Abstract
Dengue is placing huge burdens on the Malaysian healthcare system as well as the economy. With the expansion in the number of high-rise residential buildings, particularly in the urban centers, the flight range and behavior of Aedes mosquitoes may be altered in this habitat type. In this study, we aimed to expand the understanding of the vertical distribution and dispersal of Aedes in nine selected high-rise residences in Kuala Lumpur, Selangor, and Johor using ovitraps as the sampling method. We discovered that Ae. aegypti is the predominant species in all study sites. Both Ae. aegypti and Ae. albopictus are most abundant within the first three levels and could be found up to level 21 (approximately 61.1–63.0 m). Pearson correlation analyses exhibited negative correlations in eight out of nine study sites between the ovitrap indexes (OIs) within each floor level, suggesting that Aedes density decreased as the building level increased. Our findings provide information to the public health authorities on ‘hot spot’ floors for effective suppression of dengue transmission.
Keywords: vertical dispersal, Aedes, mosquito, high-rise residences, dengue, surveillance
1. Introduction
Dengue is a vector-borne infectious disease with an estimated 100–400 million infections annually [1]. Transmission of one of the four antigenically distinct serotypes of dengue virus (DENV-1 to DENV-4) may cause dengue and its severe forms—hemorrhagic fever and shock syndrome [2]. To date, efficacious and cost-effective vaccines and antiviral drugs against these four serotypes are still under various stages of research and development [3,4]. Until dengue vaccines or antiviral drugs become available, the only current method proven to be effective in dengue control and prevention is vector control measures that could be sustained through community involvement [1]. The spread of dengue globally is fueled by a combination of various factors including climate change, rapid urbanization, increased international travel and trade, as well as changes in land-use patterns [5].
Dengue is endemic in Malaysia and Malaysia is one of the most severely affected countries in South-East Asia [1]. The country had 80,615 reported dengue cases and 147 deaths in the year 2018 [6]. Selangor reported the highest number of dengue cases (45,349 cases), followed by Federal Territories of Kuala Lumpur and Putrajaya (7591 cases), Penang (6071 cases) and Johor (5885 cases) [6]. The primary mosquito vector for dengue is Aedes aegypti, while Ae. albopictus is recognized as the secondary vector [2]. These two Aedes species are prevalent in Malaysia where they can coexist in similar ecological niches [7].
Increased demand for housing due to urban sprawl and land scarcity in major urban regions resulted in an increase in high-rise residential buildings ranging from flats and apartments to luxury condominiums [8]. According to a housing statistic, 5.5 million people in Malaysia live in high-rise residences as of June 2014 [9], suggesting that a large number of Malaysians are experiencing high-rise living, also known as vertical living. The rise in the numbers of new non-landed, high-rise properties, as a result, may pose greater challenges in developing preventive and control measures against dengue, due to a change in dispersal dynamics of Aedes due to adaptation to non-landed, high-rise housing.
The vector flight range plays a key role in the dynamics of transmission. In standard approaches by the Ministry of Health (MOH), Malaysia, reduction of the proliferation of infected vectors and suppression of dengue outbreaks are achieved through vector control measures, e.g., insecticide fogging, larviciding and elimination of potential larval habitats, within a buffer zone with a radius of 200 m from the house where a dengue case is reported [10]. This is in line with standard mark-release-recapture studies showing that the mean distance of recaptured Ae. aegypti rarely went beyond 100 m [11,12]. In a review article, Ae. aegypti is said to disperse horizontally with an average distance of 83.4 m [13]. However, the standard approaches used are generally applied to low-rise housing. Slightly different vector control approaches may be required for high-rise housing. At present, specific guidelines for vector control activities in high-rises are yet to be released. But according to a circular from the MOH, fogging shall be performed within a 200 m radius from the index case house, and for buildings that have more than five levels, fogging shall be completed at least within five levels below and above the index case house [14]. For buildings that have five floors or less, the whole building shall be fogged [14].
Studies on the vertical distribution of Aedes in high-rises have been carried out in several countries, including Sri Lanka [15], Trinidad [16], Singapore [17] and Malaysia [7,18,19]. Research in Trinidad, West Indies, suggested that Aedes eggs were found mostly in 13–24 m elevations [16]. While in Sri Lanka, Aedes could be found from the ground floor to the highest floor of 130 ft (approximately 40 m), but largely were discovered at the 60 ft elevation (approximately 18 m) [15]. A similar observation was seen in Putrajaya, Malaysia in which Aedes density peaked at the height of 16.1–18 m [19]. These studies indicated that infestations of Aedes in high-rises were extensive, ranging from the ground level to higher elevations, and that they have the ability to survive in high-rise buildings.
This study aimed to expand the understanding of the vertical distribution of Aedes in nine selected high-rise residences in the Federal Territory of Kuala Lumpur and the States of Selangor and Johor using ovitraps as the sampling method. Although a few studies have been performed in Malaysia for similar purposes [7,18,19,20], the ever-increasing number of high-rise residences in Malaysia makes this study warranted. Up-to-date information gained from this study will hopefully serve as guidelines for the public health officers to identify ‘hot spot’ floors of Aedes infestation in high-rises for quick and more efficient dengue control efforts.
2. Materials and Methods
2.1. Study Sites
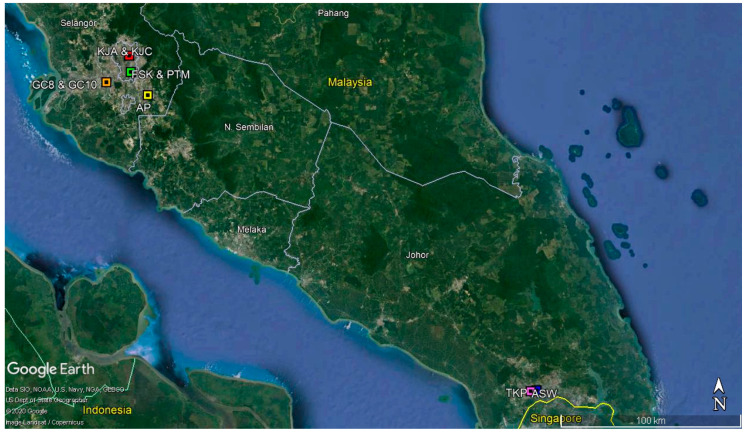
The study was conducted in nine study sites that constituted high-rise properties. Five study sites were in Selangor State—Subang Perdana Goodyear Court 8 (GC8), Goodyear Court 10 (GC10), Apartmen Pesona (AP), Flat Sri Kota (FSK) and PPR Taman Mulia (PTM); two study sites were in Johor State—PPR Taman Kempas Permai (TKP) and Apartmen Sri Wangi (ASW) and two study sites were in the Federal Territory of Kuala Lumpur—Kuarters Jalan Sultan Abdul Aziz Block A (KJA) and Block C (KJC) (Figure 1). The sites were chosen because they had prolonged dengue outbreaks between the years 2014 and 2018. All study sites were in urban areas, at which their coordinates were obtained using the Global Positioning System (GPS) (Garmin Montana® 680, Olathe, Kansas, US). The physical and geographical description as well as the building design of each study site are explained in Supplementary Table S1 and Figures S1–S6.
Figure 1.
The geographical map of the study sites. Abbreviations: GC8—Goodyear Court 8, GC10—Goodyear Court 10, AP—Apartmen Pesona, FSK—Flat Sri Kota, PTM—PPR Taman Mulia, KJA—Kuarters Jalan Sultan Abdul Aziz Block A, KJC—Kuarters Jalan Sultan Abdul Aziz, TKP—PPR Taman Kempas Permai and ASW—Apartmen Sri Wangi.
2.2. Ovitrap Surveillance
The number of Aedes larvae that represented the population densities of Aedes was assessed at different building levels via ovitraps. Ovitrapping was performed as described in Lee [21]. Each ovitrap consisted of a 250 mL cylindrical, black plastic container (7.0 cm diameter, 9.0 cm height) filled with tap water to a level of 5.5 cm. Each ovitrap was equipped with a removable oviposition paddle that was made from a thin strip of brown hardboard (10 cm × 2.5 cm × 0.3 cm). The paddle was placed diagonally with the rough surface facing upwards where the mosquitoes laid eggs above the water level.
Ovitrap monitoring was conducted separately for each study site between the years 2014 and 2018, with sampling at each site consisting of 10 weeks of data collection. The total number of ovitraps placed in each study site is described in Table 1. One ovitrap was placed randomly on each level that had minimum human, physical and environmental disturbance. We placed the ovitraps in a semi-indoor environment, defined as the area outside of the house units but is still sheltered by the roof, e.g., shared corridor and stairway. The ovitraps were collected after seven days and replaced with new ovitraps consisting of fresh tap water and egg-free oviposition paddles. Any disturbances to the exposed ovitraps, e.g., theft, vandalism or invasion by insects, were recorded.
Table 1.
The total number of ovitraps placed in each study site.
| Study Site | Total Number of Ovitraps |
|---|---|
| GC8 | 300 |
| GC10 | 500 |
| AP | 200 |
| FSK | 700 |
| PTM | 400 |
| ASW | 360 |
| TKP | 510 |
| KJA | 570 |
| KJC | 190 |
The collected ovitraps were transported back to the Institute for Medical Research (IMR), Kuala Lumpur, laboratory for further processing. The ovitrap contents along with the oviposition paddle were transferred into the plastic containers and labeled according to the study site, date of collection, and level. Beef liver powder (Difco Laboratories, MD, USA) was added into each container as larval food. Identification to species level of third or fourth instar was performed using established taxonomy keys [22,23] under a compound microscope (Nikon Eclipse® E100, Japan). The ovitrap contents were examined for species identification until no newly emerged larvae were present in the containers.
2.3. Data Analysis
Data entry and statistical analyses were conducted using Microsoft Excel and Statistical Package for Social Sciences (SPSS) Version 25.0 [24]. Ovitrap index (OI) is expressed as the percentage of positive ovitraps per the number of ovitraps recovered. Summation and the mean number of Aedes larvae for each Aedes species per ovitrap were performed in every study site. Paired samples t-test analysis was conducted to determine if there is any significant difference between the mean number of Ae. aegypti larvae and Ae. albopictus larvae per ovitrap. Single and cohabitation of Aedes spp. were also analyzed. Linear regression line for OIs in each floor level was constructed for each site. The Spearman’s rank correlation coefficient test was further conducted to investigate the correlation between the mean number of Aedes larvae and floor level. All levels of statistical significance were determined at p ≤ 0.05.
3. Results
The overall results showed that the OI varied quite considerably between the nine study sites, with AP exhibiting the highest OI (63.00 ± 3.40%), followed by TKP (55.00 ± 2.40%) and ASW (51.00 ± 2.70%) (Table 2). The lowest OI belonged to KJA with OI of 26.00 ± 1.90% (Table 2). Paired samples t-test analysis indicated that the mean number of Ae. aegypti larvae per ovitrap was significantly higher than that of Ae. albopictus in all study sites (p ≤ 0.05), demonstrating that Ae. aegypti is the predominant species in all sites studied. Collected Ae. aegypti larval instars were highest in TKP (8847 larvae), while Ae. albopictus instars were most abundant in AP (980 larvae) (Table 2). The relative abundance (ratio) of Ae. aegypti to Ae. albopictus ranged from the lowest of 1.7:1 in AP to the highest of 30.6:1 in KJA (Table 2).
Table 2.
The overall analysis of Aedes larval instars collected in each study site.
| Study Site | Ovitrap Collected | Ovitrap Index (%) | Ae. aegypti | Ae. albopictus | Ratio Ae. aegypti: Ae. albopictus | ||
|---|---|---|---|---|---|---|---|
| Total Larvae | Overall Percentage (%) | Total Larvae | Overall Percentage (%) | ||||
| GC8 | 297/300 | 48.00 ± 2.90 | 5216 | 94.02 | 332 | 5.98 | 15.7: 1 |
| GC10 | 454/500 | 41.00 ± 2.30 | 5258 | 84.63 | 955 | 15.37 | 5.5: 1 |
| AP | 200/200 | 63.00 ± 3.40 | 1640 | 62.60 | 980 | 37.40 | 1.7: 1 |
| FSK | 658/700 | 37.00 ± 1.90 | 3111 | 91.10 | 304 | 8.90 | 10.3: 1 |
| PTM | 371/400 | 37.00 ± 2.50 | 1614 | 76.17 | 505 | 23.83 | 3.2: 1 |
| ASW | 351/360 | 51.00 ± 2.70 | 4483 | 90.22 | 486 | 9.78 | 9.3: 1 |
| TKP | 448/510 | 55.00 ± 2.40 | 8847 | 92.45 | 722 | 7.55 | 12.3: 1 |
| KJA | 564/570 | 26.00 ± 1.90 | 1898 | 96.79 | 63 | 3.21 | 30.6: 1 |
| KJC | 188/190 | 31.00 ± 3.40 | 738 | 94.98 | 39 | 5.02 | 18.7: 1 |
We performed a further analysis by looking at single and cohabitation of Aedes species in the same ovitraps. Our analyses showed that single breeding of Aedes was much more prevalent than the cohabitation of Aedes spp. in all study sites (Table 3). The proportions of ovitraps positive for single breeding of Ae. aegypti were noticeably high in all study sites, ranging from 64.80% in AP to 96.62% in KJA (Table 3). It is worth noting that KJA was the only study site void of cohabitation of Aedes spp., contributing to its high percentage of single breeding of Ae. aegypti (Table 3). Meanwhile, percentages of ovitraps positive for single breeding of Ae. albopictus were, in general, higher than cohabitation of Aedes spp., with the exception of TKP (Table 3). Analysis variance of one-way ANOVA showed that there were significant differences in the mean number of Ae. aegypti larvae (p ≤ 0.05, F = 34.652) and Ae. albopictus larvae (p ≤ 0.05, F = 9.712) in single breeding as well as in cohabitation of Aedes spp. among the nine study sites (p ≤ 0.05, F = 8.628). TKP displayed the highest mean number of Ae. aegypti per ovitrap in single breeding (17.33 ± 1.50) and cohabitation of Aedes spp. (3.58 ± 0.74) (Table 3). Interestingly, PTM was the only site that exhibited a higher ratio of Ae. albopictus to Ae. aegypti in cohabitation containers (Table 3).
Table 3.
Comparisons between single breeding and cohabitation of Aedes spp. in each study site.
| Study Site | Single Breeding | Cohabitation | |||||
|---|---|---|---|---|---|---|---|
| Ae. aegypti | Ae. albopictus | ||||||
| Percentage of Positive Ovitraps (%) | Mean Larvae per Ovitrap ± SE | Percentage of Positive Ovitraps (%) | Mean Larvae per Ovitrap ± SE |
Percentage of Positive Ovitraps (%) | Mean Larvae per Ovitrap ± SE |
Ratio Ae. aegypti: Ae. albopictus | |
| GC8 | 90.97 | 16.96 ± 1.66 | 5.56 | 0.79 ± 0.37 | 3.47 | 0.93 ± 0.45 | 1.86: 1.00 |
| GC10 | 78.38 | 10.56 ± 0.99 | 13.51 | 1.40 ± 0.39 | 8.11 | 1.73 ± 0.57 | 1.45: 1.00 |
| AP | 64.80 | 7.38 ± 0.91 | 21.60 | 4.14 ± 1.18 | 13.60 | 1.59 ± 0.45 | 1.08: 1.00 |
| FSK | 90.24 | 4.55 ± 0.34 | 6.91 | 0.38 ± 0.11 | 2.85 | 0.26 ± 0.10 | 2.31: 1.00 |
| PTM | 78.68 | 4.25 ± 0.50 | 18.38 | 1.16 ± 0.31 | 2.94 | 0.30 ± 0.16 | 1.00: 1.90 |
| ASW | 79.33 | 11.32 ± 1.22 | 10.61 | 0.89 ± 0.27 | 10.61 | 1.95 ± 0.51 | 2.10: 1.00 |
| TKP | 82.26 | 17.33 ± 1.50 | 6.05 | 0.45 ± 0.16 | 11.69 | 3.58 ± 0.74 | 2.92: 1.00 |
| KJA | 96.62 | 3.37 ± 0.30 | 3.38 | 0.11 ± 0.07 | 0.00 | 0.00 ± 0.00 | 0.00: 0.00 |
| KJC | 94.92 | 3.76 ± 0.53 | 3.39 | 0.11 ± 0.09 | 1.69 | 0.27 ± 0.27 | 1.78: 1.00 |
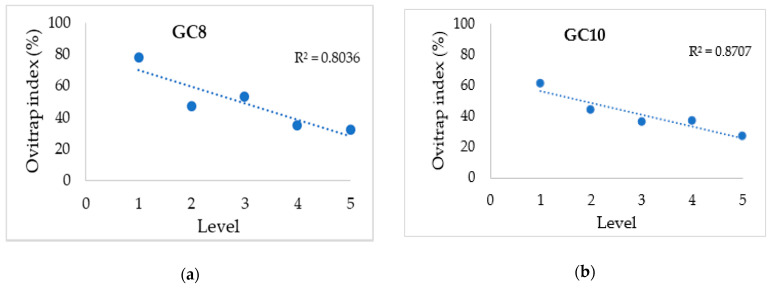
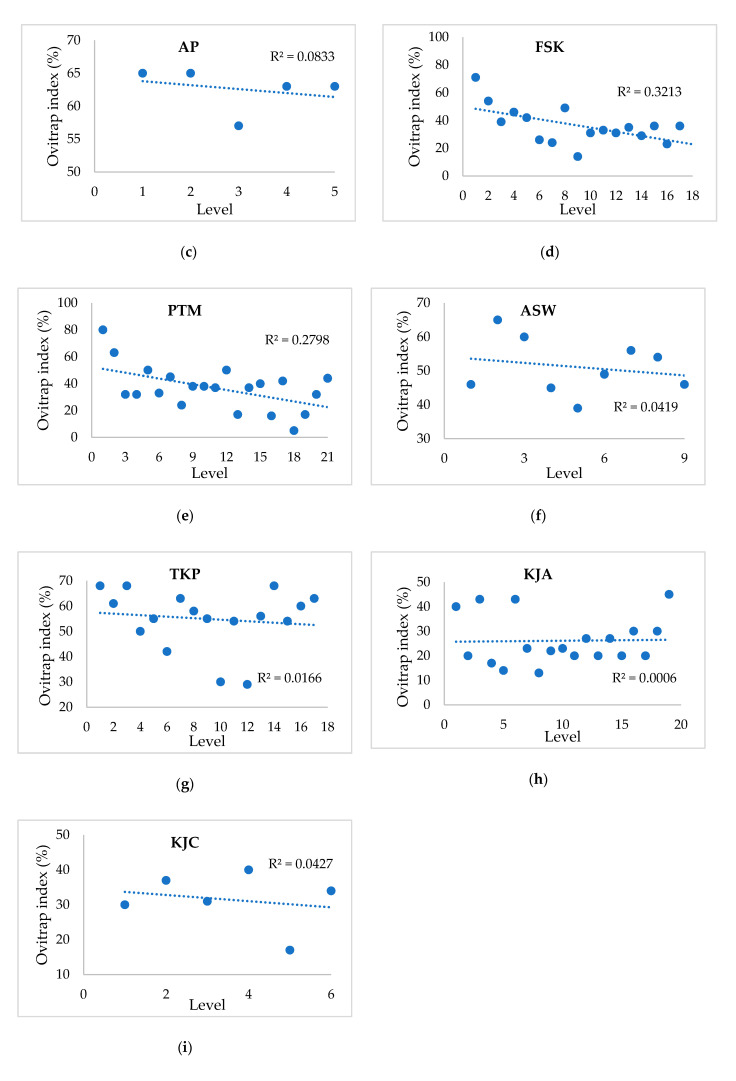
The relationship between OI and floor level was investigated in this study. For this, the linear regression line of each study site was constructed (Figure 2). Except for KJA, Pearson correlations exhibited negative correlations in all sites, suggesting that as the floor level increased, the OI decreased. Significant differences between OIs within each floor level were exhibited in GC8 (p = 0.020), GC10 (p = 0.010), FSK (p = 0.009) and PTM (p = 0.007). KJA had a positive correlation between OIs within each floor level but was insignificant (p = 0.460).
Figure 2.
Relationship between ovitrap index (%) and level in each study site. (a–i) GC8, GC10, AP, FSK, PTM, ASW, TKP, KJA, KJC.
To go deeper into this analysis, we found that six out of nine study sites—GC8, GC10, AP, FSK, PTM, and TKP—displayed the highest mean number of Aedes larvae per ovitrap on floor level 1 (0.0–3.0 m height) (Table 4). In contrast, Aedes larvae per ovitrap peaked on the top floor (54.1–57.0 m height) in KJA (Table 4). In ASW and KJC, Aedes larvae density was highest on level 7 (18.1–21.0 m height) and level 4 (9.1–12 m height), respectively (Table 4). Intriguingly, all three sites of the five-story building—GC8, GC10 and AP—showed the lowest mean number of Aedes larvae per ovitrap on the highest floor (12.1–15.0 m height) (Table 4). Likewise, ASW, KJA and KJC presented the least Aedes larvae per ovitrap within the same floor height (Table 4). It may be noted that Ae. aegypti was found on every floor level at all study sites, up to level 21 (61.1–63.0 m height). Similar to Ae. aegypti, Ae. albopictus was discovered on each floor level up to level 21, but in KJA and KJC, the species could only be found up to level 3 and level 2, respectively (Supplementary Tables S2 and S3). Similar to the OI analysis, Spearman’s rank correlation coefficient showed negative correlations in all sites except for KJA (Table 4).
Table 4.
The mean number of Aedes larvae per ovitrap on each level. Spearman’s rank correlation coefficient between the mean number of Aedes larvae and level (height) was calculated using SPSS statistics.
| Level | Approximate Height (m) | GC8 | GC10 | AP | FSK | PTM | ASW | TKP | KJA | KJC |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.0–3.0 | 36.16 ± 5.02 | 26.72 ± 3.71 | 14.90 ± 2.80 | 9.88 ± 1.40 | 14.10 ± 3.40 | 11.21 ± 3.08 | 36.29 ± 8.81 | 5.13 ± 1.73 | 4.10 ± 1.48 |
| 2 | 3.1–6.0 | 19.28 ± 3.91 | 12.99 ± 2.07 | 13.28 ± 2.68 | 6.57 ± 1.66 | 9.16 ± 2.62 | 15.83 ± 3.68 | 33.39 ± 8.59 | 1.67 ± 0.77 | 4.93 ± 1.53 |
| 3 | 6.1–9.0 | 14.22 ± 2.76 | 10.73 ± 2.25 | 14.18 ± 3.68 | 3.63 ± 1.05 | 6.05 ± 2.27 | 17.38 ± 3.82 | 20.21 ± 4.00 | 3.73 ± 1.07 | 3.76 ± 1.20 |
| 4 | 9.1–12.0 | 13.72 ± 3.34 | 10.04 ± 1.97 | 12.08 ± 3.99 | 6.23 ± 1.60 | 7.58 ± 3.56 | 9.38 ± 2.68 | 23.42 ± 7.21 | 2.59 ± 1.13 | 5.47 ± 1.52 |
| 5 | 12.1–15.0 | 10.53 ± 2.73 | 8.76 ± 2.08 | 11.08 ± 2.19 | 5.87 ± 1.42 | 7.83 ± 2.90 | 7.89 ± 2.67 | 17.90 ± 4.86 | 1.31 ± 0.91 | 2.23 ± 1.11 |
| 6 | 15.1–18.0 | 4.64 ± 1.59 | 5.44 ± 3.49 | 15.64 ± 4.04 | 14.75 ± 5.71 | 4.67 ± 1.12 | 4.31 ± 1.71 | |||
| 7 | 18.1–21.0 | 4.92 ± 1.81 | 5.45 ± 2.31 | 19.79 ± 5.25 | 25.25 ± 7.09 | 4.30 ± 1.75 | ||||
| 8 | 21.1–24.0 | 8.38 ± 1.81 | 3.12 ± 1.79 | 15.64 ± 4.41 | 17.21 ± 5.65 | 2.27 ± 1.28 | ||||
| 9 | 24.1–27.0 | 2.76 ± 1.51 | 5.50 ± 2.13 | 14.51 ± 4.21 | 12.86 ± 4.38 | 2.22 ± 0.97 | ||||
| 10 | 27.1–30.0 | 4.08 ± 1.28 | 3.25 ± 1.93 | 16.55 ± 8.33 | 4.07 ± 1.59 | |||||
| 11 | 30.1–33.0 | 1.53 ± 1.48 | 8.11 ± 3.44 | 19.61 ± 7.04 | 2.73 ± 1.09 | |||||
| 12 | 33.1–36.0 | 3.72 ± 1.29 | 9.06 ± 2.94 | 5.54 ± 2.86 | 3.03 ± 1.01 | |||||
| 13 | 36.1–39.0 | 3.15 ± 1.03 | 1.28 ± 0.80 | 23.00 ± 6.57 | 4.00 ± 1.68 | |||||
| 14 | 39.1–42.0 | 4.00 ± 1.41 | 4.58 ± 1.45 | 33.68 ± 7.43 | 2.80 ± 1.01 | |||||
| 15 | 42.1–45.0 | 5.23 ± 1.49 | 5.10 ± 2.69 | 10.86 ± 2.93 | 2.90 ± 1.23 | |||||
| 16 | 45.1–48.0 | 3.08 ± 1.10 | 0.89 ± 0.57 | 32.48 ± 8.58 | 2.97 ± 1.09 | |||||
| 17 | 48.1–51.0 | 5.31 ± 1.71 | 6.68 ± 2.46 | 17.73 ± 5.33 | 3.07 ± 1.18 | |||||
| 18 | 51.1–54.0 | 0.05 ± 0.05 | 3.90 ± 1.33 | |||||||
| 19 | 54.1–57.0 | 1.17 ± 0.76 | 8.66 ± 2.01 | |||||||
| 20 | 57.1–60.0 | 6.00 ± 2.29 | ||||||||
| 21 | 60.1–63.0 | 7.28 ± 4.52 | ||||||||
| r | −0.304 | −0.231 | −0.070 | −0.172 | −0.203 | −0.024 | −0.059 | 0.024 | −0.028 | |
| P | 0.000 | 0.000 | 0.322 | 0.000 | 0.000 | 0.657 | 0.211 | 0.576 | 0.705 |
4. Discussion
With the expansion in the number of high-rise residential buildings largely in the urban areas in Malaysia, we believe that the up-to-date information on the vertical distribution of Aedes in this habitat type is warranted. We discovered that Ae. aegypti was the predominant species in all nine study sites, in which the number of Ae. aegypti larval instars were up to 30-fold higher in comparison to Ae. albopictus. The dominance of Ae. aegypti is in line with the results of several vertical dispersal studies conducted in urban high-rise residences in Kuala Lumpur and Selangor [7,18] as well as Putrajaya [19]. This finding is not surprising, because Ae. aegypti is adapted to reside in and around human dwellings. Female Ae. aegypti also preferentially breed in artificial and domestic containers in the urban areas [25], which may be commonly found in the semi-indoor environment of our study sites. Likewise, the lower number of Ae. albopictus larval instars observed in all study sites were due to their behavior. Ae. albopictus prefers to breed in natural habitats such as tree holes and to reside where vegetation is plentiful [26], which may be especially uncommon in high-rise properties where space is limited for planting or gardening. However, recent studies in Malaysia discovered that Ae. albopictus have been acclimatizing to the domestic environment in urban areas. The species is now found both in the indoor and outdoor environments and readily breeds in artificial or man-made containers [27,28]. Therefore, it is not surprising that Ae. albopictus was found, but in a lower number.
The mixed infestation of Ae. aegypti and Ae. albopictus was detected in all sites, apart from KJA, accounting for 1.39% to 13.60% of the percentage of positive ovitraps collected. The cohabitation of Aedes spp. found in the present study suggested that their ecological niche overlapped. A study in multi-story buildings in Selangor and Kuala Lumpur by Lau [7] discovered a higher percentage of cohabitation of Aedes spp., ranging from 10.77% to 26.56% from the total percentage of positive ovitraps collected. The lower percentages of co-breeding compared to single breeding may be explained through their breeding behavior. Ae. albopictus, for instance, would avoid breeding in habitats occupied by Ae. aegypti, and vice versa, as shown in a study in Penang [29].
The discovery of Aedes on each level suggested that Aedes could disperse up to the highest floor level. Three main types of adult mosquito dispersal exist [30]. The first is unintentional dispersal by riding along with humans via airplanes or ships, involving long-distance dispersal from one continent to another. The second is mosquitoes being carried through wind-assisted dispersal, also in a long-distance and unintentional dispersal. The third type (involved in our study) is considered as active and intentional in a short-distance dispersal. In high-rise buildings, Aedes could be involved in short-distance dispersal via human transportation using stairs or lifts as suggested in other studies [7,19]. Mosquitoes may disperse to look for blood sources, nectar sources, mates, oviposition sites and resting sites [30]. The search for new oviposition sites, for example, could be represented by the ovitraps and possibly other cryptic breeding sites that drive the dispersal of female Aedes through the availability of stagnant water for the development of immature, aquatic stages of mosquitoes. Studies conducted in urban habitats in Malaysia discovered that both Aedes spp. were mostly found in domestic receptacles such as flower vases, flower pot plates, pails, bowls, refrigerator trays, plastic containers, empty paint cans and in building structures such as roofs, drains, gutters and gully traps [27,31,32]. We hypothesized that the Aedes mosquitoes in our study sites could have originated from these sources.
Additionally, the discovery of Aedes on each level suggested that the ground floor, as well as the higher elevations, support the survival of Aedes. The availability of biotic and abiotic components (ecological niche) and ecosystem in our study sites may have provided sufficient bloodmeal, resting place, and breeding sites for Aedes [16]. Biotic components include humans, pets, and plants, while abiotic components include temperature, humidity, wind speed, and building structure [16]. The denser people living in high-rises will most likely provide more blood meals for Aedes, and in turn increase their vectorial capacity to transmit dengue viruses due to higher contact with human hosts [33]. This is particularly true for Ae. aegypti, which has an anthropophilic tendency for preferential feeding on human blood and takes multiple blood-feeding in a gonotrophic cycle [34,35].
Although Aedes dispersal seemed extensive (up to level 21, 61.0–63.0 m height), the present study demonstrated that Aedes larvae were most abundant on the ground floor. A plausible reason may be due to the existence of communal areas in the ground level such as parking spaces, shops and waste disposal areas that sometimes could have poor sanitation, creating favorable mosquito breeding sites. In Singapore, for example, children residing in the ground level reportedly had higher dengue infection rates compared to those residing in higher levels because more Aedes breeding habitats were found in the communal areas [17]. This finding emphasized that the ground floor demands additional efforts during vector control measures.
Intriguingly, unlike the other eight other study sites, we found Aedes larvae were most abundant at the highest level of KJA with an elevation of approximately 54.1 to 57 m. We speculate that this might be due to the presence of an uneven rooftop floor, where heavy rain will cause puddles and create breeding sites for mosquitoes. Importantly, in the study, we found that the maximum height of Ae. aegypti dispersal was identical to Ae. albopictus (61.1–63.0 m). However, we wanted to point out that Ae. albopictus had overall a higher density closer to the ground floor. In KJA and KJC, they could only be found up to level 2 and level 3, respectively. The results suggested that Ae. albopictus preferred elevations closer to the ground floor, presumably with scattered vegetation around for breeding and resting. Furthermore, from the results, it was clear that ‘hot spot’ floors of Aedes infestation were within the first three floors. Again, we think that floors closer to the ground may create a complete ecosystem and ecological niche suitable for Aedes infestation.
It is noteworthy to point out that TKP exhibited comparably high Aedes populations at the upper levels (levels 14 to 17) as well as the first two floor levels, implying a U-shaped curve instead of a declining trend. This may have occurred owing to the existence of water tanks between levels 16 and 17. Likewise, PTM has water tanks between levels 20 and 21 that may be responsible for the considerably large Aedes populations at the upper levels.
Our study possesses some limitations. Firstly, we did not consider environmental factors such as temperature, relative humidity, wind speed and rainfall. Wind speed, for instance, could influence the flight capabilities of mosquitoes [36], while relative humidity influences the mating and feeding behavior of mosquitoes [37]. Future research incorporating these environmental factors may be warranted. Secondly, our study sites have different building designs that may have influenced our results. However, we could say that the low-cost houses, i.e., AP, FSK, TKP, and PTM, possess similar building designs (Supplementary Figures S2, S3 and S5). Because our study only included low- and medium-cost houses, future studies incorporating high-cost houses such as luxury condominiums may provide interesting new insights into the vertical dispersal of Aedes.
In conclusion, our study revealed that the dispersal of Aedes vertically in high-rise residential buildings could be considered extensive. We think that Aedes could disperse to the upper floors even under little pressure to fulfill their needs, particularly finding hosts and oviposition sites. Based on our results, we recommended that the vector control operations should be concentrated within the first three floors and that guidelines specific for high-rises should be available. Given the extensive vertical dispersal of Aedes, other levels (middle and upper) should also be taken into consideration during vector control measures, depending on the location of the index case house as recommended in the circular. Vertical vector dispersal and propagation may play key roles in dengue virus dissemination in high-rises and may require further attention to fully investigate its importance.
Acknowledgments
The authors thank the Director-General of Health, Malaysia for his permission to publish this article, and Director of Institute for Medical Research for unceasing support. The authors also thank the staff of the Medical Entomology Unit, IMR for assistance in the field.
Supplementary Materials
The following are available online at https://www.mdpi.com/2414-6366/5/3/114/s1, Table S1: Physical and geographical descriptions of all study sites. Figure S1–S6: Building designs of all study sites. Table S2: Total number and percentage of Aedes spp. on each level at KJA. Table S3: Total number and percentage of Aedes spp. on each level at KJC.
Author Contributions
Conceptualization, N.A.H., S.N.M.N., N.W.A., H.L.L.; methodology, N.A.H., H.L.L., T.O., N.W.A.; software, N.R.I., A.A.H., M.K.K.Z.; validation, N.A.H., F.D.A., T.O., N.W.A., H.L.L.; formal analysis, N.A.H., S.N.M.N., N.R.I.; investigation: A.M.B.E., A.A.H., F.A.A., S.F.A., M.K.K.Z., M.I.M.N., N.H.A., N.A.K., I.F.Z.; A.N.M.R.; resources: N.A.H., F.D.A., T.O., N.W.A., H.L.L.; data curation: N.A.H., S.N.M.N., N.R.I., R.M.R.; writing—original draft preparation, N.A.H., S.N.M.N., R.M.R.; writing—review and editing: N.A.H., F.D.A., T.O., H.L.L.; supervision, N.A.H., F.D.A., H.L.L.; project administration, N.A.H., F.D.A., T.O., N.W.A., H.L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Health Malaysia Research Grant (NMRR-13-921-17915).
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Approval
The study was approved by the Institute for Medical Research Committee (JPP-IMR) and National Institutes of Health Malaysia (JPP-NIH), and registered with the National Medical Research Register (NMRR-13-921-17915). This study met the ethical standard of the Medical Research and Ethics Committee (MREC) (Ref No: KKM/NIHSEC/800–2/2/2JldP13905).
References
- 1.WHO Dengue and Severe Dengue. [(accessed on 15 April 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- 2.WHO . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. World Health Organization; Geneva, Switzerland: 2009. [PubMed] [Google Scholar]
- 3.Deng S.Q., Yang X., Wei Y., Chen J.T., Wang X.J., Peng H.J. A review on dengue vaccine development. Vaccines. 2020;8:63. doi: 10.3390/vaccines8010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato F., Ishida Y., Oishi S., Fujii N., Watanabe S., Vasudevan S.G., Tajima S., Takasaki T., Suzuki Y., Ichiyama K., et al. Novel antiviral activity of bromocriptine against dengue virus replication. Antivir. Res. 2016;131:141–147. doi: 10.1016/j.antiviral.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 5.WHO WHO Region of the AMERICAS Records Highest Number of Dengue Cases in History; Cases Spike in Other Regions. [(accessed on 15 April 2020)]; Available online: https://www.who.int/news-room/detail/21-11-2019-who-region-of-the-americas-records-highest-number-of-dengue-cases-in-history-cases-spike-in-other-regions.
- 6.Ministry of Health Malaysia Kenyataan Akhbar Ketua Pengarah Kesihatan Malaysia. Situasi semasa Demam Denggi, Zika, dan Chikungunya di Malaysia. [(accessed on 15 April 2020)]; Available online: http://www.moh.gov.my/index.php/database_stores/store_view_page/17/1119.
- 7.Lau K.W., Chen C.D., Lee H.L., Izzul A.A., Asri-Isa M., Zulfadli M., Sofian-Azirun M. Vertical distribution of Aedes mosquitoes in multiple storey buildings in Selangor and Kuala Lumpur, Malaysia. Trop. Biomed. 2013;30:36–45. [PubMed] [Google Scholar]
- 8.Sarip A.G., Foong L. Exploring the Perception of Lifestyle Housing Development in Malaysia. The Asia Pacific Network for Housing Research; Gwangju, Korea: 2015. [Google Scholar]
- 9.New Straits Times Malaysian House Buyers Increasingly Drawn to High-Rise Living. [(accessed on 15 April 2020)]; Available online: https://www.nst.com.my/news/2016/07/161815/malaysian-house-buyers-increasingly-drawn-high-rise-living.
- 10.Ministry of Health Malaysia . Garis Panduan Halatuju Baharu Dalam Kawalan Denggi. Vector Borne Disease Sector, Disease Control Division, Ministry of Health Malaysia; Putrajaya, Malaysia: 2014. [Google Scholar]
- 11.Muir L.E., Kay B.H. Aedes aegypti survival and dispersal estimated by mark-release-recapture in northern Australia. Am. J. Trop. Med. Hyg. 1998;58:277–282. doi: 10.4269/ajtmh.1998.58.277. [DOI] [PubMed] [Google Scholar]
- 12.Harrington L.C., Buonaccorsi J.P., Edman J.D., Costero A., Kittayapong P., Clark G.G., Scott T.W. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidae) from Puerto Rico and Thailand. J. Med. Entomol. 2001;38:537–547. doi: 10.1603/0022-2585-38.4.537. [DOI] [PubMed] [Google Scholar]
- 13.Verdonschot P.F.M., Besse-Lototskaya A.A. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica. 2014;45:69–79. [Google Scholar]
- 14.Ministry of Health Malaysia . Panduan Pengabutan (fogging) di Premis/ Bangunan Melebihi Lima (5) Tingkat. Ministry of Health Malaysia; Putrajaya, Malaysia: 2010. [Google Scholar]
- 15.Jayathilake H., Wickramasinghe M., De Silva N. Oviposition and vertical dispersal of Aedes mosquitoes in multiple storey buildings in Colombo district, Sri Lanka. J. Vector Borne. Dis. 2015;52:245–251. [PubMed] [Google Scholar]
- 16.Chadee D.D. Observations on the seasonal prevalence and vertical distribution patterns of oviposition by Aedes aegypti (L) (Diptera: Culicidae) in urban high-rise apartments in Trinidad, West Indies. J. Vector Ecol. 2004;29:323–330. [PubMed] [Google Scholar]
- 17.Liew C., Curtis C.F. Horizontal and vertical dispersal of dengue vector mosquitoes, Aedes aegypti and Aedes albopictus, in Singapore. Med. Vet. Entomol. 2004;18:351–360. doi: 10.1111/j.0269-283X.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 18.Roslan M.A., Shafie A., Ngui R., Lim Y.A., Sulaiman W.Y. Vertical infestation of the dengue vectors Aedes aegypti and Aedes albopictus in apartments in Kuala Lumpur, Malaysia. J. Am. Mosq. Control Assoc. 2013;29:328–336. doi: 10.2987/13-6363.1. [DOI] [PubMed] [Google Scholar]
- 19.Wan-Norafikah O., Nazni W.A., Noramiza S., Shafa’ar-Ko’ohar S., Azirol-Hisham A., Nor-Hafizah R., Sumarni M.G., Mohd-Hasrul H., Sofian-Azirun M., Lee H.L. Vertical dispersal of Aedes (Stegomyia) spp. in high-rise apartments in Putrajaya, Malaysia. Trop. Biomed. 2010;27:662–667. [PubMed] [Google Scholar]
- 20.Sairi F.A.M., Dom N.C., Camalxaman S.N. Infestation profile of Aedes mosquitoes in multi-storey buildings in Selangor, Malaysia. Procedia Soc. Behav. Sci. 2016;222:283–289. [Google Scholar]
- 21.Lee H.L. Aedes ovitrap and larval survey in several suburban communities in Selangor, Malaysia. Mosq-Borne. Dis. Bull. 1992;9:9–15. [Google Scholar]
- 22.Parija S. Illustrated keys: Some mosquitoes of Peninsula Malaysia. Trop. Parasitol. 2014;4:70. [Google Scholar]
- 23.Pratt H.D., Stojanovich C.J., Magennis N.J. Workbook on Identification of Aedes Aegypti Larvae. U.S. Department of Health, Education and Welfare; Atlanta, GA, USA: 1964. [Google Scholar]
- 24.IBM C.R. IBM SPSS Statistics for Windows, Version 25.0. IBM Corp.; Armonk, NY, USA: 2017. [Google Scholar]
- 25.Mahmud M., Mutalip H., Lodz N.A., Shahar H. Study on key Aedes spp breeding containers in dengue outbreak localities in Cheras district, Kuala Lumpur. Int. J. Mosq. Res. 2018;5:23–30. [Google Scholar]
- 26.Estrada-Franco J.G., Craig G.B. Biology, Disease Relationships, and Control of Aedes Albopictus. Pan American Health Organization; Washington, DC, USA: 1995. [Google Scholar]
- 27.Dieng H., Saifur R.G.M., Hassan A.A., Salmah M.R.C., Boots M., Satho T., Jaal Z., Abu Bakar S. Indoor-breeding of Aedes albopictus in northern peninsular Malaysia and its potential epidemiological implications. PLoS ONE. 2010;5:e11790. doi: 10.1371/journal.pone.0011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozilawati H., Zairi J., Adanan C.R. Seasonal abundance of Aedes albopictus in selected urban and suburban areas in Penang, Malaysia. Trop. Biomed. 2007;24:83–94. [PubMed] [Google Scholar]
- 29.Hashim N.A., Ahmad A.H., Talib A., Athaillah F., Krishnan K.T. Co-breeding association of Aedes albopictus (Skuse) and Aedes aegypti (Linnaeus) (Diptera: Culicidae) in relation to location and container size. Trop. Life. Sci. Res. 2018;29:213–227. doi: 10.21315/tlsr2018.29.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Service M.W. Mosquito (Diptera: Culicidae) dispersal—The long and short of it. J. Med. Entomol. 1997;34:579–588. doi: 10.1093/jmedent/34.6.579. [DOI] [PubMed] [Google Scholar]
- 31.Nyamah M.A., Sulaiman S., Omar B. Categorization of potential breeding sites of dengue vectors in Johor, Malaysia. Trop. Biomed. 2010;27:33–40. [PubMed] [Google Scholar]
- 32.Zainon N., Mohd-rahim F., Roslan D., Abd Samat A.H. Prevention of Aedes breeding habitats for urban high-rise building in Malaysia. Plan. Malays. J. 2016;14:115–128. [Google Scholar]
- 33.Costero A., Edman J.D., Clark G.G., Scott T.W. Life table study of Aedes aegypti (Diptera: Culicidae) in Puerto Rico fed only human blood versus blood plus sugar. J. Med. Entomol. 1998;35:809–813. doi: 10.1093/jmedent/35.5.809. [DOI] [PubMed] [Google Scholar]
- 34.Dye C. Vectorial capacity: Must we measure all its components? Parasitol. Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- 35.Harrington L.C., Edman J.D., Scott T.W. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann E.J., Miller J.R. Reassessment of the role and utility of wind in suppression of mosquito (Diptera: Culicidae) host finding: Stimulus dilution supported over flight limitation. J. Med. Entomol. 2003;40:607–614. doi: 10.1603/0022-2585-40.5.607. [DOI] [PubMed] [Google Scholar]
- 37.Hales S., de Wet N., Maindonald J., Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.