Abstract
Ganoderma lucidum or Reishi is recognized as the most potent adaptogen present in nature, and its anti-inflammatory, antioxidant, immunomodulatory and anticancer activities are well known. Moreover, lately, there has been an increasing interest from pharmaceutical companies in antiaging G. lucidum-extract-based formulations. Nevertheless, the pharmacological mechanisms of such adaptogenic and regenerative actions remain unclear. The present investigation aimed to explore its molecular and cellular effects in vitro in epidermal keratinocyte cultures by applying liquid chromatography coupled to ion trap time-of-flight mass spectrometry (LCMS-IT-TOF) for analysis of ethanol extracts using ganoderic acid-A as a reference compound. The G. lucidum extract showed a keratinocyte proliferation induction accompanied by an increase of cyclic kinase protein expressions, such as CDK2 and CDK6. Furthermore, a noteworthy migration rate increase and activation of tissue remodelling factors, such as matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9), were observed. Finally, the extract showed an antioxidant effect, protecting from H2O2-induced cytotoxicity; preventing activation of AKT (protein kinase B), ERK (extracellular signal-regulated kinase), p53 and p21; and reducing the number of apoptotic cells. Our study paves the path for elucidating pharmacological properties of G. lucidum and its potential development as cosmeceutical skin products, providing the first evidence of its capability to accelerate the healing processes enhancing re-epithelialization and to protect cells from free-radical action.
Keywords: Ganoderma lucidum, wound healing, triterpenic acids, oxidative stress, cosmeceuticals
1. Introduction
Ganoderma lucidum (Ling Zhi (in China) or Reishi (in Japan)), also known as “the fungus of immortality”, is recognized among most important traditional medicinal mushrooms and most powerful adaptogens present in nature, since it acts as a regulator of biological functions [1,2,3]. The pharmacological properties of G. lucidum, such as anti-inflammatory, antioxidant, antiaging, immunomodulatory and antitumour activities [4,5,6,7], are due to its peculiar chemical composition in bioactive compounds such as polysaccharides, terpenoids, nucleotides, steroids, fatty acids, proteins and glycopeptides [8,9]. Based on that, G. lucidum was also reported to act as an adjuvant in the treatment of several diseases, i.e., anorexia, hypertension, insomnia and chronic hepatitis [10,11,12].
Among the terpenoid class, the most represented are triterpenes (ganoderic acids, ganoderoli acids, ganoderenics and lucid acids), which exhibit well-recognized anti-inflammatory, antioxidant, antitumour, anti-hepatitis, hypoglycaemic, antimalarial and antimicrobial activities [13,14,15,16,17,18,19].
Recently, bioactive extracts of G. lucidum have been having great success in the nutraceutical and cosmeceutical fields; indeed, its triterpenic acids are often found in cosmetics formulations [20]. In this respect, the G. lucidum extracts can be used to control hyperpigmentation as photoprotective agents, to suppress inflammatory skin diseases, to mitigate lipid metabolic disorders and to balance gut microbiota composition [21,22,23]. Nevertheless, the mechanism of action and the biological targets behind their beneficial dermatological effects are still unclear. Thus, a deeper understanding of the biology of the molecular interactions might be acquired.
Therefore, in this work, we aimed first to assess the chemical composition of triterpenic acids in the fruiting body of the fungus, highlighting its potential as a valuable source of bioactive compounds, and then to provide any further pharmacological evidence of how the ethanol extract of G. lucidum is able to boost the wound healing process and to prevent premature skin aging, lessening free-radical action. These findings paved the path for developing highly effective G. lucidum-based cosmeceuticals.
2. Results
2.1. Extraction and Characterization of G. lucidum Pericarp
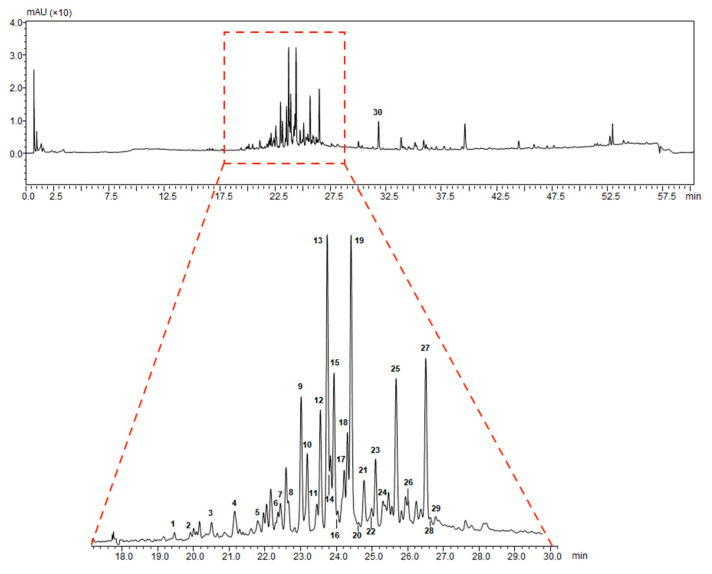
In the attempt to maximize ganoderic acid extraction, we performed an ethanol procedure operation as described in the Materials and Methods section. The ethanol extracts of G. lucidum were analysed by UHPLC-ESI-IT-TOF (Ultra-High Performance Liquid Chromatography-Electrospray ionization-Ion Trap-Time of flight) for quantification of triterpenes in G. lucidum ethanol extracts. In Figure 1, we showed the chromatographic profile acquired by UHPLC couplet to Photodiode Array Detector (PDA) (λ = 254 nm) of the G. lucidum extract.
Figure 1.
UHPLC-PDA (λ = 254 nm) chromatographic profile of the G. lucidum extract.
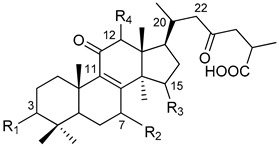
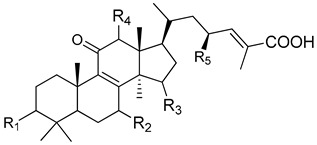
In Table 1, we showed the chemical structures of the identified triterpenoids. In Table 2, identification and quantification of triterpenes in the G. lucidum ethanol extract were reported.
Table 1.
Chemical structures of the triterpenoids identified in the G. lucidum extract.
| Peak | Retention Time (min) | Molecular Formula | [M − H]− Observed |
[M − H]− Calculated |
Error (ppm) | MS2 m/z | Tentative Identification |
|---|---|---|---|---|---|---|---|
| 1 | 19.49 | C30H46O8 | 533.3106 | 533.3120 | −2.63 | 515.3096 | 12-hydroxyganoderic acid C2 |
| 2 | 19.94 | C30H42O8 | 529.2807 | 529.2807 | 0.00 | 511.2468 | 20-hydroxyganoderic acid AM1 |
| 3 | 20.55 | C30H42O8 | 529.2843 | 529.2807 | 4.89 | 511.2698 | 12-deacetylganoderic acid H |
| 4 | 21.19 | C30H44O8 | 531.2962 | 531.2963 | −0.19 | 513.2930 | Ganoderic acid η |
| 5 | 21.75 | C30H42O8 | 529.2774 | 529.2807 | −6.23 | 511.2707; 467.2884 |
12-hydroxyganoderic acid D |
| 6 | 22.42 | C30H46O7 | 517.3189 | 517.3171 | 3.48 | 499.3131 | Ganoderic acid C2 |
| 7 | 22.49 | C30H46O8 | 529.2801 | 529.2807 | −1.13 | 511.2640; 467.2661 |
Ganoderic acid C6 |
| 8 | 22.78 | C27H40O6 | 459.2761 | 459.2752 | 1.96 | 441.2709 | Lucidenic acid N |
| 9 | 23.07 | C30H44O8 | 531.2991 | 531.2963 | 5.27 | 513.2849; 469.2961 |
Ganoderic acid G |
| 10 | 23.27 | C30H42O7 | 513.2864 | 513.2858 | 1.17 | 495.2737 | Ganoderenic acid B |
| 11 | 23.52 | C30H44O7 | 515.2979 | 515.3014 | −6.79 | 497.2841 | Ganoderic acid B |
| 12 | 23.61 | C29H40O8 | 515.2651 | 515.2650 | 0.19 | 473.2539 | Lucidenic acid E |
| 13 | 23.79 | C32H44O9 | 571.2936 | 571.2913 | 4.03 | 553.2818 | Ganoderenic acid K |
| 14 | 23.85 | C30H42O7 | 513.2847 | 513.2858 | −2.14 | 495.2708 | Ganoderic acid AM1 |
| 15 | 23.95 | C32H46O9 | 573.3067 | 573.3069 | −0.35 | 555.2977 | Ganoderic acid K |
| 16 | 24.09 | C30H42O8 | 529.2825 | 529.2807 | 3.40 | 511.2753 | Ganoderic acid derivative |
| 17 | 24.25 | C32H42O9 | 569.2750 | 569.2756 | −1.05 | 551.2746 | Ganoderic acid F |
| 18 | 24.32 | C30H44O7 | 515.3061 | 515.3014 | 4.12 | 497.2940 | Ganoderic acid A |
| 19 | 24.49 | C32H44O9 | 571.2909 | 571.2913 | −0.70 | 553.2850 | Ganoderic acid H |
| 20 | 24.68 | C30H40O8 | 527.2648 | 527.2650 | −0.38 | 509.2487 | Elfvingic acid A |
| 21 | 24.81 | C27H38O6 | 457.2620 | 457.2596 | 5.25 | 439.2390 | Lucidenic acid A |
| 22 | 24.89 | C30H44O6 | 499.3001 | 499.3065 | −6.40 | 481.3798 | Ganolucidic acid A |
| 23 | 25.17 | C30H40O7 | 511.2717 | 511.2701 | 3.13 | 493.2400 | Ganoderenic acid D |
| 24 | 25.31 | C27H36O6 | 455.2449 | 455.2439 | 2.20 | 380.2072; 301.1813 |
Lucidenic acid F |
| 25 | 25.72 | C29H38O8 | 513.2508 | 513.2494 | 2.73 | 471.2400 | Lucidenic acid D |
| 26 | 26.04 | C34H46O10 | 613.2997 | 613.3018 | −3.42 | 595.2916; 553.2831 |
3-acetylganoderic acid H |
| 27 | 26.58 | C32H42O9 | 569.2736 | 569.2697 | 3.85 | 551.2649 | 12-acetoxyganoderic acid F |
| 28 | 26.81 | C30H42O7 | 513.2859 | 513.2858 | 0.19 | 451.2844 | Ganoderic acid J |
| 29 | 28.20 | C30H44O6 | 499.3089 | 499.3065 | 4.81 | 437.2981 | Ganolucidic acid D |
| 30 | 31.98 | C30H44O5 | 483.3122 | 483.3116 | 1.24 | 439.3309; 409.2717 |
Ganolucidic acid E |
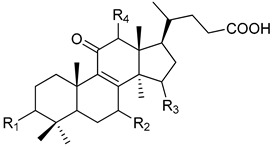
Table 2.
Identification of triterpenes in the G. lucidum ethanol extract.
|
|
|
||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| Peak | Compound Name | Type | R1 | R2 | R3 | R4 | R5 | Double Bond |
| 1 | 12-hydroxyganoderic acid C2 | A | β-OH | β-OH | α-OH | OH | - | |
| 4 | Ganoderic acid η | C | β-OH | β-OH | =O | β-OH | β-OH | |
| 5 | 12-hydroxyganoderic acid D | A | =O | β-OH | =O | OH | - | |
| 6 | Ganoderic acid C2 | A | β-OH | β-OH | α-OH | H | - | |
| 7 | Ganoderic acid C6 | A | β-OH | =O | =O | β-OH | - | |
| 8 | Lucidenic acid N | B | β-OH | β-OH | =O | H | - | |
| 9 | Ganoderic acid G | A | β-OH | β-OH | =O | β-OH | - | |
| 10 | Ganoderenic acid B | A | β-OH | β-OH | =O | H | - | Δ20,22 |
| 11 | Ganoderic acid B | A | β-OH | β-OH | =O | H | - | |
| 12 | Lucidenic acid E | B | β-OH | =O | =O | β-OAc | - | |
| 13 | Ganoderenic acid K | A | β-OH | β-OH | =O | β-OAc | - | Δ20,22 |
| 14 | Ganoderic acid AM1 | A | β-OH | =O | =O | H | - | |
| 15 | Ganoderic acid K | A | β-OH | β-OH | =O | β-OAc | - | |
| 17 | Ganoderic acid F | A | =O | =O | =O | H | - | |
| 18 | Ganoderic acid A | A | =O | β-OH | α-OH | H | - | |
| 19 | Ganoderic acid H | A | β-OH | =O | =O | β-OAc | - | |
| 20 | Elfvingic acid A | A | =O | =O | β-OH | α-OH | - | Δ20,22 |
| 21 | Lucidenic acid A | B | =O | β-OH | =O | H | - | |
| 22 | Ganolucidic acid A | A | =O | H | α-OH | H | - | |
| 23 | Ganoderenic acid D | A | =O | β-OH | =O | H | - | Δ20,22 |
| 24 | Lucidenic acid F | B | =O | =O | =O | H | - | |
| 25 | Lucidenic acid D | B | =O | =O | =O | β-OAc | - | |
| 26 | 3-acetylganoderic acid H | A | β-OAc | =O | =O | β-OAc | - | |
| 27 | 12-acetoxyganoderic acid F | A | =O | =O | =O | β-OAc | - | |
| 28 | Ganoderic acid J | A | =O | =O | α-OH | H | - | |
| 29 | Ganolucidic acid D | C | =O | H | α-OH | H | β-OH | |
| 30 | Ganolucidic acid E | C | =O | H | α-OH | H | H | |
2.2. Evaluation of G. lucidum Extract Effect in HaCaT Cells
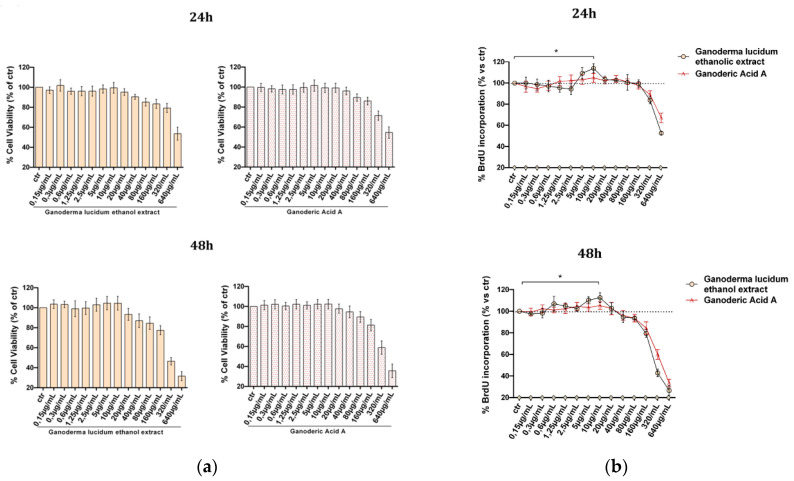
First, we evaluated the effect of the extract on human keratinocytes. HaCaT cells were cultured with increasing concentrations of the ethanol extract and standard ganoderic acid A (0–640 µg mL−1) as a control for 24 and 48 h. As reported in Figure 2, only at the highest doses did the extract show a negative effect either on cell viability evaluated by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Figure 2a) or on cell proliferation (BrdU incorporation, Figure 2b), in both cases comparable to the pure ganoderic acid A profile.
Figure 2.
Evaluation of the G. lucidum extract effect in HaCaT cells: HaCaT cells were cultured for 24 or 48 h in the presence of the indicated concentrations (0–640 µg mL−1) of the G. lucidum extract or ganoderic acid A before MTT assay (a) or BrdU incorporation (b). The results are expressed as means ± SD of independent experiments performed in triplicate and str reported as percentage vs. the untreated control (ANOVA, * p < 0.05 vs. control).
Interestingly, we identified an interval of concentrations (5 and 10 µg mL−1) where the G. lucidum extract induced an increase of DNA synthesis, hence stimulating cell proliferation both at 24 h and 48 h (p ≤ 0.05).
2.3. Improvement of the Migratory Capacity of Human Keratinocytes Exposed to G. lucidum Extract
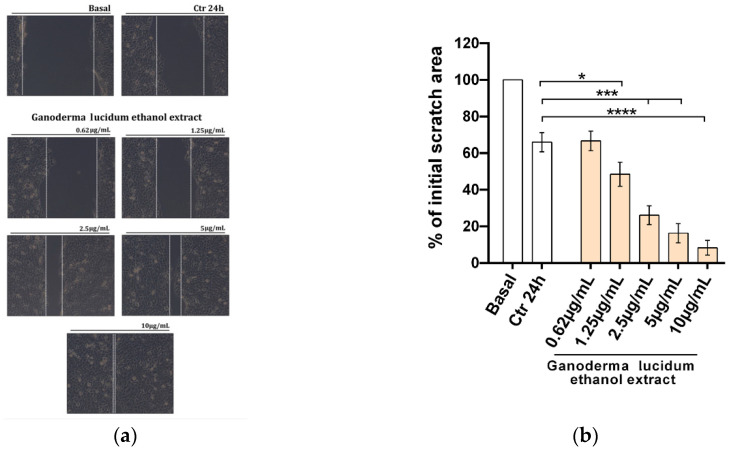
In order to assess the potential effect of G. lucidum ethanol extract on the migratory function of HaCaT cells, we performed a scratch wound assay (Figure 3) treatment for 24 h with vehicle (CTR, control) or G. lucidum ethanol extracts at five increasing concentrations (0.62–10 µg mL−1) in complete medium (Figure 3a).
Figure 3.
Improvement of the migratory capacity of human keratinocytes exposed to the G. lucidum extract: (a) wound healing assay performed in HaCaT cells treated for 24 h with vehicle (CTR) or G. lucidum extracts at the indicated concentrations (0.62–10 µg mL−1) in complete medium. Representative light microscope images from three independent experiments are shown. Dotted white lines indicate the wounded area from the initial scratch. Magnification is at ×20. (b) Histograms represent the mean scratch area observed in HaCaT cells expressed as a percent of the initial area. The measurement was made in three different experiments. The results are presented as mean ± standard error (ANOVA, * p < 0.05, *** p < 0.001 and **** p < 0.0001 vs. control).
After 24 h of cell culture, in the presence of the G. lucidum extract, we observed an enhancement of wound healing at all doses tested from 0.62 to 10 µg mL−1, with 10 µg mL−1 being the most effective dose (****p < 0.0001), as shown in light microscope images from three independent experiments (Figure 3a) and in a histogram representation of the mean scratch area (Figure 3b).
2.4. G. lucidum Ethanol Extract Induces Expression of Proteins Linked to the Control of Cell Cycle and Migration
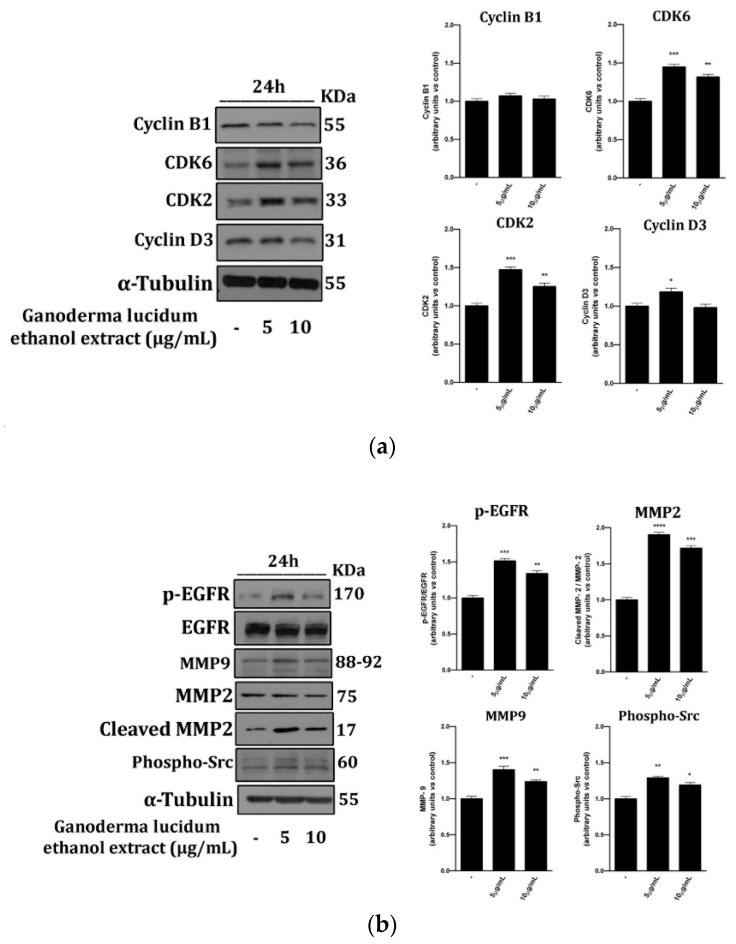
In order to investigate the molecular pathways tuned by G. lucidum extracts in the previous experiments, we determined by Western blot analysis the status of the same proteins involved in both cell cycle progression and cell migration (Figure 4).
Figure 4.
G. lucidum ethanol extracts induced cell cycle progression and migration protein expression. (a) Western blot analysis of cyclin B1, CDK6, CDK2 and cyclin D3 in whole cell extracts from HaCaT cells cultured for 24 h in the presence of the indicated concentrations of G. lucidum ethanol extract: Tubulin was used as a control for protein loading. The panel shows a representative Western blot of three different experiments performed with similar results. Histograms represent mean ± SD in densitometry units of scanned immunoblots from the 3 different experiments (ANOVA, *** p < 0.001, ** p < 0.01 and * p < 0.05). (b) Western blot analysis of p-EGFR (epidermal growth factor receptor), EGFR, MMP-2 (total and cleaved), MMP-9 and Phospho-Src in whole cell extracts from HaCaT cells cultured for 24 h in the presence of the indicated concentrations of G. lucidum ethanol extract: Tubulin was used as a control for protein loading. The panel shows a representative Western blot of three different experiments performed with similar results. Histograms represent mean ± SD in densitometry units of scanned immunoblots from the 3 different experiments (ANOVA, **** p < 0.0001, *** p < 0.001, ** p < 0.01 and * p < 0.05).
To this end, we treated cells with the G. lucidum ethanol extract at the most effective doses, 5 and 10 µg mL−1 for 24 h. According to the wound healing results, we observed that the G. lucidum ethanol extract increased the expression of cell cycle regulation proteins such as cyclin D3, CDK2 and CDK6 (Figure 4a). Besides, we showed that G. lucidum extracts induced MMPs, such as MMP2 (total and cleaved) and MMP9 expression (Figure 4b), and then subsequently triggered the EGRF signalling cascade. Activation of the downstream EGFR pathway, inducing phosphorylation of Src (Figure 4b), suggested that exposition to G. lucidum extracts provided a driving force in human keratinocyte migration.
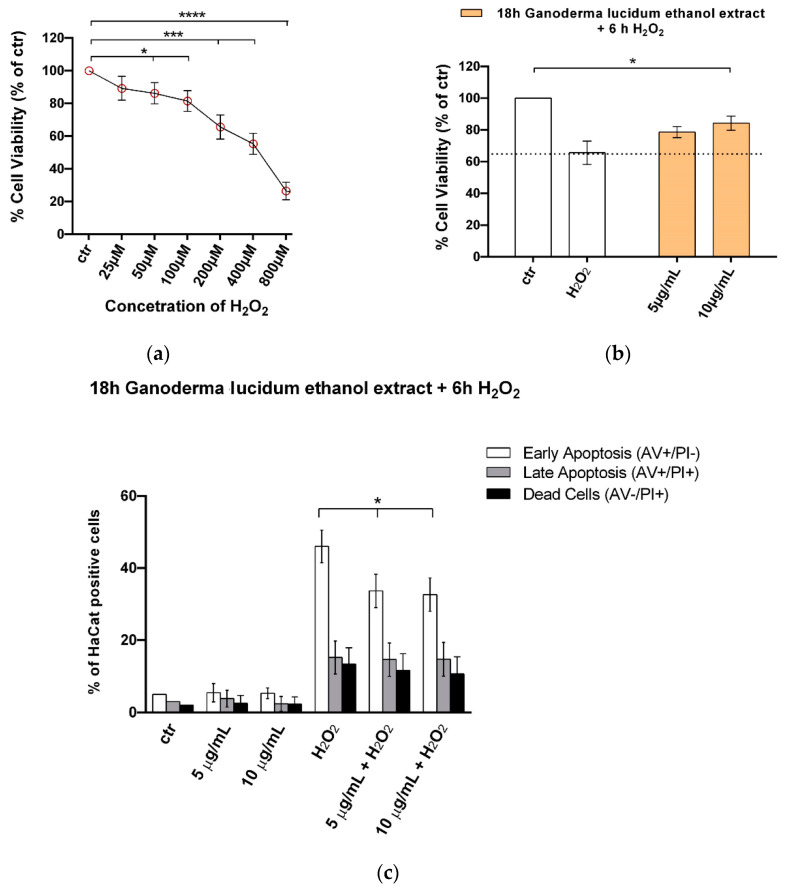
2.5. G. lucidum Ethanol Extract Ameliorates Cytotoxicity and Apoptosis Induced by H2O2 in HaCaT Cells
HaCaT cells were exposed to H2O2 (0–800 µM) for 6 h, and the MTT assay was used as an indicator of cell viability (Figure 5). H2O2 induced cytotoxicity in a dose-dependent manner. The decrease in cell viability was statistically significant at 50 µM H2O2, whereas cell viability was reduced to 35.4% at 200 µM H2O2 (Figure 5a). Scientific evidence shows that relatively low concentrations of H2O2 caused apoptotic death of more cells (maximal at 250 µM), whereas 1000 µM H2O2 resulted in a reduction in apoptosis but an increase in overall cell death [24]. Therefore, in our system, we used 200 µM H2O2 in the subsequent experiments. We observed that at 18 h pretreatment of keratinocytes with the G. lucidum ethanol extract at 5 and 10 µg mL−1 protected the cells from H2O2-induced cytotoxicity. Cell viability declined to 35.4% ± 7.3% after exposure to 200 µM H2O2 for 6 h, whereas it increased to 78.6% ± 3.5% and 84.2% ± 4.5% with the G. lucidum extract at 5 and 10 µg mL−1 doses, respectively (Figure 5b). In order to strength the data obtained with the MTT assay, we performed a cell death analysis by annexin-V and propidium iodide double staining. As shown in Figure 5c, G. lucidum pretreatment before H2O2 exposure resulted in a significant reduction of apoptosis and particularly of early apoptosis.
Figure 5.
G. lucidum ethanol extract ameliorated cytotoxicity and apoptosis H2O2 induced in HaCaT cells: (a) HaCaT cells were cultured for 6 h in the presence of the indicated concentrations of H2O2 (25–800 µM) before MTT assay. The results are expressed as means ± SD of independent experiments performed in triplicate and are reported as percentage vs. the untreated control (ANOVA, * p < 0.05,*** p < 0.001 and **** p < 0.0001 vs. control). (b) HaCaT cells were cultured for 18 h in the presence of the indicated concentrations (0, 5 and 10 µg mL−1) of the G. lucidum ethanol extract before treatment with H2O2 for 6 h. The results are expressed as means ± SD of independent experiments performed in triplicate and are reported as percentage vs. the untreated control (ANOVA, * p < 0.05 vs. control). (c) Flow cytometric analysis of annexin V and propidium iodide (PI) double staining in the G. lucidum ethanol extract-treated HaCaT cells after 18 h and H2O2 for 6 h: histograms indicate the total percentage of early (annexin V-positive cells/PI-negative cells) and late apoptotic events (annexin V/PI-double positive cells) as well as necrotic cells (annexin V-negative cells/PI-positive cells). The results are representative of four independent experiments performed in duplicate and are expressed as mean ± SD (ANOVA, * p < 0.05).
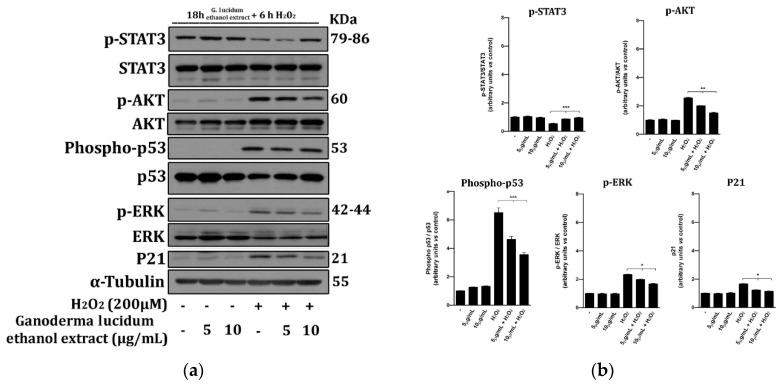
2.6. G. lucidum Extract Prevents the Activation of Cell Death Molecular Pathways
AKT, ERK, p53 and p21 are critical proteins involved in the control of cell response to external damages, i.e., those induced by free radicals, and are involved in apoptosis activation [25,26]. Aiming to elucidate cell death molecular pathways modulated by G. lucidum extract, we performed Western blot analysis of STAT3 (Signal transducer and activator of transcription 3), AKT, p53, ERK (total and phosphorylated) and p21 in whole-cell extracts from HaCaT cells (Figure 6) using hydrogen peroxide to mimic oxidative stress-induced injury (OSI) within a short period [27].
Figure 6.
Ganonderic acid extract prevented the activation of cell death molecular pathways. (a) Western blot analysis of STAT3, AKT, p53, ERK (total and phosphorylated) and p21 in whole cell extracts from HaCaT cells cultured for 18 h in the presence of the indicated concentrations of the G. lucidum ethanol extract and H2O2 for 6 h: Tubulin was used as a control for protein loading. The panel shows a representative Western blot of three different experiments performed with similar results. (b) Histograms represent mean ± SD in densitometry units of scanned immunoblots from the 3 different experiments (ANOVA, *** p < 0.001, ** p < 0.01 and * p < 0.05).
The phosphorylation status of the abovementioned and p21 proteins in 18 h pretreated cells with ganoderic extract at 5 and 10 µg mL−1 doses, H2O2 or their combination was evaluated using tubulin as a control for protein loading (Figure 6).
We observed that H2O2 treatment increased the levels of p-AKT, p-ERK, phospho-p53 and p21 whereas G. lucidum extract treatment partially reversed these effects significantly, preventing both activation and shutdown of STAT3 signalling, critical for cell survival [28] (Figure 6a,b).
3. Discussion
Bioactive extracts from G. lucidum only recently have been reported to present remarkable in vitro and in vivo pharmacological properties beneficial also to the development of cosmeceutical formulations [21]. However, despite the commercial success, the pharmacological efficacy, especially in the field of cosmetic dermatology, still needs more in-depth scientific support. Lately, an in vitro analysis of the G. lucidum ethanolic extract as a dermatological ingredient was carried out, showing its suitability for skincare formulations as the absence of toxicity in keratinocytes and fibroblasts [28,29].
In this context, the present study reported the effect of G. lucidum on human keratinocytes as an in vitro skin model for evaluation of its dermatological properties which can be transferred to the cosmetics field for cosmetic use or to the therapeutic field for possible medical applications.
The ethanol extraction of G. lucidum was selected and listed as the most robust and suitable extraction method for this class of natural compounds [30,31], and detailed identification, characterization of chemical structures and quantification of triterpenes by UHPLC-ESI-IT-TOF analysis were performed for a detailed molecular description. In vitro studies on cultures of human keratinocytes were conducted by comparing G. ludicum extract activity with ganoderic acid A, the main G. lucidum described bioactive compound and mostly present in skin products available on the market [9,32,33]. Once the lack of cytotoxicity by G. lucidum ethanol extract was confirmed, we identified an interval of concentrations (5 and 10 µg mL−1) for the induction of cellular DNA synthesis, hence stimulating cell proliferation only for the G. lucidum ethanol extract, with respect to gandoreic acid A. This activity, observed only for the G. lucidum ethanol extract, might be ascribed to other bioactive ganoderic acids present in lower quantities or also might be attributable to a synergistic effect of various active components present in the ethanol extract.
Importantly, additional lines of evidence of this proliferation induction by G. lucidum ethanol extract treatment indicate an increase of the cyclin-dependent kinase protein expressions, mainly CDK2 and CDK6 (Figure 4a), key components of the cell cycle machinery for G1 to S transition.
To evaluate if G. lucidum ethanol extract treatment could be helpful for skin care, from minor, superficial and basic skin injuries to more complicated pathological states such as ulcers or bedsores, pathologies which, in addition to cell proliferation, also required suitable cell migration [34], we evaluated whether the extract treatment influenced the expression and activity of proteins involved in cell migration. Furthermore, we reported an increase of the migration rate supported by activation of the matrix metalloproteinases (MMPs), specifically MMP-2 and MMP-9; the pathways downstream EGFR stimulation; and phospho-Src (Figure 4b), important factors for stimulation of cell migration and normal tissue remodelling [35,36]. These results were confirmed by the functional wound healing assay at all doses tested from 0.62 to 10 µg mL−1, with 10 µg mL−1 being the most effective dose (Figure 3a,b).
Since oxidative stress plays a crucial role in several diseases pathogenesis, including allergic and inflammatory skin diseases like atopic dermatitis, urticaria and psoriasis [37], and in skin aging and since regulation of reactive oxygen species (ROS) levels is essential for maintenance of healthy skin homeostasis [38], we investigated whether the G. lucidum extract was able to protect cells from H2O2-induced cytotoxicity. In our system, 6 h exposure of 200 µM H2O2 reduced more than 35% of cell viability; thus, this concentration was used in subsequent experiments. The G. lucidum ethanol extract at 5 and 10 µg mL−1 doses reverted this trend, reducing the oxidative stress-induced injury to 15% (Figure 5b). These data were corroborated by annexin-V and propidium iodide double staining cell death analysis, which showed that G. lucidum pretreatment before H2O2 exposure resulted in a significant reduction of apoptosis, particularly of early apoptosis (Figure 5c). To elucidate cell death molecular pathways modulated by G. lucidum extract, we performed Western blot analysis of STAT3, AKT, p53, ERK (total and phosphorylated) and p21 in whole-cell extracts from HaCaT cells pretreated for 18 h with the ganoderic extract (Figure 6) using H2O2 to mimic OSI within a short time period. We observed that H2O2 treatment increased the levels of p-AKT, p-ERK, phospho-p53 and p21.
Our study confirmed that pretreatment with G. lucidum extracts partially reversed these effects, significantly preventing both activation and shutdown of STAT3 signalling involved in cell damage and apoptosis activation (Figure 6a,b). These data suggested that ERK signalling plays a critical role in the induction of survival, migration and proliferation in G. lucidum extract-treated cells and that protective effects against OSI, at least partially, depended upon STAT3 inhibition.
Our results, confirming what is already reported in scientific literature for the antioxidant activity of the G. lucidum extract [39] in addition to its anticancer, antimicrobial and anti-inflammatory activities [39,40], provide the first evidence of an increase in cell migration and an accelerated healing process and, at the same time, show the proteins involved and the possible molecular mechanisms in these activities.
4. Materials and Methods
4.1. Chemicals and Materials
The fruiting bodies of G. lucidum were provided by Indena S.p.A. (Viale Ortles 12, 20139 Milan, Italy) as dry material. Ganoderic acid A was purchased from Sigma-Aldrich Inc. (St. Luis, MO, USA). The G. lucidum extract was solubilized in dimethyl sulfoxide (DMSO) (0.01% in our assays) and added to cell cultures at the reported concentrations. H2O2 was purchased from Sigma-Aldrich (Milan, Italy). For Western blot analysis, the following antibodies were used: mouse monoclonal antihuman α-Tubulin, rabbit polyclonal antihuman phospho-STAT3 (p-STAT3; Tyr705), rabbit monoclonal antihuman STAT3, rabbit monoclonal antihuman phospho-p44/42 MAPK (p-Erk1/2; Thr202/Tyr204), rabbit monoclonal antihuman p44/42 MAPK (Erk1/2), rabbit monoclonal antihuman phospho-Akt (p-Akt; Ser473), rabbit monoclonal antihuman Akt, rabbit polyclonal p53, rabbit polyclonal antibody to phosphorylated p53, rabbit monoclonal antihuman p21, mouse monoclonal antihuman CDK6, rabbit monoclonal antihuman CDK2, mouse monoclonal antihuman cyclin D3, rabbit monoclonal antihuman phospho-EGFR (p-EGFR; Tyr1068) and rabbit monoclonal antihuman EGF receptor were purchased from Cell Signaling Technology (Danvers, MA, USA). Mouse monoclonal MMP2, mouse monoclonal antihuman MMP9, rabbit polyclonal antihuman Src (phospho Y418) and rabbit monoclonal antihuman cyclin B1 were purchased from Abcam (Cambridge, UK). Secondary HRP (Horseradish Peroxidase)-linked goat anti-mouse or goat anti-rabbit IgG were also purchased from Cell Signaling Technology (Danvers, MA, USA).
4.2. Sample Preparation
The dried powder of G. lucidum (3 g) was extracted with 100 mL of ethanol by refluxing in a Soxhlet apparatus for 6 h, and the solvent was evaporated under reduced pressure. The dried ethanol residue was then extracted with ethyl acetate three times. Finally, the ethyl acetate fractions were combined, filtered, evaporated and lyophilized for 24 h (LyoQuest-55, Telstar Technologies, Terrassa, Spain), using condenser temperature at −52 °C and 0.100 mBar as vacuum value [30].
4.3. LCMS-IT-TOF Analysis of G. lucidum Extract
A Shimadzu Nexera UHPLC system consisting of a SIL-30AC autosampler, a CBM-20A controller, a DGU-20 AR5 degasser, two LC-30AD pumps, a CTO-20AC column oven and an SPD-M20A photodiode array detector was used for UHPLC-ESI-IT-TOF analyses. The UHPLC system was coupled online to an LCMS–IT-TOF mass spectrometer through an ESI source (Shimadzu, Kyoto, Japan). LC-MS data elaboration was performed by the LCMSsolution® software (Version 3.50.346, Shimadzu).
LC-MS analysis of the G. lucidum extract was carried out on Kinetex® C18 150 × 2.1 mm (100 Å), packed with 2.6 μm core-shell particles column (Phenomenex, Bologna, Italy). The injection volume was 2 µL, and the flow rate was 0.5 mL min−1. The temperature of the column oven was set to 40 °C. The following PDA parameters were applied: sampling rate, 12.5 Hz; detector time constant, 0.240 s; and cell temperature, 40 °C. Data acquisition was set in the range 190–400 nm, and chromatograms were monitored at 254 nm at maximum absorbance of the compounds of interest. The mobile phase consisted of H2O (A) and ACN (B), both acidified by formic acid 0.1% v/v. The analysis was performed in gradient elution as follows: 0.01–5.00 min, isocratic to 1% B; 5.01–53.00 min, 1–95% B; 53.01–56.00 min, isocratic to 95% B; then four minutes for column re-equilibration.
Negative ionization mode was used for MS detection operating with the following parameters: detector voltage, 1.65 kV; CDL (Curved Desolvation Line) temperature, 250 °C; block heater temperature, 250 °C; nebulizing gas flow (N2), 1.5 L/min; and drying gas pressure, 100 kPa. Full scan MS data were acquired in the range of 150–1600 m/z (ion accumulation time, 30 ms; IT (Ion trap) repeat = 2). MS/MS experiments were conducted in the data-dependent acquisition, and precursor ions were acquired in the range 100–1200 m/z; ion accumulation time, 60 ms; CID (Collision Induced Dissociation) energy, 50%; collision gas, 50%; repeat = 1; execution trigger (BPC Base Peak Chromatogram) intensity, at the 70% stop level.
Identification was carried out based on standard retention time and UV spectra and by comparing MS/MS data with those present in the literature [30]. Molecular formulas were calculated by the Formula Predictor software (Version 1.12, Shimadzu), setting a low tolerance so that most of the identified compounds were in position 1 in the list of possible candidates.
4.4. Quantitative Analysis
As an external standard, we chose ganoderic acid A for quantification of triterpenes in the G. lucidum ethanol extract. The stock solution (1 mg mL−1) was prepared in ethanol, the calibration curve was obtained in a concentration range of 200–0.5 µg mL−1 with seven concentration levels (200, 50, 25, 10, 5, 1 and 0.5 µg mL−1) and triplicate injections of each level were run. Peak areas of ganoderic acid A were plotted against corresponding concentrations (µg mL−1). The amount of compounds in the sample was expressed as milligram per gram of dried extract, and linear regression was used to generate calibration curve with r2 values ≥ 0.9999. Limits of detection (LOD) and quantification (LOQ) were calculated by the ratio between the standard deviation (SD) and the analytical curve slope multiplied by 3.3 and 10, respectively.
4.5. Cells
Human immortalized keratinocytes (HaCaT) were grown in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, Grand Island, NY, USA) and supplemented as described in detail elsewhere [41]. HaCaT cells were kindly provided by Giuseppe Monfrecola (Department of Experimental Dermatology, University of Naples, Naples, Italy).
All cell cultures were maintained at 37 °C in a humidified 5% CO2 atmosphere.
4.6. Determination of Cells Viability, MTT Assay
HaCaT cells (6 × 103/well) were cultured for 24 h into 96-well plates before the addition of the individual substances at the indicated concentrations and were cultured for an additional 24–48 h at 37 °C. The reduction of the MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) tetrazolium salts assay was employed to examine cells’ viability, as described in detail elsewhere [42]. All experiments were performed in triplicate, and the relative cell viability was expressed as a percentage in comparison with the untreated control cells.
4.7. Determination of Cells Proliferation, BrdU Assay
HaCaT cells (6 × 103/well) were cultured for 24 h into 96-well plates before the addition of the G. lucidum ethanol extract or ganoderic acid-A at the indicated concentrations and were cultured for an additional 24–48 h at 37 °C. Cell proliferation was evaluated by measuring BrdU incorporation into DNA (BrdU colorimetric assay kit; Roche Applied Science, South San Francisco, CA, USA) and was determined by an ELISA plate reader (ThermoScientific, Waltham, MA, USA) at 450 nm as described in detail elsewhere [43]. All experiments were performed in triplicate, and the relative cell growth was expressed as percentage in comparison with the untreated control cells (100%).
4.8. Scratch Wound Healing Assay
To evaluate the effect of G. lucidum extracts on HaCaT cell migration, the cells were plated in 6-well plates at a density of 5 × 103 cells/well. When the confluent cells formed a homogeneous carpet and a vertical wound in the wells using a 200 µL tip was performed, culture medium containing G. lucidum extracts at the indicated concentrations or the vehicle alone was added to the wells, after the removal of detached cells. The wound area was recorded immediately and after 24 h through microscope analysis, as previously described [41].
4.9. Apoptosis Analysis
Quantitative assessment of apoptosis of HaCaT cells was analysed by antihuman annexin V (BioLegend, San Diego, CA, USA) using propidium iodide solution (PI) staining. Briefly, cells grown in 100-mm dishes for 24 h with G. lucidum extracts, H2O2 or combined as indicated were harvested with trypsin, washed in phosphate buffer saline (PBS) and subjected to apoptosis determination by the procedure described in detail elsewhere [43].
4.10. Western Blot Analysis
Cells were grown in p60 tissue culture plates at a density of 2 × 104 cells/cm2 for 24 h. Cells were then incubated with vehicle, G. lucidum extracts (for 24 h), H2O2 (for 6 h) or their combination (G. lucidum extracts for 18 h and H2O2 for an additional 6 h), as indicated. After incubation, cells were washed with PBS, harvested and lysed in ice-cold RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% Triton X-100, 0.5% deoxycholic acid, 10 mg mL−1 leupeptin, 2 mM phenylmethylsulfonyl fluoride and 10 mg mL−1 aprotinin) and then assayed for Western blot by the procedure, which is described in detail elsewhere [44].
4.11. Statistical Analysis
Statistical analysis was performed in all experiments shown by using the GraphPad Prism 6.0 software for Windows (GraphPad software). For each type of assay or phenotypic analysis, data obtained from multiple experiments are calculated as mean ± SD and analysed for statistical significance using the 2-tailed Student t-test for independent groups or using ANOVA followed by Bonferroni correction for multiple comparisons. p values less than 0.05 were considered significant. * p < 0.05, ** p < 0.01 and *** p < 0.001.
5. Conclusions
We provided the first scientific evidence that G. lucidum ethanol extract due to its high content in triterpenes remarkably increased cell migration patterns and accelerated the healing process, principally enhancing re-epithelialization and, at the same time, protecting the skin from the action of free radicals, paving the way for possible medical applications. Moreover, the prevention of skin aging leads to considering its formulations attractive as cosmeceuticals. Additional in vivo and clinical studies are requested to develop and validate novel nutraceuticals, cosmeceuticals and pharmacological formulations.
Author Contributions
M.A. and G.P. wrote the paper, designed and conducted the research, and analysed and interpreted data; M.G.B. and V.C. performed the research; R.R. and S.P. analysed data and reviewed the paper critically; A.M.D., M.B. and W.C. provided important suggestions including some reagents along with critical reading of the paper; S.P., M.B., P.C. and M.R. provided financial support to the project; P.C. and M.R. were fully responsible for the study design and supervised the project in its entirety. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded supported by “Nutraceutica e cosmeceutica come strumenti di tutela della salute dell’uomo”—CUP: B48I17000160008—COR: 652124—F/050127/03/X32; Fondo per la Crescita Sostenibile—Horizon 2020 PON “IMPRESE E COMPETITIVITA” 2014–2020 FESR; and Associazione Italiana Ricerca sul Cancro (AIRC; IG 13312, IG 18999, AIRC and Fondazione Cariplo TRIDEO 2015 No. 17216).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wachtel-Galor S., Yuen J., Buswell J.A., Benzie I.F.F. Ganoderma Lucidum (Lingzhi or Reishi) Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Taylor and Francis Group; Milton Park, UK: 2011. [PubMed] [Google Scholar]
- 2.Chu T.T., Benzie I.F., Lam C.W., Fok B.S., Lee K.K., Tomlinson B. Study of potential cardioprotective effects of Ganoderma lucidum (Lingzhi): Results of a controlled human intervention trial. Br. J. Nutr. 2012;107:1017–1027. doi: 10.1017/S0007114511003795. [DOI] [PubMed] [Google Scholar]
- 3.Yuen J.W., Gohel M.D. Anticancer effects of Ganoderma lucidum: A review of scientific evidence. Nutr. Cancer. 2005;53:11–17. doi: 10.1207/s15327914nc5301_2. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y.L., Han F., Luan S.S., Ai R., Zhang P., Li H., Chen L.X. Triterpenoids from Ganoderma lucidum and Their Potential Anti-inflammatory Effects. J. Agric. Food Chem. 2019;67:5147–5158. doi: 10.1021/acs.jafc.9b01195. [DOI] [PubMed] [Google Scholar]
- 5.Lu S.Y., Peng X.R., Dong J.R., Yan H., Kong Q.H., Shi Q.Q., Li D.S., Zhou L., Li Z.R., Qiu M.H. Aromatic constituents from Ganoderma lucidum and their neuroprotective and anti-inflammatory activities. Fitoterapia. 2019;134:58–64. doi: 10.1016/j.fitote.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Cuong V.T., Chen W., Shi J., Zhang M., Yang H., Wang N., Yang S., Li J., Yang P., Fei J. The anti-oxidation and anti-aging effects of Ganoderma lucidum in Caenorhabditis elegans. Exp. Gerontol. 2019;117:99–105. doi: 10.1016/j.exger.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Wang J., Cao B., Zhao H., Feng J. Emerging Roles of Ganoderma Lucidum in Anti-Aging. Aging Dis. 2017;8:691–707. doi: 10.14336/AD.2017.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop K.S., Kao C.H.J., Xu Y., Glucina M.P., Paterson R.R.M., Ferguson L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry. 2015;114:56–65. doi: 10.1016/j.phytochem.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Liang C., Tian D., Liu Y., Li H., Zhu J., Li M., Xin M., Xia J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019;174:130–141. doi: 10.1016/j.ejmech.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X.R., Zhang B.J., Deng S., Zhang H.L., Huang S.S., Huo X.K., Wang C., Liu F., Ma X.C. Isolation and identification of oxygenated lanostane-type triterpenoids from the fungus Ganoderma lucidum. Phytochem. Lett. 2016;16:87–91. doi: 10.1016/j.phytol.2016.03.007. [DOI] [Google Scholar]
- 11.Liu Z., Xing J., Zheng S., Bo R., Luo L., Huang Y., Niu Y., Li Z., Wang D., Hu Y., et al. Ganoderma lucidum polysaccharides encapsulated in liposome as an adjuvant to promote Th1-bias immune response. Carbohydr. Polym. 2016;142:141–148. doi: 10.1016/j.carbpol.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Baby S., Johnson A.J., Govindan B. Secondary metabolites from ganoderma. Phytochemistry. 2015;114:66–101. doi: 10.1016/j.phytochem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Wang H.H., Nie X.T., Jiang W.R., Zhang Y.S. Sodium butyrate ameliorates lipopolysaccharide-induced cow mammary epithelial cells from oxidative stress damage and apoptosis. J. Cell. Biochem. 2018;120:2370–2381. doi: 10.1002/jcb.27565. [DOI] [PubMed] [Google Scholar]
- 14.Kladar N.V., Gavarić N.S., Božin B.N. Ganoderma: Insights into anticancer effects. Eur. J. Cancer Prev. 2016;25:462–471. doi: 10.1097/CEJ.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 15.Li H., Lou B., Zhang Y., Zhang C. Ganoderic Acid A exerts the cytoprotection against hypoxia-triggered impairment in PC12 cells via elevating microRNA-153. Phytother. Res. 2020;34:640–648. doi: 10.1002/ptr.6556. [DOI] [PubMed] [Google Scholar]
- 16.Wong K.L., Chao H.H., Chan P., Chang L.P., Liu C.F. Antioxidant activity of Ganoderma lucidum in acute ethanol-induced heart toxicity. Phytother. Res. 2004;18:1024–1026. doi: 10.1002/ptr.1557. [DOI] [PubMed] [Google Scholar]
- 17.Ruan W., Wei Y., Popovich D.G. Distinct Responses of Cytotoxic Ganoderma lucidum Triterpenoids in Human Carcinoma Cells. Phytother. Res. 2015;29:1744–1752. doi: 10.1002/ptr.5426. [DOI] [PubMed] [Google Scholar]
- 18.Van Nguyen T., Tung N.T., Cuong T.D., Hung T.M., Kim J.A., Woo M.H., Choi J.S., Lee J.H., Min B.S. Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum. Phytochem. Lett. 2015;12:69–74. doi: 10.1016/j.phytol.2015.02.012. [DOI] [Google Scholar]
- 19.Stojkovic D.S., Barros L., Calhelha R.C., Glamoclija J., Ciric A., van Griensven L.J., Sokovic M., Ferreira I.C. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int. J. Food Sci. Nutr. 2014;65:42–47. doi: 10.3109/09637486.2013.832173. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y., Choi M.H., Li J., Yang H., Shin H.J. Mushroom cosmetics: The present and future. Cosmetics. 2016;3:22. doi: 10.3390/cosmetics3030022. [DOI] [Google Scholar]
- 21.Guo W.L., Pan Y.Y., Li L., Li T.T., Liu B., Lv X.C. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. 2018;9:3419–3431. doi: 10.1039/C8FO00836A. [DOI] [PubMed] [Google Scholar]
- 22.Taofiq O., Gonzalez-Paramas A.M., Martins A., Barreiro M.F., Ferreira I.C. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016;90:38–48. doi: 10.1016/j.indcrop.2016.06.012. [DOI] [Google Scholar]
- 23.Taofiq O., Martins A., Barreiro M.F., Ferreira I.C. Mushrooms Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016;50:193–210. doi: 10.1016/j.tifs.2016.02.005. [DOI] [Google Scholar]
- 24.Turner N.A., Xia F., Azhar G., Zhang X., Liu L., Wei J.Y. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. J. Mol. Cell. Cardiol. 1998;30:1789–1801. doi: 10.1006/jmcc.1998.0743. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Qi C., Liu L., Zhao L., Cui W., Tian Y., Liu B., Li J. Taurine Protects Primary Neonatal Cardiomyocytes Against Apoptosis Induced by Hydrogen Peroxide. Int. Heart J. 2018;59:190–196. doi: 10.1536/ihj.16-372. [DOI] [PubMed] [Google Scholar]
- 26.Jin G.F., Hurst J.S., Godley B.F. Hydrogen peroxide stimulates apoptosis in cultured human retinal pigment epithelial cells. Curr. Eye Res. 2001;22:165–173. doi: 10.1076/ceyr.22.3.165.5517. [DOI] [PubMed] [Google Scholar]
- 27.Duan W., Yang Y., Yi W., Yan J., Liang Z., Wang N., Li Y., Chen W., Yu S., Jin Z., et al. New Role of JAK2/STAT3 Signaling in Endothelial Cell Oxidative Stress Injury and Protective Effect of Melatonin. PLoS ONE. 2013;8:e57941. doi: 10.1371/journal.pone.0057941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Wang D., Xu J., Wang Y., Ma F., Li Z., Liu N. Stat3 activation is critical for pluripotency maintenance. J. Cell. Physiol. 2018;234:1044–1051. doi: 10.1002/jcp.27241. [DOI] [PubMed] [Google Scholar]
- 29.Taofiq O., Rodrigues F., Barros L., Barreiro M.F., Ferreira I.C., Oliveira M. Mushroom ethanolic extracts as cosmeceuticals ingredients: Safety and ex vivo skin permeation studies. Food Chem. Toxicol. 2019;127:228–236. doi: 10.1016/j.fct.2019.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Taofiq O., Heleno S.A., Calhelha R.C., Alves M.J., Barros L., Gonzalez-Paramas A.M., Barreiro M.F., Ferreira I.C. The potential of Ganoderma lucidum extracts as bioactive ingredients in topical formulations, beyond its nutritional benefits. Pt AFood Chem. Toxicol. 2017;108:139–147. doi: 10.1016/j.fct.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 31.Ruan W., Lim A.H., Huang L.G., Popovich D.G. Extraction optimisation and isolation of triterpenoids from Ganoderma lucidum and their effect on human carcinoma cell growth. Nat. Prod. Res. 2014;28:2264–2272. doi: 10.1080/14786419.2014.938337. [DOI] [PubMed] [Google Scholar]
- 32.Chang Y., Kong R. Ganoderic acid A alleviates hypoxia-induced apoptosis, autophagy, and inflammation in rat neural stem cells through the PI3K/AKT/mTOR pathways. Phytother. Res. 2019;33:1448–1456. doi: 10.1002/ptr.6336. [DOI] [PubMed] [Google Scholar]
- 33.Cao F.R., Feng L., Ye L.H., Wang L.S., Xiao B.X., Tao X., Chang Q. Ganoderic Acid A Metabolites and Their Metabolic Kinetics. Front. Pharmacol. 2017;8:101. doi: 10.3389/fphar.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long M., Cai L., Li W., Zhang L., Guo S., Zhang R., Zheng Y., Liu X., Wang M., Zhou X., et al. DPP-4 Inhibitors Improve Diabetic Wound Healing via Direct and Indirect Promotion of Epithelial-Mesenchymal Transition and Reduction of Scarring. Diabetes. 2018;67:518–531. doi: 10.2337/db17-0934. [DOI] [PubMed] [Google Scholar]
- 35.Yang L., Zheng Z., Zhou Q., Bai X., Fan L., Yang C., Su L., Hu D. miR-155 promotes cutaneous wound healing through enhanced keratinocytes migration by MMP-2. J. Mol. Histol. 2017;48:147–155. doi: 10.1007/s10735-017-9713-8. [DOI] [PubMed] [Google Scholar]
- 36.Vuong T.T., Rønning S.B., Ahmed T.A.E., Brathagen K., Høst V., Hincke M.T., Suso H.P., Pedersen M.E. Processed eggshell membrane powder regulates cellular functions and increase MMP-activity important in early wound healing processes. PLoS ONE. 2018;13:e0201975. doi: 10.1371/journal.pone.0201975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Targets Inflamm. Allergy. 2005;4:517–519. doi: 10.2174/1568010054526386. [DOI] [PubMed] [Google Scholar]
- 38.Dunaway S., Odin R., Zhou L., Ji L., Zhang Y., Kadekaro A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018;9:392. doi: 10.3389/fphar.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cör D., Knez Ž., Knez Hrnčič M. Antitumour, Antimicrobial, Antioxidant and Antiacetylcholinesterase Effect of Ganoderma Lucidum Terpenoids and Polysaccharides: A Review. Molecules. 2018;23:649. doi: 10.3390/molecules23030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Z., Du R., Xiu L., Bian Z., Ma C., Sato N., Hattori M., Zhang H., Liang Y., Yu S., et al. Protective effect of triterpenes of Ganoderma lucidum on lipopolysaccharide-induced inflammatory responses and acute liver injury. Cytokine. 2020;127:154917. doi: 10.1016/j.cyto.2019.154917. [DOI] [PubMed] [Google Scholar]
- 41.Ciaglia E., Malfitano A.M., Laezza C., Fontana A., Nuzzo G., Cutignano A., Abate M., Pelin M., Sosa S., Bifulco M., et al. Immuno-Modulatory and Anti-Inflammatory Effects of Dihydrogracilin A, a Terpene Derived from the Marine Sponge Dendrilla membranosa. Int. J. Mol. Sci. 2017;18:1643. doi: 10.3390/ijms18081643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisanti S., Picardi P., Ciaglia E., Margarucci L., Ronca R., Giacomini A., Malfitano A.M., Casapullo A., Laezza C., Gazzerro P., et al. Antiangiogenic effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, mediated by AMPK activation. FASEB J. 2014;28:1132–1144. doi: 10.1096/fj.13-238238. [DOI] [PubMed] [Google Scholar]
- 43.Ciaglia E., Abate M., Laezza C., Pisanti S., Vitale M., Seneca V., Torelli G., Franceschelli S., Catapano G., Gazzerro P., et al. Antiglioma effects of N6-isopentenyladenosine, an endogenous isoprenoid end product, through the downregulation of epidermal growth factor receptor. Int. J. Cancer. 2017;140:959–972. doi: 10.1002/ijc.30505. [DOI] [PubMed] [Google Scholar]
- 44.Abate M., Laezza C., Pisanti S., Torelli G., Seneca V., Catapano G., Montella F., Ranieri R., Notarnicola M., Gazzerro P., et al. Deregulated expression and activity of Farnesyl Diphosphate Synthase (FDPS) in Glioblastoma. Sci. Rep. 2017;7:14123. doi: 10.1038/s41598-017-14495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]