Abstract
Adult spinal deformity causes significant health-related problems such as pain and disability in adults. Currently, there are several non-operative management strategies such as pain medications, physical modalities, exercises, bracing or interventional procedures. Specific exercises including strengthening of back and abdominal muscles, neuromuscular re-education for daily tasks, and active self-correction are vital to improve postural control and spinal stability. Bracing for a few hours a day can help reduce pain and provide spinal stability in adults. In case of severe disability and pain unresponsive to non-operative treatments, spinal fusion with instrumentation is an alternative. Postoperative rehabilitation can help to reduce pain and disability and improve return to activity or work. Protecting the spine early after surgery and timing of initiation of exercises with respect to osseointegration and bone remodeling phases are important principles of postoperative rehabilitation. In this review, rehabilitation in adult spinal deformity is discussed in the light of the literature.
Keywords: Adult spinal deformity, bracing, degenerative scoliosis, exercise, rehabilitation, spinal fusion
Adult spinal deformity (ASD) or adult scoliosis is abnormal alignment of adult spine in coronal, axial, and sagittal planes. It is diagnosed, if a curve is >10° in a skeletally mature individual.[1,2] Its prevalence ranges between 2% and 68%, being higher in elderly.[3-6] Adult spinal deformity may begin during adolescence and progress into the adulthood (progressive idiopathic scoliosis)[2]or may appear de novo (degenerative scoliosis). Age at the onset of symptoms is 70 years in degenerative scoliosis and 36 years in progressive idiopathic scoliosis.[7,8]
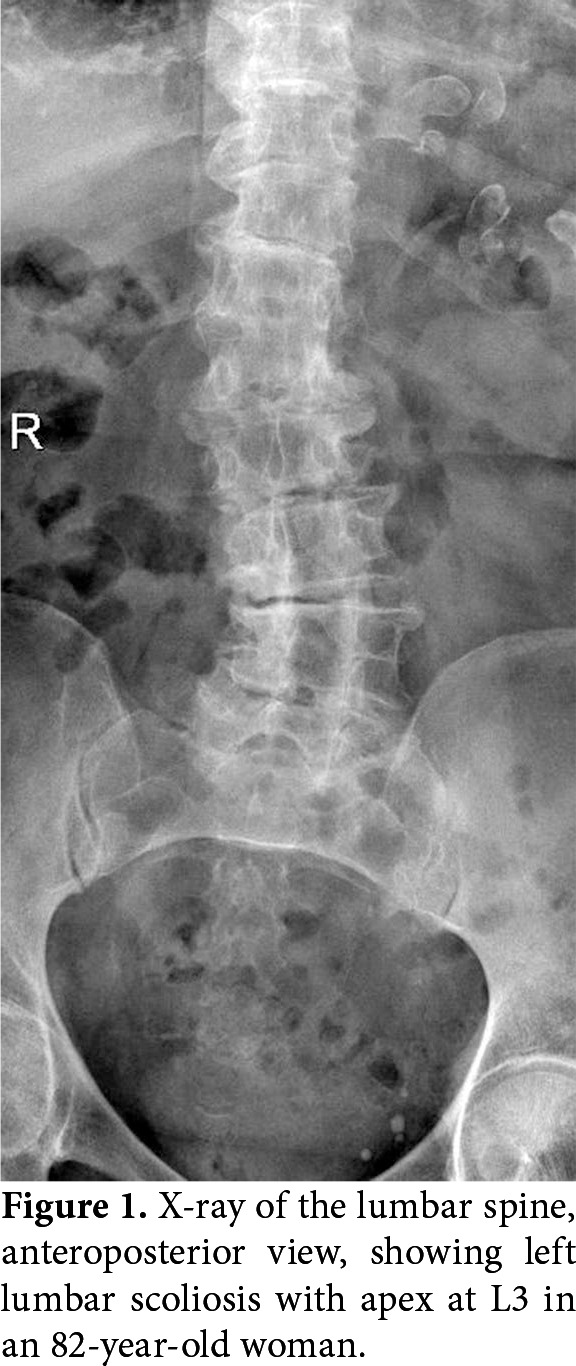
In degenerative scoliosis, an asymmetric disc degeneration is assumed to lead to asymmetric loading of the spine, further exacerbating the degeneration and deformity.[2,9] The curve is usually narrow (<50°) in the lumbar spine, where disc degeneration is common.[10] Compensatory thoracic curves are occasional. Rotation and lateral subluxation is common at the apex, usually L3-L4 level (Figure 1).[7,11] Osteophytes secondary to segmental instability may lead to spinal stenosis.[1]
Figure 1. X-ray of the lumbar spine, anteroposterior view, showing left lumbar scoliosis with apex at L3 in an 82-year-old woman.
Deformities in the sagittal plane is common and predict disability and decreased quality of life (QoL).[12] In idiopathic scoliosis, risk of progression into adulthood is greater with curves >50°.[9] Following spinal fusion, stenosis may develop in the lower adjacent segment, occasionally 15 to 20 years after surgery.[2]
Annual progression is 1.6° to 2.4° and 0.8° for degenerative and idiopathic scoliosis, respectively.[13] Some years of observation is required to accurately observe the individual curve progressions,[14] since measurement error is 5°.[15] Therefore, it is not recommended to repeat X-rays before four or five years in adults.[16]
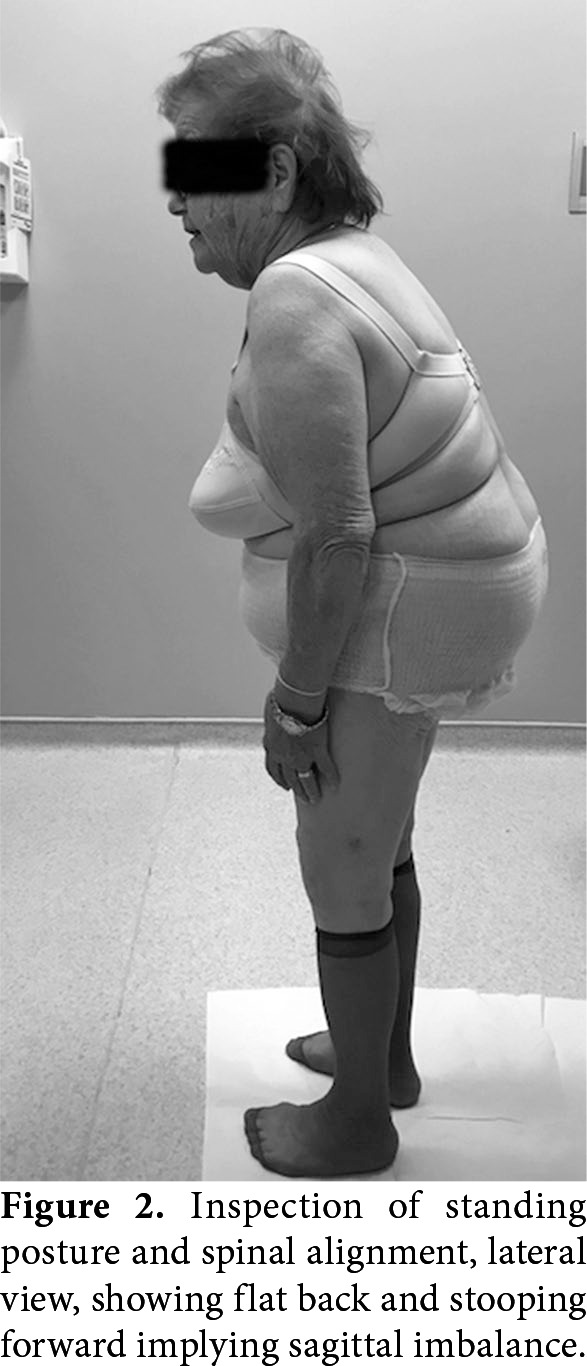
The clinical presentations of ASD are back pain, leg pain, claudication, and neurological deficit.[2] Back pain is seen in 40 to 90% of patients with degenerative scoliosis[10] and is attributed to muscle fatigue, facet and disc degeneration,[2] listhesis, and sagittal deformity.[9] The loss of lumbar lordosis leads to back muscle overactivity causing muscle fatigue and diffuse pain over areas of muscle insertion such as iliac crests, sacrum, and spinous processes (Figure 2).[2,10] Back and leg pain usually increases in upright postures due to spinal instability. Pain relief often requires lying down to take off the axial load on the spine.[2,7] Bending forward may not be sufficient to reduce claudication, but sitting with patient’s arms supported on the armchair relieves pain via unloading trunk.[1] Disability related to deformity is the most important parameter in deciding treatment, cosmetic issues play less role in degenerative scoliosis.
Figure 2. Inspection of standing posture and spinal alignment, lateral view, showing flat back and stooping forward implying sagittal imbalance.
The main goals of rehabilitation are to reduce pain and disability and to improve QoL in ASD.[2] Reducing the magnitude or progression of the curve in a skeletally mature adult is not expected.
Clinical and Research Consequences
Currently, evidence-based recommendations are not available; however, non-operative treatment is the first-line approach.[8,17] Progressive neurological deficit, severe disability, and pain unresponsiveness to treatment are indications for surgery in selected patients without significant cardiopulmonary disease, severe osteoporosis, organ failure, or cognitive disorders.[10,18]
Non-operative management in ASD involves pain medications, exercises, physical modalities, manual therapy, bracing, and interventional procedures.[10] In a systematic review, physical therapy, chiropractic care, and bracing had Level IV evidence and steroid injections had Level III evidence in the management of adult scoliosis.[19]
Pharmacological therapy
Analgesics, non-steroidal anti-inflammatory drugs, and gamma-aminobutyric acid (GABA) analogues are used with special attention to their potential side-effects.[8] Opioid analgesics can be considered for short-term pain relief due to risk of addiction and tolerance in long-term use.
Physical modalities
Physical modalities such as transcutaneous electrical nerve stimulation and thermotherapy are applied in appropriate patients, although level of evidence is indeterminate.[8]
Interventional procedures
Epidural injections, selective nerve root injections, facet blocks, and trigger point injections can be performed. Multiple segments may be responsible for pain.[2] An interval of at least three weeks is usually suggested between the injections, with maximum three or four injections within 6 to 12 months. Diagnostic injections can help to determine the extent of decompression.[7]
Manual therapy
High-quality evidence does not exist to support manual therapy in either adolescent or adult scoliosis. However, the International Scientific Society on Scoliosis Orthopaedic and Rehabilitation Treatment (SOSORT) guidelines recommend manual therapy (gentle, short-term mobilization, or releasing soft tissues techniques), only if it is associated with stabilization exercises in adolescent scoliosis.[9]
Manual therapy is suggested to help to balance and release myofascia via neurophysiological mechanisms.[20] The proposed explanation is that there is a continuous interaction between the myofascia and bones it surrounds, including the spine and ribs, and a balanced myofascia is expected to act as a three-dimensional template in which the deformed spine may realign over the years.
Manipulation alone is not sufficient to alter the curves, and exercise is usually combined.[21] Additive effects of myofascial release to exercise have been demonstrated on posture, subjective well-being, and functional activity in one case.[22] Manipulation has been suggested to play a role in improving chest wall expansion in another adult with thoracic lordoscoliosis.[23] On the other hand, there is still a concern that excessive spinal manipulation may lead to instability and curve progression.[21]
Exercises
Exercises help recover postural collapse and enhance postural control and spinal stability.[14] As adult bones are mature and stiff, reduction in curve is not expected. On the other hand, curve reduction following exercise may be observed in some adults, probably not due to the correction of bony deformity, but instead, to the correction of postural collapse component of the curve.[14] Reduction in postural collapse may reduce asymmetric loading, slow down asymmetric degeneration and, consequently, curve progression in the long-term.[14]
Scoliosis-specific exercises based on three- dimensional auto-correction and maintenance of the corrected posture in daily activities are recommended by the SOSORT in adolescent idiopathic scoliosis with curves <45° alone or in conjunction with bracing.[9] Several randomized controlled trials (RCTs) supported the effectiveness of scientific exercises approach to scoliosis (SEAS)[24] and Schroth[25] exercises in adolescent idiopathic scoliosis in reducing the curve, despite within the range of measurement error.[24-26] In adults, evidence is weaker.[27] Outcome measures are pain and curve progression, and only two studies measured disability until now, although it is the most important reason for seeking treatment in ASD.[28,29]
There is only one RCT comparing the effects of a multidisciplinary program involving active self-correction, task-oriented exercises, and cognitive behavioral therapy (CBT) with a general physiotherapy in adults with idiopathic scoliosis (Cobb angles <35°).[28] Task-oriented exercises were superior than general physiotherapy in enhancing functional outcomes and reducing disability, and CBT had an additional effect in modifying pain perception at one year. Radiological improvements were not clinically meaningful.
In a retrospective cohort of adult idiopathic scoliosis patients (mean Cobb; 56°) who refused surgery, a two-year-SEAS exercise program reduced curve severity or slowed down the progression at 25 years of follow-up. The lack of a control group, however, limits the strength of the study.[14]
Few cases are reported that Cobb angles improved >10° following exercise. A 25-year-old woman with scoliosis progressed after weaning of the brace improved following SEAS exercises. Regular exercises during bracing are important to prevent postural collapse.[16] Another woman with scoliosis secondary to a thoracic surgery at age three presented with severe pain and shortness of breath at age 23. After nine months of Schroth exercises, thoracic curve decreased from 70° to 58°.[30]
Bracing
Braces are not routine in adults, due to its questionable effects on mature spine as well as comfort issues,[9,31] and muscle deconditioning in prolonged use.[7,10] Bracing does not aim to prevent curve progression in adults, but aims to provide spine stability and reduce pain,[32] similar to a seat support during sitting.[9] Its principle is to decompress the spine by transferring the trunk weight from the ribs to the iliac crests (i.e., “hourglass effect”).[33] Some braces are designed to restore lumbar lordosis[34,35] and forces on the sagittal plane are thought to affect the coronal curves.[33]
There is no RCT or controlled study to provide an evidence-based recommendation for bracing in ASD. Patients with a mean Cobb angle of >30° are usually included in most studies. Rigid to very rigid braces[31,33] and elastic braces[32,36] were recommended for >4 to 6 h/day[33,37,38] in these studies.
Bracing reduces pain[9,32,33,35,36,38-40] for a brief duration[39] and not in minimal clinically significant levels.[9,34] In a study, one month of bracing did not change the QoL of ASD patients with chronic low back pain.[9] Two long-term studies showed that bracing might slow down the yearly progression of coronal curve,[33,37] being greater in degenerative scoliosis (from 1.5° to 0.2°) than idiopathic scoliosis (from 0.7° to 0.2°) in nine years.[37] Complementary to bracing, specific exercises,[38,40] physiotherapy[31,33,35] or chiropractic care[36] are recommended.
The rate of compliance is 17%.[31] Tolerance is poorer with long rigid braces with sternoclavicular thrust used in thoracolumbar kyphosis, and relatively better with short rigid braces ending under the breast. Newly designed braces[9] and individualized modifications in the commercial elastic orthosis by a band applying a coronal shifting force in the direction of the concavity[32] are suggested as alternatives with a higher level of comfort and tolerability.
Spinal surgery
A surgical decision is made regarding whether the complications or the benefits weigh more for the individual. Spinal fusion and instrumentation of several segments is applied to eliminate painful motion and to restore spinal stability.[41] Spinal mobility of the fused segments is largely reduced. However, total spinal range of motion is usually not limited, since fusion usually involves few segments,[41] not greater than three levels.[42,43] Bone grafts are used to stimulate bone fusion between the target vertebrae, which takes several months. During healing time, the fused spine must be kept in proper alignment. Instrumentation increases the rate of successful bone healing.[41]
Integration of the instrument to the bone tissue through histological connections is termed osseointegration and is important for stability. Osseointegration begins as tissue reactions around the implant early after surgery. Peri-implant trabecular osteogenesis starts on 10 to 14 days following instrumentation, and mixed woven and lamellar bone matrix is seen at three months.[44] Peri-implant bone remodeling may last one year. Patients are educated about appropriate safe techniques during movements and activities that require restriction before osseointegration is completed to prevent pseudoarthrosis or loosening of the instruments.[41]
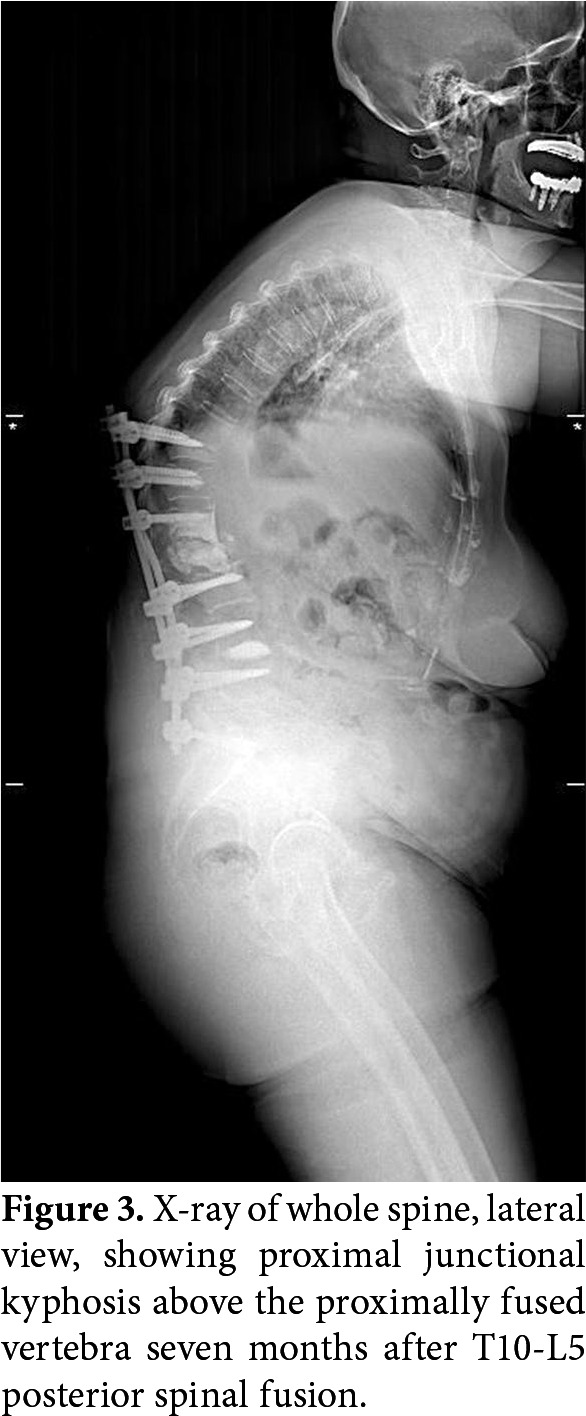
The junctional areas between the fused and unfused segments are potential sites for pain and kyphosis due to uneven distribution of the mobility in the sagittal plan (Figure 3). Preoperative multifidus atrophy is associated with postoperative sagittal malalignment[45] and progression of proximal junctional kyphosis[46] in degenerative scoliosis. Proper activation of muscles may help stabilization and reduce junctional failure.
Figure 3. X-ray of whole spine, lateral view, showing proximal junctional kyphosis above the proximally fused vertebra seven months after T10-L5 posterior spinal fusion.
Lumbar fusion affects the extensor muscle strength and trunk extensor/flexor balance adversely. Normally trunk extensors are stronger than flexors. However, in degenerative spine, lumbar extensor strength is decreased. After spinal fusion, imbalance between trunk extensor and flexors persists or even increases. The strength imbalance does not recover spontaneously with daily activity, although pain and disability improves following surgery.[47]
Besides deconditioning due to prolonged immobilization and reduced abdominal and back muscle strength, fear of movement may contribute to disability and poor QoL. Fear of movement may persist postoperatively beyond the expected healing time, thereby, leading to avoidance behavior and poor functional outcomes.[48]
Preoperative rehabilitation (prehabilitation)
Preoperative patient assessment by the physiatrist helps the surgeon in decision making and improves surgical outcomes. Assessment of bone mineral density may be helpful in elective surgeries to increase the quality of fixation. Prehabilitation is a term defining rehabilitation before surgery and its contents are provided in Table 1.
Table 1. Content of preoperative rehabilitation.
| • Advice on cessation of smoking and harmful drinking |
| • Decision and timing of surgery |
| - Evaluating physical findings and psychosocial factors |
| - Ensuring the fitness of the patient for the surgery |
| - Assessment of bone mineral density |
| - Counseling for timing of surgery (preparation of bone density and muscle strength) |
| • Information regarding; |
| - Surgical and anesthetic procedures |
| - Postoperative inpatient activities and exercises such as transfer training, mobilization, respiratory and circulatory exercises, core exercises |
| - Counseling for physical activity and time to return to work |
| • Exercise program (including aerobic, abdominal and back strengthening exercises) |
| • Cognitive behavioral therapy |
Information regarding surgery and anesthesia is given preoperatively. Cessation of smoking four to eight weeks prior to surgery[49] and alcohol abstinence four weeks prior to surgery in harmful drinkers[50] can reduce postoperative complications. Counseling and education for postoperative physical activity, including early inpatient mobilization and core exercises, and time to return to activities of daily living, sports, or work should be provided.[43,51]
In recent years, rehabilitation before surgery, termed as prehabilitation, has been widely studied. However, supporting evidence or an expert consensus does not exist. It is not a routine care in many centers; one-third of Dutch and Swedish and one of thirty British surgeons[52] refer patients to prehabilitation. Prehabilitation as a daily 30-min home-based program for a total of six weeks also including smoking cessation, alcohol abstinence programs and early postoperative rehabilitation decreased the length of stay, reached postoperative milestones earlier, and reduced costs.[50] In another study, a daily 30-min home-based prehabilitation program consisting of aerobic and abdominal and back strengthening exercises for a total of six to eight weeks before elective lumbar fusion decreased the length of stay and improved postoperative disability and QoL.[53]
Preoperative CBT may prevent fear-avoidance beliefs and pain catastrophizing. In two studies, a total of four 1-h-individual session[54] or 3-h group sessions[42] were guided by a physiotherapist[54] or a multidisciplinary team[42] before surgery. The CBT was superior to the usual care in pain coping ability with decreased analgesic use and earlier independent mobility.[42]
An RCT compared prehabilitation twice a week for a total of nine weeks to a waiting list provided an advice to keep active. Prehabilitation group received a behavioral approach and supervised exercise program (e.g., specific exercises, mobilization, tailor- made general exercises, motor control exercises, and traction) in the clinics. Prehabilitation improved pain, disability, self-efficacy, and QoL prior to surgery; however, postoperatively there were no significant differences between groups. They suggest to select the patients that would benefit from prehabilitation.[51]
Recommendations for physical activity following surgery
Pain is usually uncomfortable during the first few days and relieves gradually thereafter. Mild pain may persist, usually not require analgesics at three to six weeks following surgery.[55] During the healing process, many activities are restricted to protect the spine. No evidence-based guideline exists to recommend physical activity or return to sport after fusion. Information regarding activity recommendations following fusion in ASD is scarce and greatly varies depending on the surgeon’s opinions and experiences. Individual factors (i.e., age, preoperative functional level, occupation, expectations, motivation, anxiety, comorbidities, complications, pain severity, bone quality, speed of recovery,[52] preoperative curve magnitude, type of physical activity[56]) and surgical factors (i.e., quality and extent of fixation, type of the instrumentation,[56] level of the distal instrumented vertebra,[56,57] and perioperative pulmonary complications) affect the return to physical activities, school/work, or sports activities. Lower distal instrumented vertebra in the lumbar spine, osteoporosis, multiple comorbidities, and deconditioning are common in ASD.
Surveys among surgeons[52,58] demonstrate that activity and safety recommendations following lumbar fusion in ASD greatly vary. For the first 6 to 12 weeks, American Academy of Orthopaedic Surgeons (AAOS) recommends avoiding heavy lifting and forward bending should be kept minimum. Driving a car is usually permitted around sixth week, since enough healing and movement without a need for analgesic is required.[55] Sexual activity can be resumed by sixth week. Bending forward, extension and rotation to end range can be performed after 12th week. Cycling and swimming may be permitted by first month, running by third month and contact sports by sixth month. Time to return to work depends on the type of work and varies between six weeks to three months or until radiological healing.[58]
Non-contact sports activities such as gym, swimming, recreational sports, cross-country running, or sprinting are usually permitted four to six months after surgery.[55] Follow-up radiographs at four months showing no change in curve correction or implant position are important to decide to allow to return to sports. Contact sports such as soccer, basketball, or volleyball usually require 6 to 12 months or complete maturation of the fusion,[55] For an athlete to return to play, resolution of preoperative symptoms, normal range of motion and normal strength, painless sport-specific activity and successful completion of a structured rehabilitation are prerequisites.[57] Return to collision sports such as American football, hockey, rugby, mixed marital arts, or wrestling is not allowed, as the lowest instrumented vertebra gets more distal, and a 12-month period is required, if allowed.[56] Failure of fixation leading to a spinal cord injury is the most feared catastrophic event, although not reported yet. One case was reported with an instrumentation failure without neurological deficit following snowboarding at two weeks postoperatively.[56] Gymnastics, football, rugby, wrestling, weightlifting, skydiving, and bungee jumping are the most common sports forbidden by surgeons following fusion.[57]
Postoperative rehabilitation
Inpatient rehabilitation
Spinal fusion with rigid instrumentation provides adequate stability and allows immediate mobilization following surgery. Rehabilitation begins immediately and consists of education, and early mobility including transfers and supervised daily walk (Table 2). Walking distance is gradually increased as tolerated. Late ambulation in elderly is associated with higher complication rates, poorer functional outcomes, and longer stay.[59]
Table 2. Content of postoperative inpatient rehabilitation.
| • Information regarding |
| - How to do safely (posture and positioning for pain control) |
| - What to avoid (prolonged flexion, bending, heavy lifting) |
| • Activities of daily living |
| - Getting in and out of bed |
| - Transfers |
| - Sitting |
| - Walking |
| - Climbing stairs |
| • Exercises |
| - Circulatory |
| - Respiratory |
| - Core stability |
| • Advices before discharge |
Inpatient rehabilitation is standard care in some centers, but is only provided, if there is lack of improvement in function, mobility or balance, ongoing pain or disability in others. Physiotherapy begins either on the same, or on the postoperative Day 1.[52] Sessions last 20 to 30 min, once or twice a day supervised by a physiotherapist for three to five days.[60] Patients are reminded about the protection of the instrumented spine during daily activities such as getting out of bed, getting into a chair, walking, and climbing stairs. Patients are also instructed to sit shorter than 30 min at a time during the first four weeks,[60] and to avoid extreme trunk flexion and lifting or carrying heavy objects for postoperative two months.[61]
Nearly all spinal surgeons allowed pain after mobilization; half allow pain duration of maximum 6 h, the other half for maximum 24 h.[58] Sitting, standing, and walking with support can be usually achieved on the same day or postoperative Day 1.[52,58] Walking without support and climbing stairs are performed on postoperative Day 2 or 3 by many patients.[52,58] The most widely used discharge criterion is safe and independent mobility including climbing stairs in a medically stable patient with a dry wound.
Gentle muscle setting exercises of the immobilized area and deep breathing exercises can be performed in the early postoperative period.[60] The main goal is to maintain spinal posture and stabilization during daily activities. Task-oriented lower extremity exercises such as closed-kinetic chain exercises in functional postures can improve performance.[60]
No evidence exists to recommend corset use following fusion.[62] However, some surgeons reported corset use, with wearing durations from six weeks to three months or only during lifting and physical activities or patient’s choice.[58] The corset use was infrequent in the United Kingdom[52] and indicated to control pain, increase compliance with protecting back, and promote healing in bone problems or surgical factors such as multilevel fusion or anterior interbody fusion.[52]
Outpatient rehabilitation
Evidence for exercise following fusion surgery in improving pain, function, disability or QoL is weak.[63,64] Superiority of exercise over no exercise has not been demonstrated in RCTs, yet.[60,65-67] However, due to the overall benefits of exercise and the lack of adverse effects, exercise is recommended postoperatively by some surgeons.[57,68] Outpatient rehabilitation is not routine in many centers.[41,52,56,58] Usual postoperative care is an advice to stay active.[67] Indications of rehabilitation are ongoing pain, disability, poor trunk control, limited function or mobility, fear-avoidance, slow postoperative recovery, multiple comorbidities, or patient’s request.[52,53] A patient-centered approach is targeted to the individual’s needs.[52] Physical deconditioning exacerbated by postoperative immobilization, altered spinal biomechanics, multifidus atrophy, and reduced ratio of trunk extensor/flexor strength should be addressed.
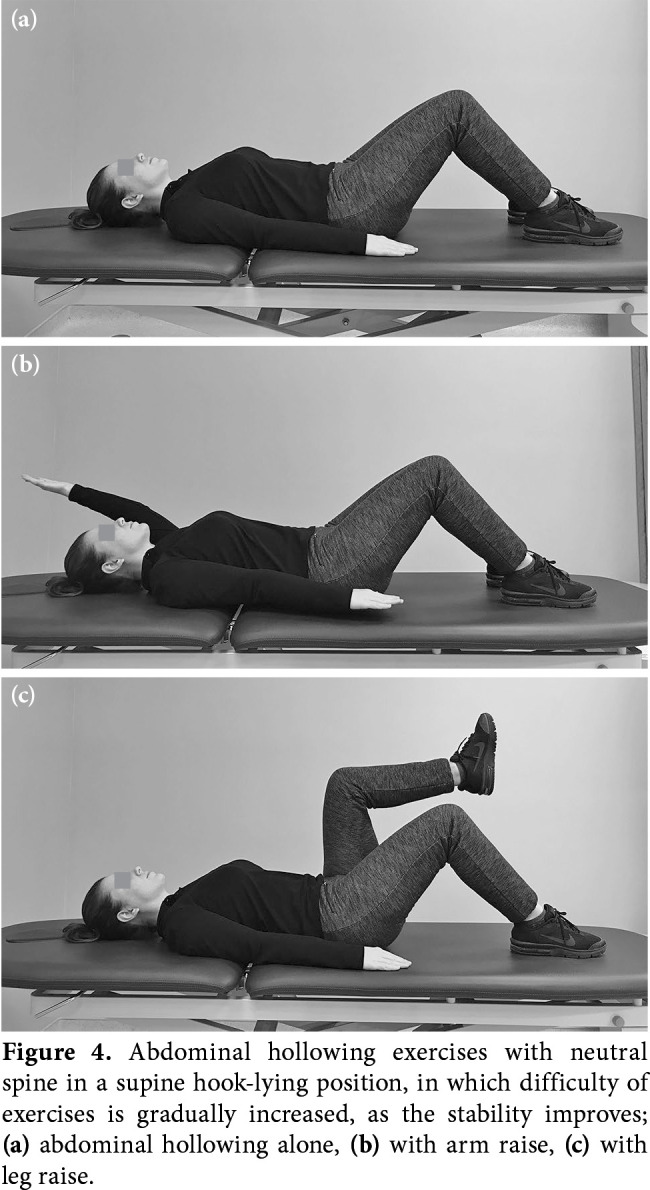
During postoperative three weeks, home- based exercises including gentle co-contraction of transversus abdominis and multifidus to splint the spine in supine lying, sitting and standing, and squatting to provide lumbopelvic stabilization are advised (Figure 4). Core stability training is the key element in spine rehabilitation. During exercises, maintaining neutral spine is crucial.[69] Pelvic tilt exercises in supine and standing position may increase the awareness about neutral spine position. Stretching (gastrosoleus, hip extensors and flexors) exercises are performed with the lumbar spine kept in the neutral position. Bending is avoided to reduce strain on the fused and adjacent segments to prevent failure of instrument or dislocation of pedicle screws.[61] Neuromuscular re-education to adapt new restrictions of lumbar motion is the basis for activities and exercises.
Figure 4. Abdominal hollowing exercises with neutral spine in a supine hook-lying position, in which difficulty of exercises is gradually increased, as the stability improves; (a) abdominal hollowing alone, (b) with arm raise, (c) with leg raise.
Rehabilitation can be commenced 6 to 12 weeks after surgery according to the AAOS.[41] Usual practice is to begin at 12 weeks,[58,60,63,70] following the evaluation of lumbar spine radiographs. Adequate time is required to ensure a safe bone healing, stable spine, and osseointegration. Some recommend beginning with core strengthening in neutral spine and non-impact aerobic activity immediately,[58] at postoperative two weeks[57] or eight weeks[67] and demonstrate to be safe during the first three months following fusion.[69] On the other hand, Oestergaard et al.[70] observed that rehabilitation started at six weeks resulted in inferior outcomes compared to the program initiated at 12 weeks. Rehabilitation started at 12 weeks led to less pain one year after surgery and better functional mobility and daily activity.
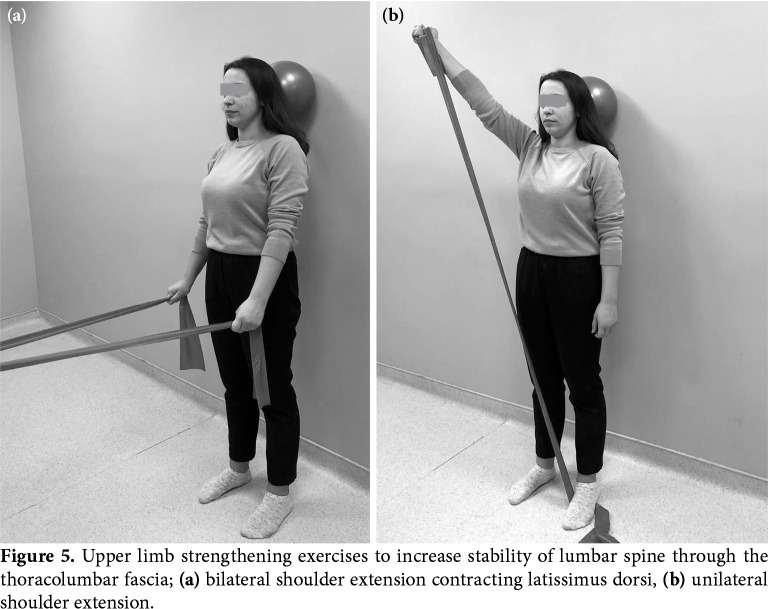
At postoperative three months, once osseointegration and fusion get stronger, spine can be gradually loaded by the increasing difficulty of the exercises, and large prime movers are integrated into the program, as the coordination of core muscles is improved.[67] Extremity movements such as lifting, pushing or pulling lead to destabilizing force on the trunk. Neutral spine position increases the spinal stability against shearing and compressive forces during these activities. Upper limb strengthening exercises retrain proper activation of trunk muscles.[61] Contraction of latissimus dorsi and external oblique muscles increases the stability of lumbar spine and sacroiliac joints via attachments to the thoracolumbar fascia (Figure 5). Bilateral shoulder extension and flexion can be used to train abdominal muscles and trunk extensors, respectively.[61] Unilateral shoulder horizontal adduction and abduction during sitting induce rotational forces to the trunk and train extensor oblique which controls the rotational forces.
Figure 5. Upper limb strengthening exercises to increase stability of lumbar spine through the thoracolumbar fascia; (a) bilateral shoulder extension contracting latissimus dorsi, (b) unilateral shoulder extension.
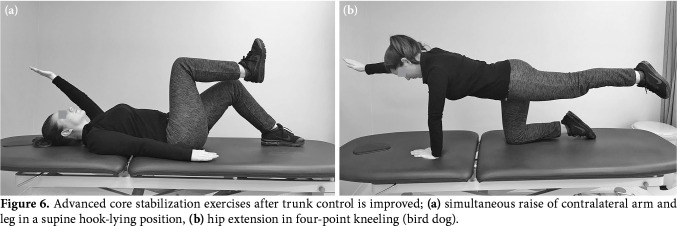
Advanced exercises such as hip extension in four-point kneeling or bird dog,[47] extension of contralateral arms and legs simultaneously in supine-lying can be prescribed, after trunk control is improved (Figure 6). Exercise balls or elastic exercise bands can be also used to modify the difficulty of exercises (Figure 7). Sport-specific training can be introduced at four to six months. It should be kept in mind that complete maturation and remodeling of bone healing may continue until 6 to 12 months.
Figure 6. Advanced core stabilization exercises after trunk control is improved; (a) simultaneous raise of contralateral arm and leg in a supine hook-lying position, (b) hip extension in four-point kneeling (bird dog).
Figure 7. Advanced exercises with elastic exercise bands and ball after proper trunk control and stability is achieved; while sitting on an exercise ball, (a) simultaneous raise of contralateral arm and leg, (b) bilateral shoulder horizontal abduction.
Aerobic exercise, usually walking, is advised. Interval walking (alternating cycles of walking at normal and brisk speeds) may be an alternative.[71] If walking is limited by symptoms, a recumbent bicycle ergometer can be recommended at one month postoperatively.[61] Aquatic exercise may be another alternative in symptomatic patients via buoyancy of water.
Rehabilitation is scheduled either at home or clinic, and two to five times a week, individually or in groups. The mean number of sessions is six (usually 4 to 10) and the mean duration of rehabilitation is four weeks, 12 weeks or 12 months in the previous studies.[63,67,69,72]
Manual therapy, chiropractic treatment, mechanical therapy (McKenzie), or massage are not advised after surgery.[58,73]
Cognitive behavioral therapy may assist to prevent or reduce fear-avoidance behavior following fusion.[69,72-74] Increasing awareness, adaptive coping strategies, and communication help in modifying catastrophic thinking and fear of movement. A gradual increase in activity is reached by motivation and follow-up in the sessions. Addition of CBT to exercise increased self- efficacy, improved general health and performance at six months,[74] produced reduction in disability at one year,[72] improved return to work at two to three years of follow-up. The first biopsychosocial “Back Café” model supplemental to usual care was an eight-week behavioral approach consisting of three 1.5-h-meetings with a physiotherapist and patients who underwent spinal fusion.[73] Results on pain and disability were better than usual care (home-based exercises) or individual supervised exercise training. This study showed the beneficial effects of interpatient learning from peer experiences and reassurance in group meetings regarding pain coping strategies and resocialization. The intense exercise training group had higher scores of leg pain at two years, indicating the role of modifying activity during episodes of increased pain and the need for an effective pain coping approach. The authors further questioned the role of traditional intense exercise training after fusion.[73]
The benefits of supplemental CBT were shown over education,[74] home-based[69] or supervised[72] exercise programs in different studies. However, some discrepancies between frequency and duration of sessions exist among studies; twice weekly 1-h sessions for four weeks,[72] weekly 30-min sessions over phone for six weeks with first session in person[74] or 90-min sessions every three weeks for a total of nine weeks.[69] Therapy was either commenced immediately,[69] six weeks[74] or three months following surgery[73] and was administered by either a psychologist[72] or a physiotherapist.[74]
To promote return to work, education should involve proper ergonomics and working posture. A work conditioning or complicating the program addressing physical, functional, behavioral, and vocational needs using real or simulated work activities may increase the rate of return to work.[75]
In conclusion, evidence-based recommendations are weak due to low-quality studies regarding rehabilitation in ASD. Pre- and postoperative rehabilitation may help to accelerate recovery and reduce the risk of complications. Future studies are needed to identify these patients with disability in the early postoperative period who would benefit from individualized rehabilitation interventions. Also, behavioral approach can be implemented to the individually-tailored rehabilitation programs in patients with fear of movement.
Footnotes
Conflict of Interest: The author received no financial support for the research and/or authorship of this article.
Financial Disclosure: The author declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Silva FE, Lenke LG. Adult degenerative scoliosis: evaluation and management. E1Neurosurg Focus. 2010;28 doi: 10.3171/2010.1.FOCUS09271. [DOI] [PubMed] [Google Scholar]
- 2.Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925–948. doi: 10.1007/s00586-005-1053-9. [DOI] [PubMed] [Google Scholar]
- 3.Kebaish KM, Neubauer PR, Voros GD, Khoshnevisan MA, Skolasky RL. Scoliosis in adults aged forty years and older: prevalence and relationship to age, race, and gender. Spine (Phila Pa 1976) 2011;36:731–736. doi: 10.1097/BRS.0b013e3181e9f120. [DOI] [PubMed] [Google Scholar]
- 4.Carter OD, Haynes SG. Prevalence rates for scoliosis in US adults: results from the first National Health and Nutrition Examination Survey. Int J Epidemiol. 1987;16:537–544. doi: 10.1093/ije/16.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Robin GC, Span Y, Steinberg R, Makin M, Menczel J. Scoliosis in the elderly: a follow-up study. Spine (Phila Pa 1976) 1982;7:355–359. doi: 10.1097/00007632-198207000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Schwab F, Dubey A, Gamez L, El Fegoun AB, Hwang K, Pagala M, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976) 2005;30:1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 7.York PJ, Kim HJ. Degenerative Scoliosis. Curr Rev Musculoskelet Med. 2017;10:547–558. doi: 10.1007/s12178-017-9445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diebo BG, Shah NV, Boachie-Adjei O, Zhu F, Rothenfluh DA, Paulino CB, et al. Adult spinal deformity. Lancet. 2019;394:160–172. doi: 10.1016/S0140-6736(19)31125-0. [DOI] [PubMed] [Google Scholar]
- 9.Negrini S, Donzelli S, Aulisa AG, Czaprowski D, Schreiber S, de Mauroy JC, et al. 2016 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018;13:3–3. doi: 10.1186/s13013-017-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong E, Altaf F, Oh LJ, Gray RJ. Adult Degenerative Lumbar Scoliosis. e930-e9Orthopedics. 2017;40 doi: 10.3928/01477447-20170606-02. [DOI] [PubMed] [Google Scholar]
- 11.Marty-Poumarat C, Scattin L, Marpeau M, Garreau de Loubresse C, Aegerter P. Natural history of progressive adult scoliosis. MSpine (Phila Pa 1976) 2007;32:1227–1234. doi: 10.1097/01.brs.0000263328.89135.a6. discussion 1235. [DOI] [PubMed] [Google Scholar]
- 12.Le Huec JC, Thompson W, Mohsinaly Y, Barrey C, Faundez A. Sagittal balance of the spine. Eur Spine J. 2019;28:1889–1905. doi: 10.1007/s00586-019-06083-1. [DOI] [PubMed] [Google Scholar]
- 13.Korovessis P, Piperos G, Sidiropoulos P, Dimas A. Adult idiopathic lumbar scoliosis. A formula for prediction of progression and review of the literature. Spine (Phila Pa 1976) 1994;19:1926–1932. [PubMed] [Google Scholar]
- 14.Negrini A, Negrini MG, Donzelli S, Romano M, Zaina F, Negrini S. Scoliosis-Specific exercises can reduce the progression of severe curves in adult idiopathic scoliosis: a long-term cohort study. Scoliosis. 2015;10:20–20. doi: 10.1186/s13013-015-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zmurko MG, Mooney JF 3rd, Podeszwa DA, Minster GJ, Mendelow MJ, Guirgues A. Inter- and intraobserver variance of Cobb angle measurements with digital radiographs. J Surg Orthop Adv. 2003;12:208–213. [PubMed] [Google Scholar]
- 16.Negrini A, Parzini S, Negrini MG, Romano M, Atanasio S, Zaina F, et al. Adult scoliosis can be reduced through specific SEAS exercises: a case report. Scoliosis. 2008;3:20–20. doi: 10.1186/1748-7161-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berven SH, Kamper SJ, Germscheid NM, Dahl B, Shaffrey CI, Lenke LG, et al. An international consensus on the appropriate evaluation and treatment for adults with spinal deformity. Eur Spine J. 2018;27:585–596. doi: 10.1007/s00586-017-5241-1. [DOI] [PubMed] [Google Scholar]
- 18.Ailon T, Smith JS, Shaffrey CI, Lenke LG, Brodke D, Harrop JS, et al. Degenerative Spinal Deformity. S75-91Neurosurgery. 2015;77(Suppl 4) doi: 10.1227/NEU.0000000000000938. [DOI] [PubMed] [Google Scholar]
- 19.Everett CR, Patel RK. A systematic literature review of nonsurgical treatment in adult scoliosis. S130-4Spine (Phila Pa 1976) 2007;32(19 Suppl) doi: 10.1097/BRS.0b013e318134ea88. [DOI] [PubMed] [Google Scholar]
- 20.Blum CL. Chiropractic and pilates therapy for the treatment of adult scoliosis. E3J Manipulative Physiol Ther. 2002;25 doi: 10.1067/mmt.2002.123336. [DOI] [PubMed] [Google Scholar]
- 21.Morningstar MW, Woggon D, Lawrence G. Scoliosis treatment using a combination of manipulative and rehabilitative therapy: a retrospective case series. BMC Musculoskelet Disord. 2004;5:32–32. doi: 10.1186/1471-2474-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis C, Doerger C, Rowland J, Sauber C, Eaton T. Myofascial release as complementary in physical therapy for two elderly patients with osteoporosis and kyphoscoliosis. J Geriatric Phys Ther. 2002;25:33–33. [Google Scholar]
- 23.Hawes MC, Brooks WJ. Improved chest expansion in idiopathic scoliosis after intensive, multiple-modality, nonsurgical treatment in an adult. Chest. 2001;120:672–674. doi: 10.1378/chest.120.2.672. [DOI] [PubMed] [Google Scholar]
- 24.Monticone M, Ambrosini E, Cazzaniga D, Rocca B, Ferrante S. Active self-correction and task-oriented exercises reduce spinal deformity and improve quality of life in subjects with mild adolescent idiopathic scoliosis. Results of a randomised controlled trial. Eur Spine J. 2014;23:1204–1214. doi: 10.1007/s00586-014-3241-y. [DOI] [PubMed] [Google Scholar]
- 25.Kuru T, Yeldan İ, Dereli EE, Özdinçler AR, Dikici F, Çolak İ. The efficacy of three-dimensional Schroth exercises in adolescent idiopathic scoliosis: a randomised controlled clinical trial. Clin Rehabil. 2016;30:181–190. doi: 10.1177/0269215515575745. [DOI] [PubMed] [Google Scholar]
- 26.Negrini S, Zaina F, Romano M, Negrini A, Parzini S. Specific exercises reduce brace prescription in adolescent idiopathic scoliosis: a prospective controlled cohort study with worst-case analysis. J Rehabil Med. 2008;40:451–455. doi: 10.2340/16501977-0195. [DOI] [PubMed] [Google Scholar]
- 27.Alanazi MH, Parent EC, Dennett E. Effect of stabilization exercise on back pain, disability and quality of life in adults with scoliosis: a systematic review. Eur J Phys Rehabil Med. 2018;54:647–653. doi: 10.23736/S1973-9087.17.05062-6. [DOI] [PubMed] [Google Scholar]
- 28.Monticone M, Ambrosini E, Cazzaniga D, Rocca B, Motta L, Cerri C, et al. Adults with idiopathic scoliosis improve disability after motor and cognitive rehabilitation: results of a randomised controlled trial. Eur Spine J. 2016;25:3120–3129. doi: 10.1007/s00586-016-4528-y. [DOI] [PubMed] [Google Scholar]
- 29.Glassman SD, Carreon LY, Shaffrey CI, Polly DW, Ondra SL, Berven SH, et al. The costs and benefits of nonoperative management for adult scoliosis. Spine (Phila Pa 1976) 2010;35:578–582. doi: 10.1097/BRS.0b013e3181b0f2f8. [DOI] [PubMed] [Google Scholar]
- 30.Lebel A, Lebel VA. Severe progressive scoliosis in an adult female possibly secondary thoracic surgery in childhood treated with scoliosis specific Schroth physiotherapy: Case presentation. Scoliosis Spinal Disord. 2016;11(Suppl 2):41–41. doi: 10.1186/s13013-016-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Mauroy JC, Lecante C, Barral F, Pourret S. Bracing in adult with scoliosis: experience in diagnosis and classification from a 15 year prospective study of 739 patients. Scoliosis Spinal Disord. 2016;11(Suppl 2):29–29. doi: 10.1186/s13013-016-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polastri M, Romano M. Lumbar scoliosis: Reducing lower back pain and improving function in adulthood. A case report with a 2-year follow-up. J Bodyw Mov Ther. 2017;21:81–85. doi: 10.1016/j.jbmt.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 33.de Mauroy JC, Lecante C, Barral F, Pourret S. Prospective study of 158 adult scoliosis treated by a bivalve polyethylene overlapping brace and reviewed at least 5 years after brace fitting. Scoliosis Spinal Disord. 2016;11(Suppl 2):28–28. doi: 10.1186/s13013-016-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss HR, Dallmayer R. Brace treatment of spinal claudication in an adult with lumbar scoliosis--a case report. Stud Health Technol Inform. 2006;123:586–589. [PubMed] [Google Scholar]
- 35.Gallo D. Case reports: orthotic treatment of adult scoliosis patients with chronic back pain. Scoliosis. 2014;9:18–18. doi: 10.1186/1748-7161-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcotte L, Rivard CH, Coillard C. Adult scoliosis and the SPINECOR® dynamic brace: some early results on efficiency. Scoliosis. 2009;4(Suppl 1):56–56. [Google Scholar]
- 37.Palazzo C, Montigny JP, Barbot F, Bussel B, Vaugier I, Fort D, et al. Effects of Bracing in Adult With Scoliosis: A Retrospective Study. Arch Phys Med Rehabil. 2017;98:187–190. doi: 10.1016/j.apmr.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Weiss HR, Moramarco K, Moramarco M. Scoliosis bracing and exercise for pain management in adults-a case report. J Phys Ther Sci. 2016;28:2404–2407. doi: 10.1589/jpts.28.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss HR, Dallmayer R, Stephan C. First results of pain treatment in scoliosis patients using a sagittal realignment brace. Stud Health Technol Inform. 2006;123:582–585. [PubMed] [Google Scholar]
- 40.Papadopoulos D. Adult scoliosis treatment combining brace and exercises. Scoliosis. 2013;8(Suppl 2):8–8. [Google Scholar]
- 41.Park DK. Spinal Fusion. American Academy of Orthopaedic Surgeons. Available at: https://orthoinfo.aaos.org/en/treatment/spinal-fusion/ . Last reviewed June 2018. [Accessed: March 12, 2020] [Google Scholar]
- 42.Rolving N, Nielsen CV, Christensen FB, Holm R, Bünger CE, Oestergaard LG. Preoperative cognitive-behavioural intervention improves in-hospital mobilisation and analgesic use for lumbar spinal fusion patients. BMC Musculoskelet Disord. 2016;17:217–217. doi: 10.1186/s12891-016-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lotzke H, Brisby H, Gutke A, Hägg O, Jakobsson M, Smeets R, et al. A Person-Centered Prehabilitation Program Based on Cognitive-Behavioral Physical Therapy for Patients Scheduled for Lumbar Fusion Surgery: A Randomized Controlled Trial. Phys Ther. 2019;99:1069–1088. doi: 10.1093/ptj/pzz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. Biology of implant osseointegration. J Musculoskelet Neuronal Interact. 2009;9:61–71. [PubMed] [Google Scholar]
- 45.Yagi M, Hosogane N, Watanabe K, Asazuma T, Matsumoto M, Keio Spine Research Group The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J. 2016;16:451–458. doi: 10.1016/j.spinee.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Vives MJ. The paraspinal muscles and their role in the maintenance of global spinal alignment. Another wrinkle in an already complex problem. Spine J. 2016;16:459–461. doi: 10.1016/j.spinee.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Tarnanen S, Neva MH, Kautiainen H, Ylinen J, Pekkanen L, Kaistila T, et al. The early changes in trunk muscle strength and disability following lumbar spine fusion. Disabil Rehabil. 2013;35:134–139. doi: 10.3109/09638288.2012.690496. [DOI] [PubMed] [Google Scholar]
- 48.Archer KR, Wegener ST, Seebach C, Song Y, Skolasky RL, Thornton C, et al. The effect of fear of movement beliefs on pain and disability after surgery for lumbar and cervical degenerative conditions. Spine (Phila Pa 1976) 2011;36:1554–1562. doi: 10.1097/BRS.0b013e3181f8c6f4. [DOI] [PubMed] [Google Scholar]
- 49.Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. CD002294Cochrane Database Syst Rev. 2014;2014 doi: 10.1002/14651858.CD002294.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen PR, Andreasen J, Asmussen M, Tønnesen H. Costs and quality of life for prehabilitation and early rehabilitation after surgery of the lumbar spine. BMC Health Serv Res. 2008;8:209–209. doi: 10.1186/1472-6963-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindbäck Y, Tropp H, Enthoven P, Abbott A, Öberg B. PREPARE: presurgery physiotherapy for patients with degenerative lumbar spine disorder: a randomized controlled trial. Spine J. 2018;18:1347–1355. doi: 10.1016/j.spinee.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 52.Rushton A, White L, Heap A, Heneghan N. Evaluation of current surgeon practice for patients undergoing lumbar spinal fusion surgery in the United Kingdom. World J Orthop. 2015;6:483–490. doi: 10.5312/wjo.v6.i6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen PR, Jørgensen LD, Dahl B, Pedersen T, Tønnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil. 2010;24:137–148. doi: 10.1177/0269215509347432. [DOI] [PubMed] [Google Scholar]
- 54.Lotzke H, Jakobsson M, Brisby H, Gutke A, Hägg O, Smeets R, et al. Use of the PREPARE (PREhabilitation, Physical Activity and exeRcisE) program to improve outcomes after lumbar fusion surgery for severe low back pain: a study protocol of a person-centred randomised controlled trial. BMC Musculoskelet Disord. 2016;17:349–349. doi: 10.1186/s12891-016-1203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Surgeons AAoO. Surgical Treatment for Scoliosis. Available at: http://orthoinfo.aaos.org/topic.cfm?topic=A00638 . [Accessed: July 25, 2019]. [Google Scholar]
- 56.Lehman RA Jr, Kang DG, Lenke LG, Sucato DJ, Bevevino AJ, Spinal Deformity Study Group Return to sports after surgery to correct adolescent idiopathic scoliosis: a survey of the Spinal Deformity Study Group. Spine J. 2015;15:951–958. doi: 10.1016/j.spinee.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Hresko MT. Lumbar spine surgery in athletes:: outcomes and return-to-play criteria. Clin Sports Med. 2012;31:487–498. doi: 10.1016/j.csm.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 58.van Erp RMA, Jelsma J, Huijnen IPJ, Lundberg M, Willems PC, Smeets RJEM. Spinal Surgeons' Opinions on Pre- and Postoperative Rehabilitation in Patients Undergoing Lumbar Spinal Fusion Surgery: A Survey-Based Study in the Netherlands and Sweden. Spine (Phila Pa 1976) 2018;43:713–719. doi: 10.1097/BRS.0000000000002406. [DOI] [PubMed] [Google Scholar]
- 59.Adogwa O, Elsamadicy AA, Fialkoff J, Cheng J, Karikari IO, Bagley C. Early Ambulation Decreases Length of Hospital Stay, Perioperative Complications and Improves Functional Outcomes in Elderly Patients Undergoing Surgery for Correction of Adult Degenerative Scoliosis. Spine (Phila Pa 1976) 2017;42:1420–1425. doi: 10.1097/BRS.0000000000002189. [DOI] [PubMed] [Google Scholar]
- 60.Chen CY, Chang CW, Lee ST, Chen YC, Tang SF, Cheng CH, et al. Is rehabilitation intervention during hospitalization enough for functional improvements in patients undergoing lumbar decompression surgery? A prospective randomized controlled study. . S41-6Clin Neurol Neurosurg. 2015;129(Suppl 1) doi: 10.1016/S0303-8467(15)30011-1. [DOI] [PubMed] [Google Scholar]
- 61.Tarnanen SP, Neva MH, Häkkinen K, Kankaanpää M, Ylinen J, Kraemer WJ, et al. Neutral spine control exercises in rehabilitation after lumbar spine fusion. J Strength Cond Res. 2014;28:2018–2025. doi: 10.1519/JSC.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 62.Dailey AT, Ghogawala Z, Choudhri TF, Watters WC 3rd, Resnick DK, Sharan A, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 14: brace therapy as an adjunct to or substitute for lumbar fusion. J Neurosurg Spine. 2014;21:91–101. doi: 10.3171/2014.4.SPINE14282. [DOI] [PubMed] [Google Scholar]
- 63.Ilves O, Häkkinen A, Dekker J, Pekkanen L, Piitulainen K, Järvenpää S, et al. Quality of life and disability: can they be improved by active postoperative rehabilitation after spinal fusion surgery in patients with spondylolisthesis. A randomised controlled trial with 12-month follow-up. Eur Spine J. 2017;26:777–784. doi: 10.1007/s00586-016-4789-5. [DOI] [PubMed] [Google Scholar]
- 64.Rushton A, Eveleigh G, Petherick EJ, Heneghan N, Bennett R, James G, et al. Physiotherapy rehabilitation following lumbar spinal fusion: a systematic review and meta-analysis of randomised controlled trials. e000829BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aalto TJ, Leinonen V, Herno A, Alen M, Kröger H, Turunen V, et al. Postoperative rehabilitation does not improve functional outcome in lumbar spinal stenosis: a prospective study with 2-year postoperative follow-up. Eur Spine J. 2011;20:1331–1340. doi: 10.1007/s00586-011-1781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGregor AH, Doré CJ, Morris TP, Morris S, Jamrozik K. ISSLS prize winner: Function After Spinal Treatment, Exercise, and Rehabilitation (FASTER): a factorial randomized trial to determine whether the functional outcome of spinal surgery can be improved. Spine (Phila Pa 1976) 2011;36:1711–1720. doi: 10.1097/BRS.0b013e318214e3e6. [DOI] [PubMed] [Google Scholar]
- 67.Mannion AF, Denzler R, Dvorak J, Müntener M, Grob D. A randomised controlled trial of post-operative rehabilitation after surgical decompression of the lumbar spine. Eur Spine J. 2007;16:1101–1117. doi: 10.1007/s00586-007-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rousing R, Jensen RK, Fruensgaard S, Strøm J, Brøgger HA, Degn JDM, et al. Danish national clinical guidelines for surgical and nonsurgical treatment of patients with lumbar spinal stenosis. Eur Spine J. 2019;28:1386–1396. doi: 10.1007/s00586-019-05987-2. [DOI] [PubMed] [Google Scholar]
- 69.Abbott AD, Tyni-Lenné R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine (Phila Pa 1976) 2010;35:848–857. doi: 10.1097/BRS.0b013e3181d1049f. [DOI] [PubMed] [Google Scholar]
- 70.Oestergaard LG, Nielsen CV, Bünger CE, Sogaard R, Fruensgaard S, Helmig P, et al. The effect of early initiation of rehabilitation after lumbar spinal fusion: a randomized clinical study. Spine (Phila Pa 1976) 2012;37:1803–1809. doi: 10.1097/BRS.0b013e31825a17ab. [DOI] [PubMed] [Google Scholar]
- 71.Tarnanen S, Neva MH, Dekker J, Häkkinen K, Vihtonen K, Pekkanen L, et al. Randomized controlled trial of postoperative exercise rehabilitation program after lumbar spine fusion: study protocol. BMC Musculoskelet Disord. 2012;13:123–123. doi: 10.1186/1471-2474-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monticone M, Ferrante S, Teli M, Rocca B, Foti C, Lovi A, et al. Management of catastrophising and kinesiophobia improves rehabilitation after fusion for lumbar spondylolisthesis and stenosis. A randomised controlled trial. Eur Spine J. 2014;23:87–95. doi: 10.1007/s00586-013-2889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christensen FB, Laurberg I, Bünger CE. Importance of the back-café concept to rehabilitation after lumbar spinal fusion: a randomized clinical study with a 2-year follow-up. Spine (Phila Pa 1976) 2003;28:2561–2569. doi: 10.1097/01.BRS.0000097890.96524.A1. [DOI] [PubMed] [Google Scholar]
- 74.Archer KR, Devin CJ, Vanston SW, Koyama T, Phillips SE, Mathis SL, et al. Cognitive-Behavioral-Based Physical Therapy for Patients With Chronic Pain Undergoing Lumbar Spine Surgery: A Randomized Controlled Trial. J Pain. 2016;17:76–89. doi: 10.1016/j.jpain.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole K, Kruger M, Bates D, Steil G, Zbreski M. Physical demand levels in individuals completing a sports performance-based work conditioning/hardening program after lumbar fusion. Spine J. 2009;9:39–46. doi: 10.1016/j.spinee.2008.07.007. [DOI] [PubMed] [Google Scholar]