Abstract
During the coronavirus disease-19 pandemic, the demand for specific medical equipment such as personal protective equipment has rapidly exceeded the available supply around the world. Specifically, simple medical equipment such as medical gloves, aprons, goggles, surgery masks, and medical face shields have become highly in demand in the health-care sector in the face of this rapidly developing pandemic. This difficult period strengthens the social solidarity to an extent parallel to the escalation of this pandemic. Education and government institutions, commercial and noncommercial organizations and individual homemakers have produced specific medical equipment by means of additive manufacturing (AM) technology, which is the fastest way to create a product, providing their support for urgent demands within the health-care services. Medical face shields have become a popular item to produce, and many design variations and prototypes have been forthcoming. Although AM technology can be used to produce several types of noncommercial equipment, this rapid manufacturing approach is limited by its longer production time as compared to conventional serial/mass production and the high demand. However, most of the individual designer/maker-based face shields are designed with little appreciation of clinical needs and nonergonomic. They also lack of professional product design and are not designed according to AM (Design for AM [DfAM]) principles. Consequently, the production time of up to 4 – 5 h for some products of these designs is needed. Therefore, a lighter, more ergonomic, single frame medical face shield without extra components to assemble would be useful, especially for individual designers/makers and noncommercial producers to increase productivity in a shorter timeframe. In this study, a medical face shield that is competitively lighter, relatively more ergonomic, easy to use, and can be assembled without extra components (such as elastic bands, softening materials, and clips) was designed. The face shield was produced by AM with a relatively shorter production time. Subsequently, finite element analysis-based structural design verification was performed, and a three-dimensional (3D) prototype was produced by an original equipment manufacturer 3D printer (Fused Deposition Modeling). This study demonstrated that an original face shield design with <10 g material usage per single frame was produced in under 45 min of fabrication time. This research also provides a useful product DfAM of simple medical equipment such as face shields through advanced engineering design, simulation, and AM applications as an essential approach to battling coronavirus-like viral pandemics.
Keywords: Medical face shield, Personal protective equipment, Product design, Additive manufacturing, Coronavirus disease-19
1 Introduction
On December 31, 2019, the World Health Organization (WHO) Country Office in China was informed of cases of pneumonia which had unknown etiology from Wuhan City, Hubei Province of China. Following the identification and confirmation of a new type of coronavirus called 2019-nCoV by the Chinese authorities[1], the WHO officially named the disease caused by the coronavirus as coronavirus disease (COVID)-19, which stands for “COVID 2019” on February 11, 2020[2], and declare the pandemic on March 11, 2020[3]. Since the date of the first case of this virus spreading, the world has been struggling with this emergent state. During this period, international and national authorities have been announcing public advice and putting in place legal regulations regarding social behavioral habits and the use of personal protective equipment (PPE) for public and health-care services. During this pandemic, health-care institutions have become one of the most hazardous environments to work in, especially for healthcare workers (HW) who deliver care and services to the sick and ailing either directly as medical doctors and nurses or indirectly as aides, helpers, laboratory technicians, or even medical waste handlers, who are considered to be in the high-risk groups[4]. The outbreaks of serious airborne infectious diseases, such as severe acute respiratory syndrome (SARS), Avian Influenza and now the COVID-19, as well as severe infectious agents associated with body fluid exposures (e.g., Ebola virus) have called for increased attention to face/eye protection as the face is the most common body part exposed to the acutely-expelled aerosols of patient body fluids during HW-patient interaction[5].
Although it is understood that wearing a surgical face mask may provide protection during distanced interaction in the patient’s room between the HW and patient who has suspected or confirmed COVID19, the use of additional PPE for closer operations potentially involving acutely-expelled aerosols of body fluids would be a necessity. Therefore, many international and national health service authorities/organizations advise the use of personal PPE for respiratory, eye/mouth/face, body, and hand protection while interacting with COVID-19 patients to avoid or minimize any likely contact, droplet, and airborne transmission[6-8]. The WHO also published a guide on the recommended types of PPE to be used in the context of SARS-CoV-2 (the virus causing COVID19), according to the setting, personnel, and type of activity[9].
For instance, the research carried out with cough aerosol and breathing simulators loaded with influenza virus (aerosol volume mean diameter of 8.5 μm) indicated 96% and 92% reductions in the risk of inhalational exposure immediately after a cough if a face shield at distances of 46 cm and 183 cm was used, respectively. In the case of a smaller aerosol diameter of 3.4 μm, the protection of the face shield is 68% at 46 cm immediately after the cough, and the protection rate decreases to 23% over 1 – 30 min post-cough (in the case of remaining airborne particles)[10].
It is understood that, in addition to face shield equipment, the use of surgical masks such as N95, filtering facepiece (FPP2), and FFP3 will give more effective and thorough protection during closer HW-patient interaction. Although there are risky cases for airborne transmitted viruses (which could have the ability to remain in the air for extended periods), in the case of larger aerosol droplet explosion, face shield (visor) products which have a simple design and manufacturable features would provide superior protection.
This face shield (visor) equipment could be designed and produced for single-use (disposable) or reusable following disinfection. In fact, before this pandemic, millions of HW, dental providers, veterinary care personnel, laboratory workers, pre-hospital emergency medical providers, police, firefighters, and custodial staff dealing with spills and contaminated waste have already been classified as the potential users of face shields. In addition to meeting the demand of this pre-existing group of face shield users, the need for this type of PPE in many countries, including Turkey and the United Kingdom, has increased drastically since the COVID-19 pandemic. On March 3, 2020, the WHO expressed the concern over the shortage of PPE that could endanger HW worldwide; therefore, announced a call for increased manufacture of face shield by 40 % to meet the rising global demand for this type of PPE[11]. Based on WHO modeling, an estimated 89 million medical masks are required for tackling COVID-19 each month[11]. About 76 million of examination gloves are required, and the international demand for goggles (including face shield equipment) now stands at 1.6 million/month.
In the face of this high demand for PPE (specifically for face shield equipment), visors that are commercially produced by conventional manufacturing methods (mostly plastic injection molding) could be supplied on time, and the high demand for this PPE has raised the unit product costs and the shipping rate. Conversely, this situation strengthens our social solidarity to address the issues pertaining to this pandemic. Many educational and government institutions, commercial and noncommercial organizations, and home/individual makers produced face shield products (not mass/serial production) and shipped/donated them to personal-public users and health-care sectors. In many cases, additive manufacturing (AM) technology was utilized for their prototype designs as it is the fastest way to obtain a usable product/prototype. The use of this manufacturing technology with easily accessible relatively professional (trademarks) and original equipment manufacturer (OEM) 3D printers has become very popular and is productive enough to meet the high demand for PPE.
A face shield (visor) product has simple structural and functional design features, which consist of two main components: The frame (metal- or plastic material-based) and the transparent protective visor shield (mostly plastic material-based). However, additional components such as elastic bands (frame-head holder), face contact softening materials (sponge, foam, rubber, etc.), and clips for fastening the transparent shield to the frame can also be included.
Various concept designs for face shield products generated by AM for rapid prototyping/manufacturing have been introduced in addition to commercially existing ones (Figure 1). However, most of these products were not carefully designed with due consideration to professional and ergonomic product design principles, and design for AM (DfAM) approaches. A design can be functional and may correspond to the needs within pre-defined design specifications; however, this may not indicate that the design has structurally and functionally optimized features. For most of the designs generated, it was not known where or how they were approved. These designs also have relatively longer production and component assembly times per unit product (more than 4 – 5 production hours; about 2 h in average), and some of the products are heavier considering their custom designs. Besides, most of them, which are equipped with additional components such as elastic bands, softening materials, and fastening clips could cause user discomfort after using for long working hours and constant taking on/off operations. These facts appear as disadvantages for a product within the context of ergonomics and total AM-based production time. In addition, from a post-prototyping perspective, most of these products do not have convenient structural design features that are amenable for commercial mass/serial production using conventional manufacturing methods such as plastic injection molding. Therefore, a lighter, more ergonomic, and single frame medical face shield without extra components for assembly would be useful for homemakers, individual designers/makers, and noncommercial producers (primary target) to provide effective, functional rapid-prototyped products, which may have a commercial potential (secondary target) in a relatively shorter time. The disadvantages of the current PPE manufacturing method were the main sources of motivation for this design study as the product designs are dynamic, and can be seen changing stages on an existing product over time, in shape and function. These changing stages can be in different range and sudden, others move step by step. In any case, the major aim of the change is to improve the design, to make the product more effective or, put simply, just more appropriate for use in the current pandemic (i.e., low-cost, quick to produce, and disposable).
Figure 1.

Examples of commercial and custom-made face shield products.
Considering the afore-mentioned specific product design issues, the aim of the current study was to design a competitively lighter, relatively more ergonomic, and easy-to-use original medical face shield which can be produced within relatively shorter production times, assembled without extra components (such as elastic bands, softening materials, and clips), fully amenable to production using AM technology, and directed to more conventional manufacturing methods, by means of advanced computer-aided design, engineering, and AM applications.
2 Materials and methods
2.1 Background, design process, and original design details
Although international standards for industrial PPE (such as ANSI/ISEA Z87.1-2020, “American National Standard for Occupational and Educational Personal Eye and Face Protection” and British Standards of BS 7028:1999 BS EN 168:2002, BS EN 166:2002; BS EN 13921:2007, Statutory Instrument 2002 No. 1144 for eye protection regulations and specifications) are available, it is reported that there is currently no universal standard for eye/face protection from biological hazards during medical applications[10,12]. The common point related to the product descriptions and functionality. From the infection control standpoint, protector components serve to minimize or prevent eye and face exposure of the wearer to sprays, splashes, or droplets of blood, body fluids, excretions, secretions, and other potentially infectious materials in occupational and educational environments, where biological hazards are expected. Hence, in this perspective, various face shield design samples and patent registrations can be found from a simple internet search; however, the number of informative scientific publications for user guides, design details, and AM and conventional or non-conventional manufacturing applications of face shield are very limited. Within the limited literature, a useful scientific review regarding face shields used for infection control to assist in the selection and proper utilization of this type of PPE was published by Roberge (2016). In addition, the WHO, NHS England, and Texas Medical Association Turkey have been updating their advisory guidelines/publications for the use of medical PPE. These are the sources available to collect reference information related to design and structure, regulatory standards, and guidelines to proper use and selection of the PPE and describe the product as PPE that provides barrier protection to the facial area and related mucous membranes (eyes, nose, and lips). Consequently, these sources were carefully considered in describing the requirements for the design study detailed in this paper.
For the term “design,” there is no single universal description in the product development applications; however, design is commonly considered as a total iterative process within the scopes of engineering design, product design, and industrial design applications; this begins with an idea or requirement and ends with a fully described product (or process or services). As shown schematically in Figure 2 adapted from two of well-known references, Budynas and Nisbett (2011) outline the idealized, iterative, and top-down phases in design[13] and Kamrani and Nasr (2010) draws an engineering design process path which indicates a similar process for the product design[14]. In addition, the result of the design activity is often expected to be original, adding value to the existing designs by solving problems in new ways,[15] which is the emphasis in this research on the original design features of a PPE product through a product-specific, redefined generic design process.
Figure 2.
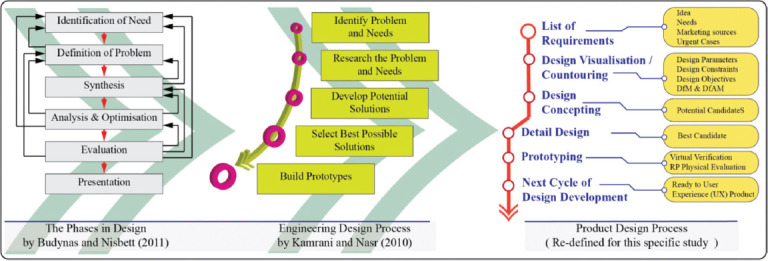
Product-specific redefined generic design process.
For the first stage of the design process, essential design function objectives for an original face shield product compatible with an AM production approaches were defined as follows:
-
F1.
Original ergonomic design, easy-to-use form with stable holding elasticity and equipped with as few components as possible
-
F2.
Single frame design which is convenient for AM production (DfAM approach)
-
F3.
Specific product originality feature: Comfortable, with non-classic goggle nose pad, and no ear hook structural features
-
F4.
No additional components used in the assembly of the frame and the transparent shield (such as softening materials, and fastening clips)
-
F5.
As little as possible additional processing time in preparation/assembly of the linkage points between the frame and the transparent visor
-
F6.
Biodegradable thermoplastic polymer/ polylactic acid (PLA) material-based production (for AM process)
-
F7.
Less than 10 g product weight (no supports during AM production)
-
F8.
Less than 60 min AM production time through OEM machine (at 60 m/s layering speed, in-fill rate of 100%) (production time may well be shorter with industrial AM equipment)
-
F9.
Minimum 8 products per day from one OEM machine during AM production stage (8 working h)
-
F10.
Through simple design changes/revisions, generated frame design should conveniently have the potential for conventional manufacturing methods (such as plastic injection molding) in addition to AM-based prototyping.
The innovation process, which can provide solutions for design needs, normally starts at the stage of conceptual design. A concept solution as the basis for the final design under pre-defined design needs and constraints can be generated from a “stand-off” between two sets of influences: The basic objective is to keep to the required size, shape, and “look” (esthetic) of the design, while improving on cost (such as material weight), lifetime, and integrity (Figure 3)[16]. In this regard, five design candidates, which have the potential to meet with these pre-defined objectives, were designed and proposed at the concept stage. These candidates were analyzed through technical functional analysis (TFA), and the best candidate design was selected for prototype testing. The core design contours and the concept design candidates are shown in Figure 3.
Figure 3.
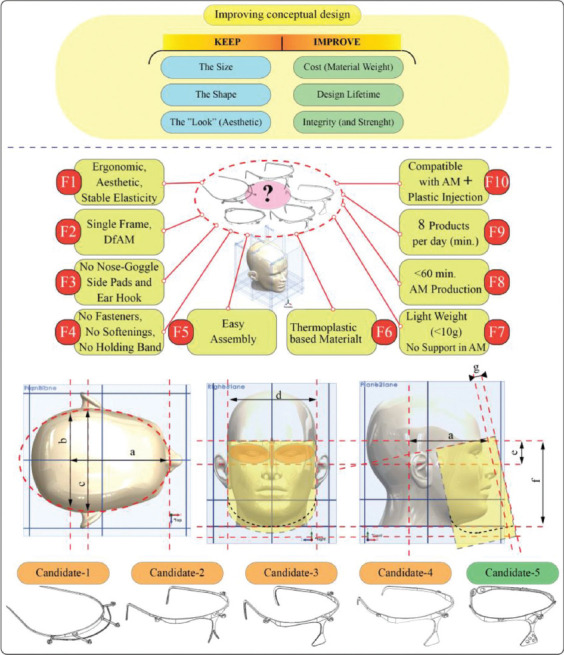
The concept design candidates.
2.2 TFA
Redesigning/improving an existing product (different design for the same functionality) on the market can pose significant challenges even to the most experienced professional. Considering the importance of the product application as discussed within this work, the authors based their redesign/design improvement or new design decisions on the guidelines given from the value analysis (VA) methodology developed by Miles in 1948[17]. More specifically, TFA was deployed to develop the optimal technical solution that best meets the concrete expression of the customers” need, at minimum cost. The external and internal functional analysis takes into consideration the main functions that the face shield needs to perform to meet both customer expectations and manufacturer specifications[18]. TFA offers a variety of tools and instruments, but for the purpose of this study, the following main stages were deployed: Function identification and characterization; ranking and valuation of the functions; economic dimensioning; result and critical evaluation of the functions; and proposal of concepts. TFA can be conducted as an iterative method, which allows product performance improvement with each new step, thus obtaining at the end of the process an optimized concept. Function analysis system technique (FAST) diagrams[19] were used for all lifecycle stages of the face shield to accurately identify the main functions. Based on FAST and in relation to the main ten design objectives identified before for the face shield’s functions were defined.
2.3 Functions importance levels
A TFA team comprised ten medical experts and industry specialists established the importance levels of the face shield by objectively grading and ranking each function in relation to the others. The valuation matrix is composed of weighting the answers of all ten team members, and their final rankings are displayed in Table 1.
Table 1.
Valuation matrix - value weighting of the functions for redesigning a face shield.
| Functions | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 1 | 0.3 | 0.8 | 0.9 | 0.6 | 0.3 | 0.7 | 0.6 | 0.5 | 0.8 | 6.5 |
| F2 | 0.7 | 1 | 0.7 | 0.9 | 0.8 | 0.5 | 0.5 | 0.9 | 0.9 | 0.8 | 7.7 |
| F3 | 0.2 | 0.3 | 1 | 0.6 | 0.5 | 0.3 | 0.5 | 0.2 | 0.3 | 0.5 | 4.4 |
| F4 | 0.1 | 0.1 | 0.4 | 1 | 0.3 | 0.4 | 0.6 | 0.4 | 0.3 | 0.5 | 4.1 |
| F5 | 0.4 | 0.2 | 0.5 | 0.7 | 1 | 0.4 | 0.5 | 0.3 | 0.3 | 0.6 | 4.9 |
| F6 | 0.7 | 0.5 | 0.7 | 0.6 | 0.6 | 1 | 0.6 | 0.8 | 0.8 | 0.8 | 7.1 |
| F7 | 0.3 | 0.5 | 0.5 | 0.4 | 0.5 | 0.4 | 1 | 0.4 | 0.5 | 0.5 | 5 |
| F8 | 0.4 | 0.1 | 0.8 | 0.6 | 0.7 | 0.2 | 0.6 | 1 | 0.5 | 0.9 | 5.8 |
| F9 | 0.5 | 0.1 | 0.7 | 0.7 | 0.7 | 0.2 | 0.5 | 0.5 | 1 | 0.9 | 5.8 |
| F10 | 0.2 | 0.2 | 0.5 | 0.5 | 0.4 | 0.2 | 0.5 | 0.1 | 0.1 | 1 | 3.7 |
Calculated weighted value 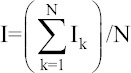
|
4.5 | 3.3 | 6.6 | 6.9 | 6.1 | 3.9 | 6 | 5.2 | 5.2 | 7.3 | 55 |
Global importance factor 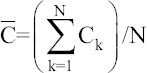
|
0.08181 | 0.06 | 0.12 | 0.12545 | 0.11091 | 0.07091 | 0.10909 | 0.09455 | 0.09455 | 0.13273 | 1 |
Global weight 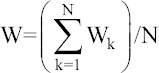
|
8.18% | 6.00% | 12.00% | 12.55% | 11.09% | 7.09% | 10.91% | 9.46% | 9.46% | 13.27% | 100% |
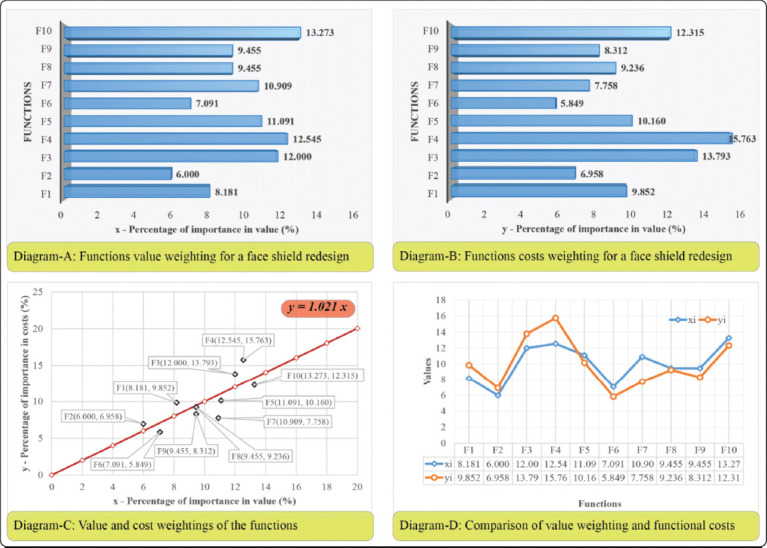
The final valuation matrix shows that F10 and F4 are the most important functions in value, namely, that the redesigned face shield should allow an easy transition from AM processes to plastic injection molding and it should have a clean, simple one-part design and avoid fasteners, softening, and holding bands. This specific TFA stage highlights the functions of the redesign that should be focused on, from the value point of view. Function value weightings are noted with xFi, where i = 1÷10. Based on the results obtained from the valuation matrix, the following percentage values were obtained: xF1 = 8.181%, xF2 = 6.0%, xF3 = 12.0%, xF4 = 12.545%, xF5 = 11.091%, xF6 = 7.091%, xF7 = 10.909%, xF8 = 9.455%, xF9 = 9.455%, xF10 = 13.273%.
2.4 Economic dimensioning of the functions
For the second step of TFA, economic dimensioning of the functions was conducted in accordance with thorough benchmarking in the medical device sector. Costs were estimated based on Ruffo et al. (2006) and Hopkinson and Dicken’s (2003) models[20,21], in which they propose that the total cost (C) is the sum of the cost of the raw materials and the indirect costs, as shown in equation (1). The indirect cost of hourly activities is shown in Table 2.
Table 2.
The indirect cost of activities.
| Activity | Cost/hour (monetary units) |
|---|---|
| Production work/time machine | 7.99 |
| Machine costs | 14.78 |
| Fixed and variable costs | 5.9 |
| Administrative costs | 0.41 |
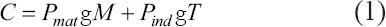
where Pmat is the price of the raw material, measured in monetary units per kilogram;
M is the mass of the 3D printed product, measured in kilograms;
Pind cost rate, measured in monetary units/hours;
T is the total production time of one part, measured in hours.
TFA is usually applied to existing products to increase their value or lower their costs, through redesign strategies. In the case of the face shield, economic dimensioning was based on the hypothesis of evaluating the average values of the main characteristics, which can include product weight, product performance, direct and indirect costs, and manufacturing time. After TFA is deployed, these average values are stated as final requirements and metrics with ideal values. Thus, market research considered different types of materials and their individual characteristics, costs, life span, recycling procedures, production volumes, and other particular features of the face shield and of the production process. The research team selected material extrusion (MEx, also referred to as Fused Deposition Modeling [FDM] or Fused Filament Fabrication) as the primary AM process to produce the redesigned face shield. PLA material was selected due to the ease of printing and an advantageous cost-performance ratio. Considering that singlepart weighs an average of 20 g (weight of reference products) and it can be manufactured in 60 min from medium grade PLA filament with a price of 170 monetary units per kilogram, the total cost of manufacturing one face shield structure is 32.48 monetary units. Based on the findings of the market analysis, the total calculated costs were assigned by a TFA specialist, as presented in Table 3. Each function participates in the total cost with a percentage value, noted with yFi, i = 1÷10. For the redesign of a face shield, the percentage values of the functions participation in the total costs are: yF1 = 9.852%, yF2 = 6.958%, yF3 = 13.793%, yF4 = 15.763%, yF5 = 10.160%, yF6 = 5.849%, yF7 = 7.758%, yF8 = 9.236%, yF9 = 8.312%, yF10 = 12.315%.
Table 3.
Functions costs distribution matrix for redesigning a face shield.
| No. | Main constructing features | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | Feature cost* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Main body frame | 1 | 1 | - | 1.92 | 1.1 | 0.53 | 0.72 | 1 | 0.75 | 1.2 | 9.22 |
| 2. | Coronal plane positioning system | 1 | 0.56 | 1.74 | 1 | - | 0.46 | 0.4 | 0.54 | 0.3 | 0.8 | 6.8 |
| 3. | Shield orientation and fixing system | 0.2 | - | - | 0.6 | 1.6 | - | 0.2 | - | 0.2 | 0.5 | 3.3 |
| 4. | Lateral gripping features | 0.5 | 0.2 | 1.74 | - | 0.3 | 0.41 | 0.4 | 0.46 | 0.7 | 0.5 | - |
| 5. | Forehead stabilizing rest | 0.5 | 0.5 | 1 | 1.6 | 0.3 | 0.5 | 0.8 | 1 | 0.75 | 1 | 7.95 |
| Total cost | 3.2 | 2.26 | 4.48 | 5.12 | 3.3 | 1.9 | 2.52 | 3 | 2.7 | 4 | 32.48 | |
| Ratio | 0.09852 | 0.06958 | 0.13793 | 0.15763 | 0.1016 | 0.05849 | 0.07758 | 0.09236 | 0.08312 | 0.12315 | 1 | |
| Cost of functions (%) | 9.852 | 6.958 | 13.793 | 15.763 | 10.16 | 5.849 | 7.758 | 9.236 | 8.312 | 12.315 | 100 | |
Economic dimensioning addresses the comparison between the functions value and cost weightings, aimed at the identification of three main function status: A function is too expensive in relation to other functions; a function is too expensive compared to its contribution in the products’ value; a function is too expensive in relation to the existing manufacturing technical possibilities. To accurately analyze the relationship between function costs and value, four diagrams are plotted (Figure 4).
Figure 4.
The relationship between functions cost and value.
2.5 Diagrams
At this stage, the smallest squares method is used to plot the diagrams necessary for TFA. To properly deploy the smallest squares method, the following parameters need to be calculated:
• The regression line:
y = agx (2)
where xi represents the functions value weighting;
yi represents functions cost weighting;
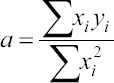 represents the regression parameter.
represents the regression parameter.
• The estimator S is determined with the smallest squares method:
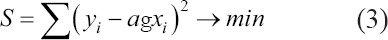
• The dispersion S’ must be as close as possible to zero value to validate the solution.
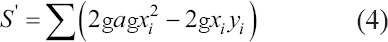
• The angle α of the regression line:

All computational elements used in the smallest squares method are presented in Table 4.
Table 4.
Computational elements for a face shield.
| No. | Calculated elements | Functions | Total value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |||
| 1. | xi | 8.181 | 6 | 12 | 12.545 | 11.091 | 7.091 | 10.909 | 9.455 | 9.455 | 13.273 | 100 |
| 2. | yi | 9.852 | 6.958 | 13.793 | 15.763 | 10.16 | 5.849 | 7.758 | 9.236 | 8.312 | 12.315 | 99.996 |
| 3. | xi2 | 66.929 | 36 | 144 | 157.377 | 123.01 | 50.282 | 119.006 | 89.397 | 89.397 | 176.173 | 1051.571 |
| 4. | xi * yi | 80.599 | 41.748 | 165.516 | 197.747 | 112.685 | 41.475 | 84.632 | 87.326 | 78.59 | 163.457 | 1053.775 |
| 6. | S | 2.735 | 0.894 | 3.125 | 10.187 | 0.911 | 1.58 | 10.073 | 0.057 | 1.352 | 0.972 | 31.886 |
| 7. | S’ | -27.06 | -11.345 | -42.428 | -80.08 | 21.167 | 17.825 | 69.247 | 4.516 | 21.989 | 26.17 | 0 |
After processing, the information in Table 4, the following values were obtained: a = 1.0021, α = 45.05998, S = 31.886, and S’ = 0.
In Figure 4, Diagram A shows the functions t for the redesign process of a face shield. The diagram was constructed using the rankings of the functions by their value, as obtained in the valuation matrix. Diagram B represents the ranking of the functions by their functional cost, as defined in the cost distribution matrix. Both diagrams are used by the TFA team to compare the functions costs in relation to their contribution to the value of the product. It is important for the redesign process to identify the most expensive functions and those with the highest weighting in the total cost of the product. This way, secondary functions that are very expensive in relation to the objective functions, or even more expensive than these, can be identified for further improvement. The weighting of effort for a certain function must match its weighting in the total value of the product. Further, to validate these assumptions, regression analysis was used, and the regression line was plotted for further analysis.
The real situation is represented in Diagram C by plotting the regression line y = 1.0021*x, with a slope angle of α = 45.05998. The smallest squares method presumes that the estimator S should tend to a minimum value and the S’ dispersion is zero. To diminish the S value, the points represented by the function weightings must be aligned as perfectly as possible along the regression line. The objective of TFA is to redesign and diminish costs or increase the value for the functions corresponding to the points above the regression line. By changing those specific points and re-plotting the diagram, the slope of the regression line modifies, and a new situation of TFA can be evaluated. As stated before, the process is iterative, and it is undertaken until the requirements of both customers and manufacturers are met.
The critical evaluation of the functions presented in Diagram D presents the most expensive functions in relation to their value. Functions F4, F1, and F2 have disproportionate costs in relation to their value contribution. Function F4 was also identified in the first TFA stage as being the second most important in value, and thus, the designers will focus on the face shield features that address this specific function.
Based on the above diagrams, the TFA team concluded that functions F1, F2, F3, and F4 need either a lower cost or an increase in value. This implies redesigning and providing new constructive solutions for ensuring the structure and the ergonomics of the frame. The redesign process will target ergonomics, single frame, and AM compatible face concepts. TFA was further used to define the final product requirements, which must be followed while designing the face shield. A specification is constructed from a metric and a value[18], which correspond to a specific function of the product. A function can have one or more metrics and values. The final list of specification objectives in relation to the TFA results is given in Table 5.
Table 5.
Final product specifications for a face mask.
| Functions | Requirements | Metric | Units | Limit values | Ideal values |
|---|---|---|---|---|---|
| F1 | Elasticity | Tensile strength at yield | MPa | >35.9 | 34 |
| Ease of usage | Ergonomic design | Yes/ No | Yes | Yes | |
| F2 | Respect DfAM principles | AM technologies | - | MEX, SLA, SLS | MEX |
| F3 | Comfortable | Wear period | hours | >6 | 8 |
| Maintains position | Ensures user protection | Yes/No | Yes | Yes | |
| Prevents condensation | Ensures ventilation features | Yes/No | Yes | Yes | |
| F4 | Single frame design | Number of components | No. | 3 | 2 |
| Allow reuse | Sterilization procedure | - | Autoclave, UV, disinfectant liquids, ozone | Disinfectant liquids, ozone | |
| F5 | Ease of maintenance | Maintenance manual | Yes/No | Yes | Yes |
| Ease of assembly | Assembly/disassembly time | minutes | 0.5 | 0.5 | |
| F6 | Biodegradable filaments | Thermoplastics | - | PLA | PLA |
| Environmental friendly | Recyclable | Yes/No | Yes | Yes | |
| F7 | Light weight | Total weight | g | <20 | 10 |
| F8 | AM produced | Total production time (3D printing+post processing+assembly) | minutes | <60 | 45 |
| F9 | AM produced | Production rate | products/day/machine | <10 | 8 |
| F10 | AM/plastic injection | Modular/parametric design | Yes/No | Yes/No | Yes |
The analysis of alternative designs was undertaken using TFA instruments to select the best candidate for further investigation. The selection criteria and their weights were set by the TFA team. As shown in Table 6, Candidate 5 obtained the best total score, best ensuring the functional characteristics required.
Table 6.
Analysis of the alternative designs for a face shield.
| No. | Analysis criteria | Weight (%) | Candidate 1 | Candidate 2 | Candidate 3 | Candidate 4 | Candidate 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade* | Weighted grade | Grade* | Weighted grade | Grade* | Weighted grade | Grade* | Weighted grade | Grade* | Weighted grade | |||
| 1 | Cost | 15 | 2 | 0.3 | 3 | 0.45 | 3 | 0.45 | 5 | 0.75 | 5 | 0.75 |
| 2 | Functional characteristics | 20 | 2 | 0.4 | 3 | 0.6 | 5 | 1 | 5 | 1 | 5 | 1 |
| 3 | Life span | 10 | 4 | 0.4 | 4 | 0.4 | 4 | 0.4 | 4 | 0.4 | 4 | 0.4 |
| 4 | Re-usage | 5 | 4 | 0.2 | 4 | 0.2 | 4 | 0.2 | 3 | 0.15 | 3 | 0.15 |
| 5 | Complexity | 10 | 2 | 0.2 | 3 | 0.3 | 4 | 0.4 | 4 | 0.4 | 4 | 0.4 |
| 6 | DfAM characteristics | 20 | 2 | 0.4 | 3 | 0.6 | 3 | 0.6 | 3 | 0.6 | 4 | 0.8 |
| 7 | Ergonomics | 5 | 3 | 0.15 | 4 | 0.2 | 4 | 0.2 | 4 | 0.2 | 4 | 0.2 |
| 8 | Environmental effect | 5 | 4 | 0.2 | 4 | 0.2 | 4 | 0.2 | 4 | 0.2 | 4 | 0.2 |
| Total score | 2.25 | 2.95 | 3.45 | 3.7 | 3.9 | |||||||
| Rank | 2 | 3 | 4 | 5 | 1 | |||||||
| Selected model | - | - | - | - | √ | |||||||
Grading scale: 1 – least important; 5 – most important
2.6 Prototyping
Once the first functional prototype of a detail design is exhibited, prototype testing can be performed to validate the proposed design solution. If the design solution cannot meet the required design objectives, the product design process is repeated until a satisfactory desired solution is reached. At this stage, it is possible to undertake both virtual prototype and physical prototype-based design verifications.
2.7 Virtual prototyping: Finite element analysis (FEA) verification
After approval of the design details of Candidate 5, a 3D parametric solid model was constructed in the virtual environment, and then virtual prototype testing for the product’s elastic deformation ability was realized. The findings from the tests were evaluated in the virtual environment for potential design changes. In this study, the virtual prototype was tested to determine head holding force and deformation behavior. To evaluate the deformation behavior of the prototype, the finite element method (FEM)-based structural deformation analysis (FEA) was carried out. The structural module of the ANSYS Workbench FEM-based commercial code was employed for the FEA. In the FEA scenarios, head wearing and head holding positions were simulated. The FEA was set up using assumptions of a linear static loading and homogeneous, linear isotropic material model. At the meshing operation, a curvature meshing strategy was utilized and the skewness metric, which is one of the primary mesh quality measures in a FEA, was checked. The shape and asymmetry of distribution can be measured by its skewness, which can be considered as the mesh quality verification of a FEM[22]. The skewness value of zero indicates an equilateral cell (best) and a value of one indicates a completely degenerate cell (worst)[23]. At the final meshing operation, FEM was created with minimum element size of 0.4 mm, and the average skewness value of 0.227 was obtained. This value indicated that the FE model used in the loading scenarios has an excellent mesh quality. Details related to the FEA set up, and simulation outputs are given in Figure 5.
Figure 5.
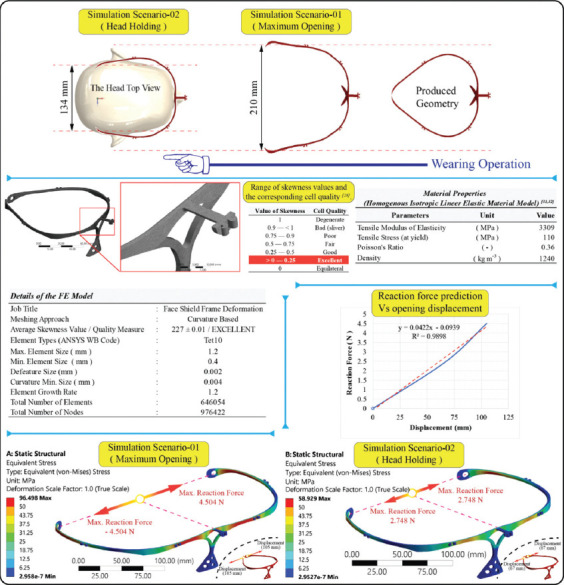
Finite element analysis verification virtual prototype.
The results of the FEA scenarios revealed that there was no plastic deformation (permanent deformation) during the maximum opening condition of wearing on the head and at the head holding positions. Maximum equivalent (Von-Mises) stress during the maximum opening condition and at the head holding position were 96.498 MPa and 58.929 MPa, respectively, which are lower than the material tensile stress at yield (which is 110 MPa). These numerical and visual results obtained from the simulation indicated that the stress distribution on the product is uniform and the elastic deformation ability of the product is satisfactorily within the design limits. For the second scenario, the head holding force after wearing was calculated as 2.744 N against each side displacements. This finding was also interpreted as the head holding capability of the product and was in a satisfactory comfort range.
2.8 Physical prototyping
As a dimensionally accurate physical part, the AM prototype is able to give the designer a sense of the appropriateness of form and fit before continuing with production[24]. The fastest technique to produce a physical prototype, most especially for a complex geometric structure, is utilizing AM technology. In short, models were created by adding successive layers of material together. To evaluate the physical prototype of the face shield, an AM approach was utilized in this study; however, the critical point here is that DfAM is a challenge for most designers as the convenient design methods that consider the unique capabilities of AM technologies are needed. Depending on the capabilities of the AM technology being utilized, DfAM can be described as a type of design method whereby functional performance and/or other key product lifecycle considerations such as manufacturability, reliability, and cost can be optimized[25]. In this regard, a useful worksheet/application guide designed for novices to AM was published by Booth et al. (2017)[26]. In using the DfAM method, some important approaches are in macroscale, mesoscale or microscale design studies: Structural optimization approach (i.e., size, shape, and topological) and manufacturability related to AM technology type, AM machine, material, build orientation, surface quality needs, production time, etc.
The production of the prototype was realized using an OEM - FDM machine with a production volume capacity of 200 mm × 220 mm × 220 mm, nozzle diameter of 0.4 mm at 210°C nozzle temperature. The production material was PLA thermoplastic with a filament diameter of 1.75 mm. Solid modeling and AM setup procedures for the face shield product handled in this study were conducted under consideration of these key approaches related to the DfAM methodology. To obtain time efficiency (short production), optimally designed (geometry and topology), and a functionally readytouse prototype with satisfying surface quality, production trials were made on STL conversion quality (geometrical parameters) and production layer heights during AM operations. The trials showed that despite the rearrangements, more precise (use of smaller triangles) STL conversion parameters and shorter production layer heights gave a smoother surface quality, the fine level of STL conversion (deviation tolerance: 0.099 mm, angle tolerance: 10°, and number of triangles: 16,736) and the layer height of 0.25 provided satisfactory results in the use of the physical prototype when considering the time efficiency approach (approximately 35 min production time per unit). Details related to the AM procedures and prototypes produced are given in Figure 6.
Figure 6.

Fabrication of prototype product for physical evaluation.
2.9 Physical prototype trials
The model of Candidate 5 was highlighted through the TFA and the prototyping stage was successfully realized. In addition, to obtain first-hand user opinions about the prototype of Candidate 5, a survey was conducted. The survey was completed by health service workers (medical doctors, nurses, and the other health service workers) at Antalya Training and Research Hospital, Antalya, Turkey in April 2020. Ten questions related to the face shield product evaluation were answered by 15 HWs who used the product in routine daily hospital activities. The survey results were interpreted as having a high satisfaction level since the answers given to question no. 9 related to satisfaction of using this product were very positive overall, in addition to the positive responses to the other questions (40% of those completing the survey found the product to be “excellent,” 40% found the product to be “good,” 17% found the product “average,” 7% found the product to be “poor,” and no-one found the product “insufficient”). Thus, for the final evaluation, it was decided that Candidate 5 as the final product design should be approved and the design evaluation undertaken in this research was finalized. The participation details, survey questions, variation of the answers given to these questions, and the pictures obtained from the trials are provided in Figure 7.
Figure 7.
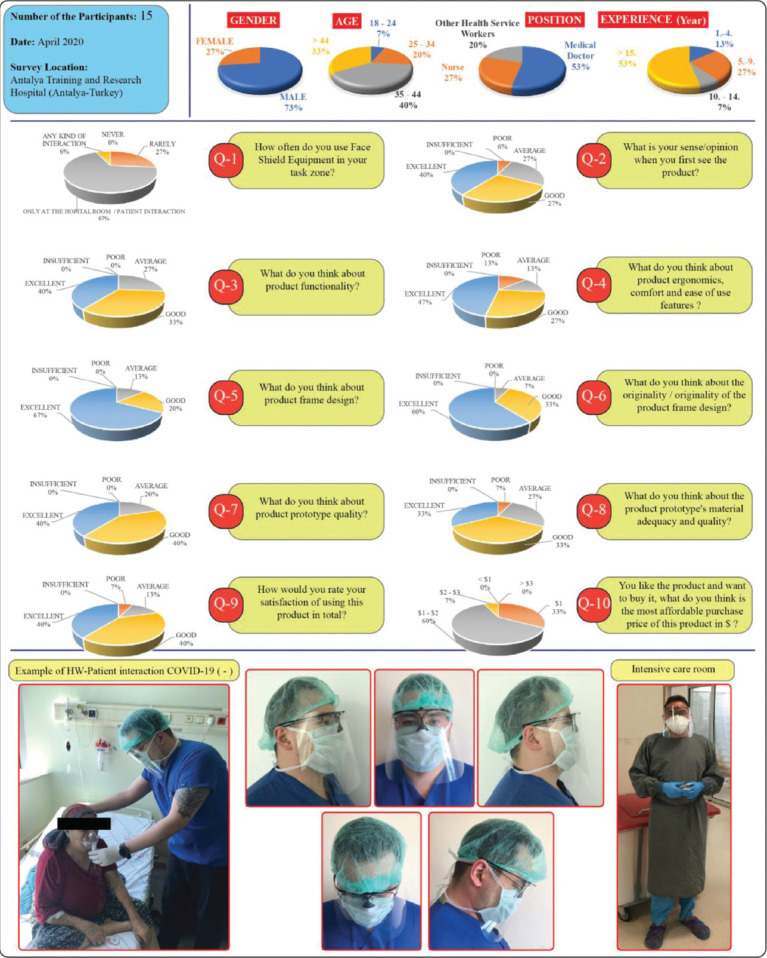
Product evaluation survey and user trials.
3 Results and discussion
After initial sketching and concept evaluation, TFA results suggested an optimum design: Candidate 5. Structural design verification was approved in the virtual environment and a physical prototype was tested by the experts and a product satisfaction survey was completed by HWs during real, in situ hospital activities. The product satisfaction survey revealed positive responses related to product design features and usability (Figure 7). The frame design was rated with 67% “excellent,” 20% “good,” and 13% “average” satisfaction. No user indicated an insufficiency rate for any of the survey questions. Under these considerations, the product design work detailed in this paper was completed, and the detailed design for Candidate 5 was approved (Figure 8). The single-frame design used <10 g FDM filament and took approximately 35 min to build. AM production time and only two-hole stamping operations are required to assemble the transparent shield to the frame. These product features can be interpreted as the originality and advantages achieved for this product against competitors as detailed in the myriad of social media.
Figure 8.

Technical drawing of Candidate 5.
During the product design process, advanced computer-aided design, engineering, and DfAM methods were successfully applied. The objectives given in the design process section were achieved. Critically, light weight and relatively shorter AM production times were accomplished. When considering the existing products being showcased in social media, the biggest disadvantages were perceived to be relatively longer AM production times per unit. Many approaches and concepts for face shield products produced using AM technologies have been proposed during the COVID-19 pandemic to support the health services. Although all of them provide some level of functionality, many of their design features lack adherence to professional design principles, optimum structural topology, ergonomics, and the application of DfAM approaches. A common goal in an industrial product is to improve product quality, shorten the product development cycle, and reduce product cost/time. As such, the product detailed in this study has satisfactorily met these conditions. Realistically, industry nowadays will not survive in a globally competitive marketplace unless they introduce new products of better quality, at a lower cost, and with shorter lead times[27].
AM brings unique considerations for engineers in the design process for medical PPE. An important issue in the use of PPE for medical purposes is biological consideration (including cleaning, sterilization, and biocompatibility). In consideration of unregulated PPE production during this COVID-19 pandemic, recently, a discussion was reported by MIT news on the limitations and dangers of using AM/3D printing for PPE fabrication[28]. The text reports that one of the biggest risks with AM-fabricated products for COVID-19 is the false sense of hope as quickly produced PPE inevitably fails to meet any of the needs previously discussed. This is crucial when considering critical PPE products such as filtration masks, which may need expert design consideration and medical/health service authority approvals, may do more harm than good. The additional risk from unregulated PPE also exists as many FDM filaments retain ambient moisture, which could pose a paradoxically increased risk for virus transmission during use or reuse[29]. Similar risks and concerns were also reviewed and reported by Clifton et al. (2020)[30]. The authors reported that during this pandemic, the open distribution and propagation of PPE prototypes happened before validation and hypothesis formulation (in the context of both engineering and biological considerations) that emphasized on the fundamentally important factors for prototype testing, such as number needed to treat and reduce harm for patients, and the approval of health service authorities had not been considered. Hence, it was possible to highlight the need for universal standardization for PPE, such as medical face shield products. True innovation can prevail over brief notoriety and avoid unintentional harm from good intentions led by poor science[30]. This study provided an original design which was developed based on scientific principles of advanced engineering design and AM methods, with the product being convenient for single-use (means of economic base) and sterilization (with limited cycles) in case of reuse in a risk-based environment such as COVID-19 (+) with close HW-patient interaction.
4 Conclusion
In this study, a competitively lighter, relatively more ergonomic, and easy-to-use medical face shield design which can be assembled without extra components (such as elastic bands, softening materials, and clips) and has a relatively shorter AM based production time were successfully realized. The motivation for this study was to provide an original AM compatible PPE product (primary target), which was designed in accordance with professional product design principles to support social solidarity against COVID-19 pandemic and potential future needs. The product design feature is also compatible with plastic injection molding-based serial production (secondary target). The survey carried out in a health service environment revealed that the product can be used for medical purposes with a good level of user/HW satisfaction. Taken together, this design study satisfactorily responded to the design requirements for a face shield PPE. Although the product provides safety features (dimensional features) described by the WHO, health safety risks should be carefully considered while using this product in health service facilities and public areas. This research provides a useful product design case study for informing further research on design, prototyping, and manufacture of simple medical equipment such as face shields for battling coronavirus-like viral pandemics by employing advanced engineering design, simulation, and AM applications.
Acknowledgments
This research study was supported financially by The Scientific Research Projects Coordination Unit of Akdeniz University, Antalya, Turkey. In addition, the authors wish to acknowledge the Department of Agricultural Machinery and Technology Engineering at Akdeniz University, Antalya, Turkey, for the contribution to the product design and prototyping stages, Department of Manufacturing at University Politehnica of Bucharest, Bucharest, Romania, for setting up the product function analysis algorithm, Antalya Training and Research Hospital, Antalya, Turkey, for medical product evaluations and Lancaster Product Development Unit (LPDU) at Lancaster University, Lancaster, United Kingdom, for technical evaluation processes employed in this research paper. The frame features and transparent shield assembly process of the face shield product detailed in this study was originally designed by Dr. H. Kursat CELIK (70%) and Prof. İbrahim AKINCI (30%). The product was submitted for consideration to the Turkish Patent and Trademark Office for Design Registration/Copyright Protection (Application No: 2020/03321; Application Date: May 15, 2020). Authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.World Health Organisation. [Last accessed on 2020 Apr 14];Novel Coronavirus (2019-nCoV):Situation Report-1 (21 January 2020) 2020 Available from:https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 .
- 2.World Health Organisation. [Last accessed on 2020 Apr 14];Novel Coronavirus (2019-nCoV):Situation Report-22 (11 February 2020) 2020 Available from:https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2 .
- 3.World Health Organisation. [Last accessed on 2020 Apr 14];Novel Coronavirus (Covid-19):Situation Report-51 (11 March 2020) 2020 Available from:https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 .
- 4.Joseph B, Joseph M. The Health of the Healthcare Workers. Indian J Occup Environ Med. 2016;20:71–2. doi: 10.4103/0019-5278.197518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talikwa L. Facing up to Wearing Facial Protection Equipment. Manag Inf Control. 2002;2:38. [Google Scholar]
- 6.European Centre for Disease Prevention and Control. [Last accessed on 2020 Apr 15];Guidance for Wearing and Removing Personal Protective Equipment in Healthcare Settings for the Care of Patients with Suspected or Confirmed COVID-19. 2020 Available from:https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-guidance-wearing-and-removing-personal-protective-equipment-healthcare-settings-updated.pdf . DOI:10.12996/gmj.2020.74.
- 7.National Health Service-England. [Last accessed on 2020 Apr 15];COVID-19:Visual Guide to Safe PPE. 2020 https://www.assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/≌56/PHE_COVID-19_visual_guide_poster_PPE.pdf .
- 8.TMA. [Last accessed on 2020 Apr 15];Turkish Medical Association:Use of Personal Protective Equipment (PPE) with Suspected or Confirmed COVID-19. 2020 https://www.ttb.org.tr/kollar/COVID19/index.php .
- 9.World Health Organisation. [Last accessed on 2020 Apr 15];Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19). (WHO Reference Number:WHO/2019-nCov/IPC PPE_use/2020.1) 2020 https://www.apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf . DOI:10.5222/bmj.2020.22931.
- 10.Roberge RJ. Face Shields for Infection Control:A Review. J Occup Environ Hyg. 2016;13(4):23542. doi: 10.1080/15459624.2015.1095302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organisation. [Last accessed on 2020 Apr 14];Shortage of Personal Protective Equipment Endangering Health Workers Worldwide. 2020 Available from:https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide . DOI:10.26616/nioshpub2009106.
- 12.Health Protection Scotland. [Last accessed on 2020 Apr 22];Standard Infection Control Precautions Literature Review:Personal Protective Equipment (PPE) Eye/Face Protection. 2020 Available from:https://www.hpspubsrepo.blob.core.windows.net/hpswebsite/nss/2605/documents/1_sicp-lr-eyewearv2.1.pdf .
- 13.Budynas RG, Nisbett JK. 9th ed. McGraw-Hill; New York, USA: 2011. Shigley's Mechanical Engineering Design. [Google Scholar]
- 14.Kamrani AK, Nasr EA. Engineering Design and Rapid Prototyping. Springer Science and Business Media; Berlin, Germany: 2010. pp. 442–6. [Google Scholar]
- 15.Yilmaz S, Seifert CM. Creativity through Design Heuristics:A Case Study of Expert Product Design. Des Stud. 2011;32:384–415. DOI:10.1016/j.destud.2011.01.003. [Google Scholar]
- 16.Matthews C. Case Studies in Engineering Design. Elsevier, Arnold; London, UK: 1998. pp. 272–4. [Google Scholar]
- 17.Miles LD. 3rd ed. Lawrence D. Miles Value Foundation; USA: 1989. Techniques of Value Analysis and Engineering. [Google Scholar]
- 18.Eppinger S, Ulrich K. 5th ed. McGraw Hill Publishing Company Ltd.; New York: 2012. Product Design and Development. [Google Scholar]
- 19.Lupeanu ME, Rennie AE, Neagu C. 12th Rapid Design, Prototyping and Manufacturing Conference; Lancaster, UK: 2011. Additive Manufacturing Technologies and Functional Analysis Used in Product Development Optimization; pp. 105–12. CRDM Ltd., High Wycombe. [Google Scholar]
- 20.Ruffo M, Tuck C, Hague R. Cost Estimation for Rapid Manufacturing Laser Sintering Production for Low to Medium Volumes. Proc Inst Mech Eng B J Eng Manuf. 2006;220(9):141727. DOI:10.1243/09544054jem517. [Google Scholar]
- 21.Hopkinson N, Dickens P. Analysis of Rapid Manufacturing Using Layer Manufacturing Processes for Production. Proc Inst Mech Eng C J Mech Eng Sci. 2003;217(1):31–9. [Google Scholar]
- 22.Brys G, Hubert M, Struyf A. A Robust Measure of Skewness. J Comput Graph Stat. 2004;13(4):996–1017. [Google Scholar]
- 23.A NSYS Documentation. Release 2020 R1, Ansys Inc; USA: 2020. Meshing User's Guide:Skewness. [Google Scholar]
- 24.Chua CK, Teh SH, Gay RK. Rapid Prototyping Versus Virtual Prototyping in Product Design and Manufacturing. Int J Adv Manuf Technol. 1999;15(8):597–603. DOI:10.1007/s001700050107. [Google Scholar]
- 25.Tang Y, Zhao YF. A Survey of the Design Methods for Additive Manufacturing to Improve Functional Performance. Rapid Prototyp J. 2016;22(3):569–90. [Google Scholar]
- 26.Booth JW, Alperovich J, Chawla P, et al. The Design for Additive Manufacturing Worksheet. J Mech Des. 2017;139(10):100904. [Google Scholar]
- 27.Cang KH. Academic Press, Elsevier Inc; USA: 2014. Product Design Modeling using CAD/CAE the Computer Aided Engineering Design Series; pp. 438–9. [Google Scholar]
- 28.Gallagher MB. 3 Questions:The Risks of Using 3D Printing to Make Personal Protective Equipment. MIT News. [Last accessed on 2020 Apr 29];2020 Available from:http://www.news.mit.edu/2020/3q-risks-using-3d-printing-make-personal-protective-equipment-0326 . DOI:10.17504/protocols.io.bd77i9rn.
- 29.Jurischka C, Dinter F, Efimova A, et al. An Explorative Study of Polymers for 3D Printing of Bioanalytical Test Systems. Clin Hemorheol Microcirc. 2020;74:1–28. doi: 10.3233/CH-190713. [DOI] [PubMed] [Google Scholar]
- 30.Clifton W, Damon A, Martin AK. Considerations and Cautions for Three-Dimensional-Printed Personal Protective Equipment in the COVID-19 Crisis. 3D Print Addit Manuf. 2020;1:1–3. doi: 10.1089/3dp.2020.0101. DOI:10.1089/3dp.2020.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oo-Kuma. [Last accessed on 2020 Apr 23];3D Printing Filaments Technical Data Sheet (ISO) PLA (Polyactic Acid) 2020 http://www.oo-kuma.com/uploads/1/4/0/8/14080379/elitepla.pdf .
- 32.Żur P, Kołodziej A, Baier A. Finite Elements Analysis of PLA 3D-Printed Elements and Shape Optimization. Eur J Eng Sci Technol. 2019;2(1):5964. [Google Scholar]


