Abstract
Cyst nematodes (Globodera spp. and Heterodera spp.) are highly evolved sedentary endoparasites that are considered as harmful pests worldwide. The hatching of the dormant eggs of cyst nematodes occurs in response to hatching factors (HFs), which are compounds that are secreted from the roots of host plants. Solanoeclepin A (SEA), a triterpene compound, has been isolated as HF for potato cyst nematode (PCN) eggs, whereas other compounds, such as steroidal glycoalkaloids (SGAs), are also known to show weak hatching stimulation (HS) activity. However, the structures of both compounds are different and the HF-mediated hatching mechanism is still largely unknown. In the present study, we observed specific hatching of PCN eggs stimulated by the hairy root culture media of potato and tomato, revealing the biosynthesis and secretion of HFs. SGAs, such as α-solanine, α-chaconine, and α-tomatine, showed significant HS activity, despite being remarkably less activities than that of SEA. Then, we evaluated the contribution of SGAs on the HS activities of the hairy root culture media. The estimated SGAs content in the hairy root culture media were low and nonconcordant with the HS activity of those, suggesting that the HS activity of SGAs did not contribute much. The analysis of structure–activity relationship revealed that the structural requirements of the HS activity of SGAs are dependent on the sugar moieties attached at the C3-hydoroxyl group and the alkaloid property of their aglycones. The stereochemistry in the EF rings of their aglycone also affected the strength of the HS activity.
Keywords: hatching factor, hatching of egg, potato cyst nematode, solanoeclepin A, steroidal glycoalkaloids
Introduction
Plant parasitic nematodes are one of the most harmful pests, and their damage to crops is estimated at $US80 billion per year (Nicol et al. 2011). Among them, the most economically important nematodes are root-knot nematodes and cyst nematodes. Cyst nematodes (Globodera spp. and Heterodera spp.) are highly evolved sedentary endoparasites, which cause serious losses in important crop production in over 50 countries (Jones et al. 2013; Nicol et al. 2011; Williamson and Gleason 2003). Unlike other plant parasitic nematodes, such as root-knot nematodes, the host range of cyst nematodes is narrow and strictly host-specific (Jones et al. 2013; Sullivan et al. 2007). For example, soybean cyst nematodes (SCNs), Heterodera glycines Ichinohe, parasitize only a few Fabaceae plants, such as soybean (Glycine max), azuki bean (Vigna angularis), and common bean (Phaseolus vulgaris), and potato cyst nematodes (PCNs) specifically parasitize a few Solanaceae plants, such as potato (Solanum tuberosum) and tomato (Solanum lycopersicum). Two species of PCNs, Globodera rostochiensis and G. pallida, have been reported in Japan. The life cycle of cyst nematodes is highly synchronized to that of the host plants (Nicol et al. 2011). The dormant eggs are covered with a cyst, which is the dead body of female nematode. The hatching of the cyst nematode eggs occurs in response to the hatching factors (HFs) secreted from the host plant roots, and then the second-stage juveniles (J2) migrate to host plant roots. This hatching behavior is a key feature to determine the high host specificity of cyst nematodes by detect the presence of host plants near the cyst nematode eggs.
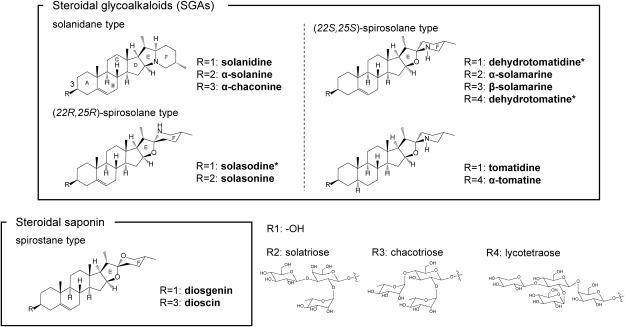
To date, three HFs for SCN, namely glycinoeclepin A (GEA), glycinoeclepin B (GEB) and glycinoeclepin C (GEC), have been identified and isolated from the extracts of common bean roots (Figure 1) (Fukuzawa et al. 1985; Masamune et al. 1982, 1987). In addition, solanoeclepin A (SEA) has been isolated from the root leachates of potatoes and tomatoes and identified as a HF for PCN eggs (Mulder et al. 1992; Schenk et al. 1999). In terms of their chemical structures, all the four HFs are classified as triterpenoids. The total organic synthesis of GEA (Corey and Houpis 1990; Mori and Watanabe 1989; Murai et al. 1988; Shiina et al. 2010; Watanabe and Mori 1991) and SEA (Tanino et al. 2011) has been developed. However, it is difficult to develop the synthetic analogs of HFs because of their structural complexities. Therefore, the HF-mediated hatching mechanism of cyst nematode eggs is still largely unknown. In addition to SEA, it has been reported that several kinds of natural compounds, such as α-solanine and α-chaconine, show weak but significant hatching stimulation (HS) activity toward PCNs eggs (Figure 1) (Byrne et al. 2001; Devine et al. 1996). Both α-Solanine and α-chaconine are well known steroidal glycoalkaloids (SGAs) that are specifically present in Solanaceae plants, and these SGAs are classified as triterpenoids, similar to SEA.
Figure 1. Hatching factors (HFs) of cyst nematode eggs. The upper group includes HFs which were isolated using hatching stimulation (HS) activity as an indicator. The lower group includes HFs reported to show weak but significant HS activity.

In this study, we analyzed the HS activity of culture medias of hairy roots of potato and tomato toward G. rostochiensis eggs. We determined the SGA concentrations in the culture medias, and evaluated the contribution of SGAs (α-solanine, α-chaconine, and α-tomatine) to the detected HS activity. We also investigated the HS activities of other related triterpene compounds and estimated whether SEA and SGAs stimulate the hatching of PCN eggs in a similar manner.
Materials and methods
Chemicals
Solanoeclepin A (SEA) was synthesized as described previously (Tanino et al. 2011). The authentic samples of α-solanine, α-chaconine, solanidine, solasonine, dioscin, and diosgenin were purchased from Sigma-Aldrich Japan (Tokyo, Japan). α-Tomatine and tomatidine were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and ChromaDex (IIrvine, CA, USA), respectively. The contaminants in α-tomatine and tomatidine were removed using high-performance liquid chromatography (HPLC). α-Solamarine and β-solamarine were purified from diploid potato clone 97H32-6 in our laboratory and identified with liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) analyses as described in Supplementary data.
Plant materials and Generation of hairy roots
The tomato plant used in this study was S. lycopersicum cv Micro-Tom (TOMJPF00001), obtained from the National BioResource Program (NBRP) (MEXT, Japan). The surface-sterilized seeds of tomato were germinated and cultivated under the condition of 25°C with 16/8-h light/dark photoperiod on Murashige and Skoog (MS) medium containing 1.5% (w/v) sucrose and 0.3% (w/v) Gelrite (Wako). The potato plant used in this study was S. tuberosum cv Sassy. The Sassy in vitro-grown shoots were grown at a temperature of 20°C with 16/8-h light/dark on MS medium containing 2% (w/v) sucrose and 0.3% (w/v) Gelrite. The common bean plant used in this study was Phaseolus vulgaris cv Honkintoki purchased from Takii & Co. (Kyoto, Japan). The common bean seeds were germinated and cultivated in a way similar to that of the tomato described above. The hairy roots of tomato and potato were obtained by inoculation of the stems with Agrobacterium rhizogenes strain ATC C15834 as described by Thagun et al. (2016). The generation of common bean hairy root was conducted in the same manner as that of the tomato and potato plants, with minor alterations as follows: the 5-day-old seedlings were infected by A. rhizogenes strain K599. The infected seedlings and hairy roots that emerged from the infected sites were subcultured every 2 weeks on a medium containing MS salts, B5 vitamins, 500 µg ml−1 carbenicillin, 2% (w/v) sucrose, and 0.3% (w/v) Gelrite.
SGAs analysis
The hairy roots of tomato and potato were cultured for 14 days with 50 ml of liquid B5 medium containing 2% (w/v) sucrose and 250 µg ml−1 cefotaxime in 200-ml glass flasks. The hairy root media were used for SGAs analysis and hatching assays. The SGAs secreted from the hairy roots were extracted from the 500 µl culture media with equal volume of n-butanol three times. The extracts were evaporated, dissolved in methanol, and analyzed by LC-MS analysis as previously described (Lee et al. 2019). Selected ion monitoring (SIM) modes with m/z 578.6, 868.9, and 852.8 were used to detect α-tomatine, α-solanine, and α-chaconine respectively.
Hatching assays
The common bean hairy roots were suspended for 2 weeks with 50 ml of liquid medium containing MS salts, B5 vitamins, 2% (w/v) sucrose, and 250 µg ml−1 cefotaxime in 200-ml glass flasks. Then, 1 ml of culture media was collected and frozen at −30°C. The hatching stimulation activities of the hairy root media and each SGA compound toward Globodera rostochiensis eggs were investigated. G. rostochiensis pathotype Ro1, a population originally isolated from a field in Kucchan-cho, Hokkaido, Japan, was propagated on greenhouse-grown potato cultivar Irish Cobbler. After the potato had died, the soil containing the cysts was lightly dried and stored at 4°C for 6 months at least in order to break dormancy. The cysts were isolated from the soil by the dry flotation method, picked out from the isolate under a binocular, and incubated in distilled water for 2 weeks at 18°C. Subsequently, an egg suspension was prepared by crushing the cysts and removing broken pieces of cysts, dead eggs, and hatched juveniles as completely as possible. After the egg density of the suspension was adjusted to approximately 600 eggs per milliliter, Tween 20 (polyoxyethylene[20] sorbitan monolaurate) was added to the egg suspension with 0.05% concentration to prevent nematode eggs aggregation. Then, 1 ml of the egg suspension were added to each glass vials (volume; 13.5 ml, inside diameter; 21 mm, height; 50 mm) and 1 ml of each sample was added with double concentration of the test conditions; SGAs: 1 and 5 µM, tomato hairy root culture medium: 1 : 1000, 1 : 5000, and 1 : 25000 dilution, potato hairy root culture medium: 1 : 100, 1 : 500, and 1 : 2500 dilution, common bean hairy root culture medium: 1 : 250 and 1 : 1000 dilution. The glass vials were added 1 ml of distilled water or 1 : 20 diluted B5 medium containing 250 µg ml−1 cefotaxime were prepared as negative controls. We also prepared the glass vials containing SEA with the concentrations of 1 and 100 pg ml−1 as positive controls. Each vial was incubated at 18°C for approximately 2 weeks. Subsequently, 1 ml of each vial was taken out and transferred to Syracuse dishes, and the hatched juveniles and unhatched eggs were counted under a binocular. Although each assay was conducted in duplicate, the negative control assay was conducted in triplicate.
Results and discussion
HS activity of potato and tomato hairy root culture media
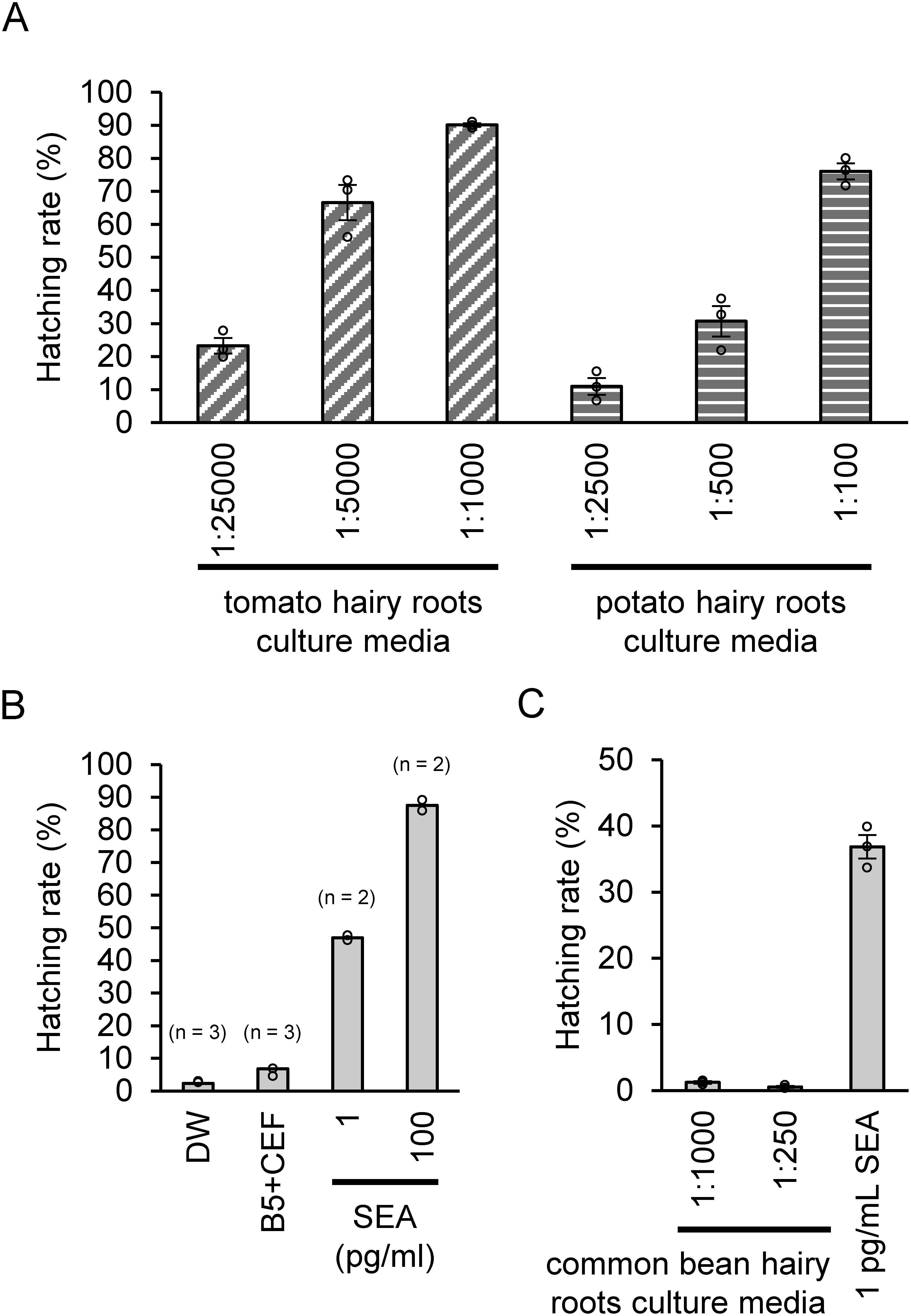
In this study, we used the hairy roots as plant materials because of their fast and bacteria-free growth. We first estimated the HS activity of the potato and tomato hairy root culture media toward PCN (Gr) eggs. Deionized water almost did not induce a hatching response of the PCN eggs, whereas the PCN eggs hatched in response to SEA at a quite low concentration (100 pg ml−1). The tomato and potato hairy root culture media showed HS activity for PCN eggs, whereas the culture media without hairy roots did not show any HS activity (Figure 2A, B). The tomato hairy root culture media showed HS activity that was ten times higher compared with that of the potato hairy root culture media. The solution of tomato culture media that was diluted 1,000 times with water exhibited a HS activity equivalent to 100 pg ml−1 of SEA, suggesting that the tomato hairy root culture media contained HFs that possess a HS activity comparable to SEA. The common bean hairy root culture media showed no significant HS activity for PCN eggs (Figure 2C), indicating that the HFs for PCN eggs are not secreted from the hairy roots of a non-host plant, such as the common bean.
Figure 2. HS activity of the hairy root culture media. The hatching rates (%) of PCN eggs incubated in culture media (A) with host hairy roots, (B) without hairy roots, and (C) with non-host hairy roots are shown. (A) The values are presented as mean±SE The samples were harvested from three independent flasks, and three series of dilution are shown for the culture media. The hatching assay was conducted in duplicate. (B) The values are presented as mean±SE The hatching assay was conducted in triplicate for distilled water (DW) and 1 : 20 diluted B5 medium containing 250 µg ml−1 cefotaxime (B5+CEF), and in duplicate for each concentration of SEA. (C) The hatching assay was conducted in triplicate. Two series of dilutions are shown for the common bean hairy root culture media.
As previously reported by Ashikawa et al. (1991), we showed that bacteria-free culture media of the host plant hairy roots can strongly induce the hatching of the PCN eggs, indicating that the HFs are definitely biosynthesized and secreted by the hairy roots. Hairy roots are well used materials in the field of plant secondary metabolism because of its stable production of metabolites. Moreover, gene disruption using genome editing tools, such as CRISPR/Cas9, has been successfully established (Nakayasu et al. 2018). Therefore, hairy roots are suitable materials to reveal the biological and physiological biosynthesis and secretion of HFs.
HS activity of α-tomatine, α-solanine and α-chaconine
We then analyzed the HS activities of steroidal glycoalkaloids (SGAs), which are one of the most abundant secondary metabolites specifically biosynthesized in Solanaceae plants. The hatching assays were performed at 1 or 5 µM of SGAs in water because the organic solvent inhibits the hatching of the PCN eggs. As previously reported by Devine et al. (1996), α-solanine and α-chaconine at 1 or 5 µM exhibited significant HS activities, and α-tomatine also showed HS activity at 5 µM. The hatching rate treated with 5 µM of α-chaconine was 48.6±1.1%, while SEA at 100 pg ml−1 (about 2 pM) induced the hatching of PCN eggs over 90% (Figures 2, 3), indicating that the HS activities of SGAs are quite weaker compared with that of SEA.
Figure 3. HS activity of steroidal glycoalkaloids (SGAs). The hatching rates (%) of PCN eggs incubated with 0, 1, and 5 µM of α-tomatine, α-solanine, and α-chaconine, respectively, are shown. The values are presented as mean±SE in triplicate.

In order to evaluate the contribution of SGAs to the HS activities detected in the hairy root culture media, we estimated the SGA levels in culture media of tomato and potato hairy roots by LC-MS analysis (Figure 4). α-Tomatine was detected in the tomato hairy root culture media, and its concentration was estimated to be 2.90±0.36 µM. Similarly, α-solanine and α-chaconine were detected in the potato hairy root culture media, and those concentrations were estimated to be 0.14±0.0061 µM and 0.071±0.0033 µM, respectively. The 1 : 1000 diluted solution of the tomato culture media contained less than 1 nM α-tomatine, whereas the hatching rate of the diluted solution was about 90%, indicating that α-tomatine almost do not affect the HS activity detected in the tomato hairy root culture media. Similarly, α-solanine and α-chaconine are of little contribution to the HS activities detected in the potato hairy root culture media. In field-grown plants, the secretion of SGAs could be higher than that in the hairy root culture, and the concentration of SGAs accumulated in the rhizosphere might be affected by their stability and microbial degradation in soils. SGA-modified and/or SGA-deficient plants by gene disruption of SGA biosynthetic genes, which we previously identified and characterized in tomato and potato (Akiyama et al. 2019; Lee et al. 2019; Nakayasu et al. 2017, 2018; Sawai et al. 2014; Umemoto et al. 2016; Yasumoto et al. 2019) can be examined to clarify the contribution of SGAs in the fields for future experiments.
Figure 4. The analysis culture media of (A) tomato and (B) potato hairy roots. The selected ion monitoring (SIM) chromatograms obtained by LC-MS analysis are shown.

Structure–activity relationship of SGAs for HS activity
Next, we analyzed the HS activities of several steroidal compounds, such as SGAs, steroidal saponins, and their aglycones (Figure 5). Tomatidine, the aglycone of α-tomatine, did not induce the hatching of PCN eggs at 1 and 5 µM, and solanidine, that of α-solanine and α-chaconine, also showed little HS activity. These results suggested that the sugar moieties attached at the C3-hydoroxyl group of the aglycones are required for the HS activity by SGAs (Figure 6A). Moreover, α-solamarine, β-solamarine, and solasonine, which are the members of SGAs that accumulated in the Solanum species, possessed significant HS activities, and solasonine showed the strongest HS activity among all the tested SGAs (Figure 6B). The hatching rates of α-chaconine and β-solamarine were higher than that of α-solanine or α-solamarine, respectively, suggesting that chacotriose is more active than solatriose as the sugar moieties attached at the C3-hydoroxyl group of the aglycones (Figure 6B). In contrast, dioscin and its aglycone diosgenin did not show significant HS activity (Figure 6A). The sugar moiety of dioscin is chacotriose, which is identical to those of α-chaconine and β-solamarine, but the aglycone of dioscin does not contain nitrogen. Therefore, these results suggested that the HS activity of SGAs depend not only on the presence of the sugar moieties but also on the alkaloid property of the aglycone. The HS activity of solasonine was more than 5 times stronger than that of α-solamarine; while these two SGAs share the same sugar moieties, namely solatriose (Figure 6B). The aglycones of solasonine and α-solamarine are (22R,25R)-spirosolane and (22S,25S)-spirosolane, respectively, suggesting that the stereochemistry in the EF rings also affects the strength of the HS activity of SGA.
Figure 5. Several kinds of triterpenoid compound are shown. *shows the compound that was not tested in this study.
Figure 6. HS activity of several kinds of triterpene compound. (A) The hatching rates (%) of PCN eggs incubated with 0, 1, and 5 µM of SGAs; steroidal saponins, and their aglycone. The values are presented as mean±SE in triplicate. (B) The hatching rates (%) of PCN eggs incubated with 5 µM SGAs, which were confirmed to exhibit HS activity. The values are presented as mean±SE in triplicate. The different letters indicate statistical differences by Tukey’s test (p<0.05).
Both of SEA and SGAs are classified as triterpenoids; however, their structures are quite different. SEA is a unique and highly oxygenated triterpenoid consisting of heptacyclic skeleton with three- to seven-membered carbocycles. The 6,6-dimethyl-7-oxabicyclo[2.2.1]heptan-2-one in the AB-ring is one of the characteristics of SEA. On the other hands, SGAs have the sugar moieties attached to C3-hydroxyl group in the A-ring, and the aglycone is a canonical steroidal skeleton with a single nitrogen. Our results of the hatching assays using SGAs suggested that the prerequisite of the sugar moieties and nitrogen in the EF ring for the HS activity, whereas SEA does not have a sugar moiety or nitrogen. This inconsistency might be due to the occurrence of different recognition mechanisms for SGAs and SEA by the PCN eggs.
Concluding Remark
Unlike other plant parasitic nematodes, cyst nematodes also establish strict host specificity in terms of the hatching mechanism of their eggs. This feature considerably enables efficient proliferation and alternation of generations. Plants produce diverse kinds of specialized metabolites which have ecological functions in plant-microbe communication in rhizosphere (Sugiyama and Yazaki 2014; van Dam and Bouwmeester 2016). Therefore, they could be suitable indicators of the presence of host plants near the dormant eggs of cyst nematodes. The hatching response of PCN eggs for a broad range of triterpenoid compounds suggested that there might have been co-evolution between triterpenoid biosynthesis in host plants and the recognition mechanism in cyst nematodes, and that cyst nematodes might evolutionally select SEA as a specific HF. Our findings are helpful for further research focusing on the biosynthesis and secretion mechanism of hatching factors, and hairy roots are suitable materials for these studies.
Acknowledgments
This research was partly supported by grants from the Project of the Bio-oriented Technology Research Advancement Institution, NARO (the special scheme project on advanced research and development for next-generation technology), and by JSPS Grant-in-Aid for Scientific Research (C) Grant number JP18K05459.
Abbreviations
- PCN
potato cyst nematode
- HF
hatching factor
- HS
hatching stimulation
- SGA
steroidal glycoalkaloid
- SEA
solanoeclepin A
Supplementary Data
References
- Akiyama R, Lee HJ, Nakayasu M, Osakabe K, Osakabe Y, Umemoto N, Saito K, Muranaka T, Sugimoto Y, Mizutani M (2019) Characterization of steroid 5α-reductase involved in α-tomatine biosynthesis in tomatoes. Plant Biotechnol 36: 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikawa I, Fukuzawa A, Murai A, Kamada H, Koshi M (1991) Production of potato cyst nematode hatching stimulus by hairy root cultures of tomato. Agric Biol Chem 55: 2025–2029 [Google Scholar]
- Byrne JT, Maher NJ, Jones PW (2001) Comparative responses of Globodera rostochiensis and G. pallida to hatching chemicals. J Nematol 33: 195–202 [PMC free article] [PubMed] [Google Scholar]
- Corey EJ, Houpis IN (1990) Total synthesis of glycinoeclepin A. J Am Chem Soc 112: 8997–8998 [Google Scholar]
- Devine KJ, Byrne J, Maher N, Jones PW (1996) Resolution of natural hatching factors for golden potato cyst nematode, Globodera rostochiensis. Ann Appl Biol 129: 323–334 [Google Scholar]
- Fukuzawa A, Matsue H, Ikura M, Masamune T (1985) Glycinoeclepins B and C, nortriterpenes related to glycinoeclepin A. Tetrahedron Lett 26: 5539–5542 [Google Scholar]
- Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-Lopez R, Palomares-Rius JE, Wesemael WML, et al. (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14: 946–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Nakayasu M, Akiyama R, Kobayashi M, Miyachi H, Sugimoto Y, Umemoto N, Saito K, Muranaka T, Mizutani M (2019) Identification of a 3β-hydroxysteroid dehydrogenase/3-ketosteroid reductase involved in α-tomatine biosynthesis in tomato. Plant Cell Physiol 60: 1304–1315 [DOI] [PubMed] [Google Scholar]
- Masamune T, Anetai M, Takasugi M, Katsui N (1982) Isolation of a natural hatching stimulus, glycinoeclepin A, for the soybean cyst nematode. Nature 297: 495–496 [Google Scholar]
- Masamune T, Fukuzawa A, Furusaki A, Ikura M, Matsue H, Kaneko T, Abiko A, Sakamoto N, Tanimoto N, Murai A (1987) Glycinoeclepins, natural hatching stimuli for the soybean cyst nematode, Heterodera glycines. II. Structural elucidation. Bull Chem Soc Jpn 60: 1001–1014 [Google Scholar]
- Mori K, Watanabe H (1989) Recent results in the synthesis of semiochemicals: Synthesis of glycinoeclepin A. Pure Appl Chem 61: 543–546 [Google Scholar]
- Mulder JG, Diepenhorst P, Plieger P, Bruggemann-Rotgans IEM (1992) Hatching agent for the potato cyst nematode. CT Int. Appl. WO 93 02 083. Chem Abstr 118: 185844z [Google Scholar]
- Murai A, Tanimoto N, Sakamoto N, Masamune T (1988) Total synthesis of glycinoeclepin A. J Am Chem Soc 110: 1985–1986 [Google Scholar]
- Nakayasu M, Akiyama R, Lee HJ, Osakabe K, Osakabe Y, Watanabe B, Sugimoto Y, Umemoto N, Saito K, Muranaka T, et al. (2018) Generation of α-solanine-free hairy roots of potato by CRISPR/Cas9 mediated genome editing of the St16DOX gene. Plant Physiol Biochem 131: 70–77 [DOI] [PubMed] [Google Scholar]
- Nakayasu M, Umemoto N, Ohyama K, Fujimoto Y, Lee HJ, Watanabe B, Muranaka T, Saito K, Sugimoto Y, Mizutani M (2017) A Dioxygenase catalyzes steroid 16α-hydroxylation in steroidal glycoalkaloid biosynthesis. Plant Physiol 175: 120–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol JM, Turner SJ, Coyne DL, den Nijs L, Hockland S, Maafi ZT (2011) Current nematode threats to world agriculture. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and Molecular Genetics of Plant-Nematode Interactions. Springer, Dordrecht, pp 21–43
- Sawai S, Ohyama K, Yasumoto S, Seki H, Sakuma T, Yamamoto T, Takebayashi Y, Kojima M, Sakakibara H, Aoki T, et al. (2014) Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant Cell 26: 3763–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk H, Driessen RAJ, de Gelder R, Goubitz K, Nieboer H, Brüggemann-Rotgans IEM, Diepenhorst P (1999) Elucidation of the structure of Solanoeclepin A, a natural hatching factor of potato and tomato cyst nematodes, by single-crystal X-ray diffraction. Croat Chem Acta 72: 593–606 [Google Scholar]
- Shiina Y, Tomata Y, Miyashita M, Tanino K (2010) Asymmetric total synthesis of glycinoeclepin A: Generation of a novel bridgehead anion species. Chem Lett 39: 835–837 [Google Scholar]
- Sugiyama A, Yazaki K (2014) Flavonoids in plant rhizospheres: Secretion, fate and their effects on biological communication. Plant Biotechnol 31: 431–443 [Google Scholar]
- Sullivan MJ, Inserra RN, Franco J, Moreno-Leheude I, Greco N (2007) Potato cyst nematodes: Plant host status and their regulatory impact. Nematropica 37: 193–202 [Google Scholar]
- Tanino K, Takahashi M, Tomata Y, Tokura H, Uehara T, Narabu T, Miyashita M (2011) Total synthesis of solanoeclepin A. Nat Chem 3: 484–488 [DOI] [PubMed] [Google Scholar]
- Thagun C, Imanishi S, Kudo T, Nakabayashi R, Ohyama K, Mori T, Kawamoto K, Nakamura Y, Katayama M, Nonaka S, et al. (2016) Jasmonate-responsive ERF transcription factors regulate steroidal glycoalkaloid biosynthesis in tomato. Plant Cell Physiol 57: 962–975 [DOI] [PubMed] [Google Scholar]
- Umemoto N, Nakayasu M, Ohyama K, Yotsu-Yamashita M, Mizutani M, Seki H, Saito K, Muranaka T (2016) Two Cytochrome P450 monooxygenases catalyze early hydroxylation steps in the potato steroid glycoalkaloid biosynthetic pathway. Plant Physiol 171: 2458–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam NM, Bouwmeester HJ (2016) Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci 21: 256–265 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Mori K (1991) Triterpenoid total synthesis: Part 2. Synthesis of glycinoeclepin A, a potent hatching stimulus for the soybean cyst nematode. J Chem Soc, Perkin Trans 1 12: 2919–2934 [Google Scholar]
- Williamson VM, Gleason CA (2003) Plant–nematode interactions. Curr Opin Plant Biol 6: 327–333 [DOI] [PubMed] [Google Scholar]
- Yasumoto S, Umemoto N, Lee HJ, Nakayasu M, Sawai S, Sakuma T, Yamamoto T, Mizutani M, Saito K, Muranaka T (2019) Efficient genome engineering using Platinum TALEN in potato. Plant Biotechnol 36: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.