Li D, Webb R, Sinclair KD. B-vitamin and homocysteine status determines ovarian response to gonadotropin treatment in sheep. Biol Reprod. 2009;80:743–752. doi: 10.1095/biolreprod.108.072074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.