Abstract
Lipocalins are very important proteins for stress resistance in plants. To better understand the function of tomato lipocalins, we observed responses to oxidative stress using over-expressed SlTIL1, SlTIL2, SlCHL, and silenced-plants. Significant differences in reactive oxygen species accumulation (oxidative damage) were observed in all tested plants under heat stress. Plants with over-expressed SlTIL1, SlTIL2, and SlCHL showed less oxidative damage compared with wild-type plants under heat stress. The expression of SlSODs was induced in over-expressed SlTIL1, SlTIL2, and SlCHL plants under normal and heat stress conditions. Furthermore, silenced PDS, SlTILs, and SlCHL plants showed slightly increasing oxidative damage under heat stress alongside with lower SlSODs under normal and stress conditions. These results suggest that SlTIL1, SlTIL2, and SlCHL were involved in antioxidant defense by eliminating ROS in tomato plants.
Keywords: gene silencing, lipocalins, SlCHL, SlTILs, tomato
Increasing temperatures are known to play an important role in yield reduction (Lipiec et al. 2013), suggesting that climate change and a rise in global temperatures will significantly impact global food security. Comprehensive research on plant heat tolerance, stress response, and adaptive capacity is essential (Buchner et al. 2013). Heat stress causes the formation of reactive oxygen species (ROS) in plants (Munne Bosch and Penuelas 2003; Pucciariello et al. 2012). Excessive ROS production can irreversibly damage plant cells through the oxidation of cellular components, such as lipids, proteins, and DNA (Apel and Hirt 2004). Therefore, excessive ROS needs to be removed as soon as possible. Several studies have examined the various ROS prevention and repair mechanisms, which exist to protect plants against heat stress (Larkindale and Knight 2002; Suzuki and Mittler 2006). To prevent ROS accumulation, plants have developed detoxification enzymes (e.g., superoxide dismutase, catalase, peroxidase, glutathione reductase, ascorbate peroxidase) and non-enzymatic antioxidants (e.g., ascorbate, glutathione, carotene, and tocopherol; Gill and Tuteja 2010; Suzuki et al. 2012). Anthocyanins are a class of flavonoids with antioxidative properties, due to the presence of hydroxyl groups in aglycon moiety (Yokozawa et al. 1998). In tropical countries such as Indonesia, heat stress is a major environmental factor that limits wheat crop productivity (Altuhaish et al. 2014).
Temperature-induced lipocalin (TIL) proteins have been identified from wheat and Arabidopsis plants (Charron et al. 2002). Lipocalins are a group of proteins found in bacteria, invertebrates, and vertebrate animals, which play a major role in response to environmental stress, membrane formation and fixation, apoptotic induction, regulation of immune response, cell growth, and metabolic adjustment. Recent studies related to lipocalin show that it is involved in the tolerance response to abiotic oxidative stress (He et al. 2015; Levesque-Tremblay et al. 2009). However, this protein and its various functions remain mostly unknown. According to Charron et al. (2005), the tissue specifications of Triticum aestivum TIL (TaTIL), Triticum aestivum CHL (TaCHL); and their accumulated transcripts protect plants against stress damage. Lipocalin chloroplastic in Arabidopsis (AtCHL) is involved in the antioxidative response of Arabidopsis leaves against light-induced oxidative stress. Some lipocalin members are thought to be found in chloroplasts (Levesque-Tremblay et al. 2009). The cell structure of vascular bundles in over-expressed SlTILs and SlCHL consists of wider (tenuous) cells compared to wild-type vascular bundle cells. These findings indicate that cells in over-expressed SlTILs and SlCHL grow more rapidly (Wahyudi et al. 2019). Lipocalins in Arabidopsis (AtTIL and AtCHL) play a major role in lipid protection, which is essential for stress resistance and survival (Boca et al. 2014).
In this study, we report that tomato lipocalins are involved in heat stress and oxidative tolerance using over-expressed and silenced SlTIL1, SlTIL2, SlCHL plants.
This study used planting material: the “Micro-Tom” plant (Solanum lycopersicum cv “Micro-Tom”). “Micro-Tom” seeds were obtained from University of Tsukuba, Japan. The fruit ripening process was divided into four stages: the green fruit stage (30–33 DPA), the yellow fruit stage (32–33 DPA), the orange fruit stage (33–35 DPA), and the red fruit stage (41–45 DPA) (Suzuki et al. 2015). Leaves, flowers, roots, and fruits from each stage were frozen in liquid nitrogen, then stored at −80°C until further analysis. The samples were then planted under 24–28°C, 60±10% relative humidity, and light : dark cycle of 16 : 8 h. Their cultivation was carried out using a pot with sterile soil media and a hydroponic system using half-aqueous Enshi formula [808 mg l−1 KNO3, 492 mg l−1 MgSO47H2O, 944 mg l−1 Ca(NO3)4H2O, 152 mg l−1 NH4H2PO4, and 50 mg l−1 Otsuka house 5 (Otsuka Agri Techno. Co., Ltd,.)]. The heat treatment was carried out under the above conditions after heating at 37°C for 5 days.
Qualitatively, the level hydrogen peroxide (H2O2) was determined using a 2,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) assay. The H2DCFDA test to detect ROS (predominantly H2O2) was carried out according to the Kaur method (Kaur et al. 2016; www.bio-protocol.org/e2061). For the detection of O2− and H2O2, over-expressed and silenced SlTILs and SlCHL plant leaf samples were cut, then immediately immersed in a 2-ml 6 mM NBT solution prepared in sodium citrate (pH 6) in a petri dish (35 mm) using tweezers. Samples were then infiltrated for 10 min at a pressure of 60 kPa, then incubated at room temperature for 10 min under ambient light. After incubation, samples were dipped in absolute ethanol, then stored in a water bath (100°C) until chlorophyll was completely removed from the cells. Samples were dipped to be cooled in 20% glycerol, then observed using a stereomicroscope under magnification up to 40× objective.
In the H2DCFDA assay, samples were observed under a confocal microscope (LSM 700, Carl Zeiss) using a laser beam of a 488-nm wavelength. Green fluorescence indicated the presence of H2O2, while red was the autofluorescence of chlorophyll.
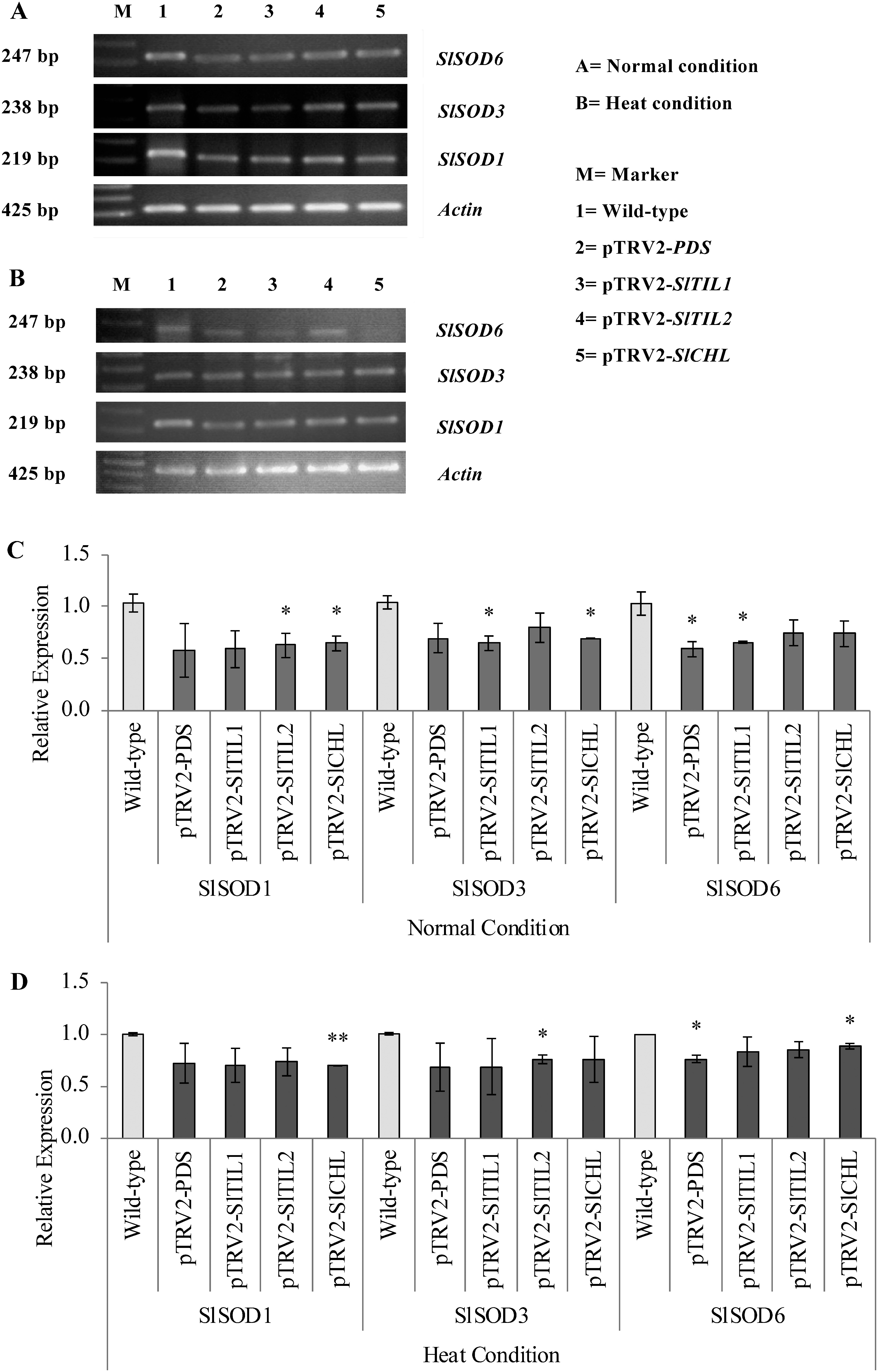
In this study, the expression of superoxide dismutases (SODs) genes was detected using the RT-PCR method. Total RNA was isolated from the leaves and fruits using the RNeasy mini kit (Qiagen, Germany) following the manufacturer’s instructions, then treated extensively with RNase-free DNase I. First-strand cDNA was synthesized from 1 µg total RNA using PrimeScript™ first-strand cDNA synthesis kit (TaKaRa, Japan). PCR was carried out under standard conditions: 30 cycles of 10 s at 98°C, 15 s at 55°C, and 1 min at 68°C, using the primers SlSOD1-F (5′-TCT GGC CTA AAA CCT GGA CT-3′), SlSOD1-R (5′-ACC AGT GAG AGG AAT CTG CT-3′); SlSOD3-F (5′-CTC CTG GAC TTC ACG GGT TT-3′), SlSOD3-R (5′-CAC AAG TGC TCG TCC AAC AA-3′), SlSOD6-F (5′-AGG ACA GCC ATC TGG TGA AC-3′), and SlSOD6-R (5′-TGG CGA GTA ATC CCA AAC GA-3′). ACTIN cDNA (425 bp) as an internal standard of gene expression was amplified using Actin-F (5′-AGA TGG TGT CAG CCA CAC AG-3′) and Actin-R (5′-ACC ACC ACT GAG GAC GAT GT-3′). RT-PCR analyses were performed in triplicates.
The results of RT-PCR were quantified using the ImageJ software and normalized with the value of ACTIN. The gene expression of different tissues in wild-type plants was used as calibrators (value=1). The data shown are the mean values for the three separate experiments±standard deviation. * and ** indicate significant differences from the wild-type at the p<0.05 and p<0.01 levels, respectively, as calculated by the Student’s t-test.
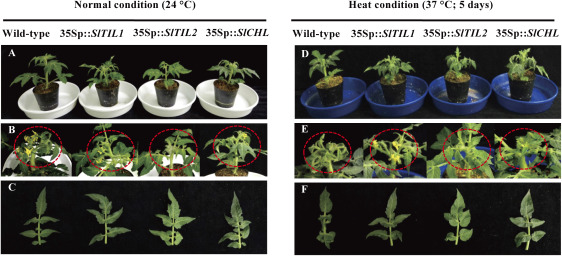
When the morphological phenotypes were compared with over-expressed SlTIL1, SlTIL2, SlCHL, and wild-type plants under heat stress, over-expressed SlTILs and SlCHL plants showed leaves curling but no wilting. Their over-expressed plants had bigger flowers and fruits, with fruits and seeds emerging earlier after 5 days of heat stress. The wild-type phenotype showed leaf wilting after heat stress treatment (Figure 1). Furthermore, we used the pTRV-based VIGS system in these studies to silence PDS, SlTIL1, SlTIL2, and SlCHL. The silenced PDS plant was used as a positive control for the VIGS system, inhibiting carotenoid biosynthesis and in turn causing a photo-bleached phenotype in leaves, flowers, and fruits in both conditions (Figure 2). The white-colored regions on the leaves and petals showed that PDS was silenced by VIGS at least until the flowering stage. The silenced SlTIL1, SlTIL2, and SlCHL plants showed different phenotypes compared with wild-type plants used as control under heat stress (Figure 2). Silenced SlTIL1, SlTIL2, and SlCHL led to delayed fruit ripening (Figure 2). SlTIL1, SlTIL2, SlCHL and PDS silenced-plants showed leaves with curling, wilting and shorter leaves compared to wild-type plants under heat stress (Figure 2B, D). Silenced plant growth was slower compared than that in wild-type plants (Figure 2).
Figure 1. Phenotypes of over-expressed SlTIL1, SlTIL2, and SlCHL plants under normal (24°C) and heat stress conditions (37°C for 5 days). (A, D) Phenotypes of over-expressed plants grown for 2 weeks after transplantation; (B, E) phenotypes of over-expressed plants after 5 days of heat treatment; (C, F) expanded leaves of over-expressed plants.
Figure 2. Phenotypes of silenced-plants grown in soil under normal (24°C) and heat stress conditions (37°C for 5 days). (A, C) Phenotypes of silenced-plants grown for two weeks after infiltration; (B, D) expanded leaves of silenced-plants.
In a previous study, over-expressed SlTILs and SlCHL plants subjected to light stress showed early flowering and fruit set, an increased number of flowers, inflorescences, and fruits, as well as larger peduncle, flower, fruit, and leaves with curling, longer terminal leaflet, bullwhip phenotype compared to the wild-type plants (Wahyudi et al. 2018). The over-expressed SlTIL1 showed the longest leaves with specific curling. The over-expressed SlTIL2 flowered earlier and the over-expressed SlCHL ripened earlier (Wahyudi et al. 2018). In this study, the phenotypes of over-expressed SlTIL1, SlTIL2, and SlCHL plants showed curling — but not wilting — leaves, bigger flower and fruits, early fruit setting, and making seed under heat stress for 5 days (Figure 1). The silenced SlTIL1, SlTIL2, and SlCHL under high light stress in Wahyudi et al. (2018) had patchy fruit coloring with areas exhibiting different shades of yellow and red color. The lipocalins-silenced-plants exhibited symptoms of the stress condition (e.g., leaf curling and faster leaf senescence). Moreover, silenced-plants grew slower than wild-type plants (Figure 2), implying that the SlTIL1, SlTIL2, and SlCHL genes may play an important role in the oxidative stress response and Arabidopsis chloroplastic lipocalin AtCHL (Abo-Ogiala et al. 2014; Levesque-Tremblay et al. 2009).
Superoxide ions (O2−) and hydrogen peroxide (H2O2) are vital ROS molecules involved in plant growth and development, including abiotic stress tolerance (Kaur et al. 2016). To investigate the effects of lipocalins on ROS, the results of NBT staining and H2DCFDA assay showed that the levels of ROS in over-expressed SlTILs and SlCHL plants under heat stress were lower than wild-type plants (Figures 3, 4), suggesting that the over-expressed SlTILs and SlCHL plants had a higher tolerance for ROS under heat stress. On the other hand, NBT staining and H2DCFDA assays in SlTILs and SlCHL silenced-plants showed that their ROS levels were slightly higher than wild-type plants under heat stress (Figures 3, 4). The superoxide ions were accumulated at the lamina and top of leaves in wild-type plants under heat stress, but they were accumulated at all part of leaves (petiole, vein and lamina) in the silenced-plants (Figure 3). These results suggested that increasing expression of SlTILs and SlCHL was effective in removing ROS.
Figure 3. Stereomicroscope image of the NBT assay of over-expressed SlTIL1, SlTIL2, and SlCHL leaves under normal (24°C) and heat stress conditions (37°C for 5 days). The presence of ROS (predominantly superoxide) is indicated by the presence of blue colored formazan. Scale bar=50 mm.
Figure 4. Confocal H2DCFDA staining images of over-expressed SlTIL1, SlTIL2, and SlCHL leaves under heat stress conditions (37°C for 5 days). The green color indicates ROS (predominantly hydrogen peroxide); the red color indicates chlorophyll; merge indicates merged ROS and chlorophyll. Scale bar=50 µm.
To better understand the relation of lipocalins and superoxide dismutase (SOD), the expression of SlSODs was determined in over-expressed and silenced SlTIL1, SlTIL2, and SlCHL plants by RT-PCR. In over-expressed SlTIL1, SlTIL2, and SlCHL plants, SlSOD1, SlSOD3, and SlSOD6 were highly expressed in the leaves under heat stress (Figure 5). Furthermore, the expression of SlSOD1, SlSOD3, and SlSOD6 dramatically decreased in the leaves of silenced SlTIL1, SlTIL2, and SlCHL plants (Figure 6). The expression of SlSOD1, SlSOD3, and SlSOD6 also decreased in silenced PDS plants. Silenced PDS plants, used as a positive control for the VIGS system, did not accumulate carotenoids and exhibited a photo-breaching phenotype. Carotenoids are known to prevent singlet oxygen and lipid peroxidation and affect redox status (Cao et al. 2015; Shimizu et al. 1996). SODs are critical antioxidant enzymes which protect organisms from ROS produced under adverse conditions (i.e., stress-induced) and are widely found in the cytoplasm, chloroplast, and mitochondria of eukaryotic and prokaryotic cells (Feng et al. 2016). In recent years, studies have reported that SODs can protect plants against abiotic and biotic stress such as heat, cold, drought, salinity, abscisic acid, and ethylene (Feng et al. 2016). In this study, the expression analyses of SlSODs (SlSOD1, SlSOD3, and SlSOD6) showed high expression in the leaves of over-expressed SlTIL1, SlTIL2, and SlCHL plants (Figure 5), but low expression in the silenced SlTIL1, SlTIL2, and SlCHL plants (Figure 6).
Figure 5. The expression of SlSODs in the leaves of over-expressed SlTIL1, SlTIL2, and SlCHL plants. (A, C) The expression and relative expression of SlSODs under normal conditions (24°C); (B, D) the expression and relative expression of SlSODs under heat stress conditions (37°C for 5 days).

Figure 6. The expression of SlSODs in the leaves of silenced SlTIL1, SlTIL2, and SlCHL plants. (A, C) The expression and relative expression of SlSODs under normal conditions (24°C); (B, D) the expression and relative expression of SlSODs under heat stress conditions (37°C for 5 days).
The generation of ROS is a common cellular response under stressful conditions. H2O2 is a central signaling molecule in the stress-induced response in plants (Neill et al. 2002). On the other hand, the over-production of ROS might cause serious oxidative damage, which can alter the normal functioning of plant metabolism (Jaspers and Kangasjarvi 2010). In this study, over-expressed SlTIL1, SlTIL2, and SlCHL plants showed lower oxidative damage under heat stress, similarly to findings reported in the literature. The over-expressed plants did not accumulate ROS under normal conditions or heat stress (Figures 3, 4) and increased the expression of SlSODs (Figure 5). Silenced SlTIL1, SlTIL2, and SlCHL plants increased oxidative damage, ROS under normal condition and heat stress (Figures 3, 4) and decreased the expression of SlSODs (Figure 6). These results of SlSODs expression indicated that over-expressed SlTILs and SlCHL plants could induce the antioxidant defense system to eliminate ROS, suggesting that lipocalins play an important role in the abiotic oxidative tolerance in tomato. These findings contribute to our understanding of the functional analyses of lipocalin proteins in tomato and provide clues for the study of abiotic stress response such as heat stress and oxidative tolerance in tomato.
Acknowledgments
We would like to thank Prof. Nakagawa (Shimane University) for providing pGWB8 vectors to make over-expressed tomato plants. This work was also supported by the Ministry of Education, Culture, Sports, Science and Technology (Japan) [Grants-in-Aid for Scientific Research (No. 23580039 to R M)] and Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Directorate General of Resources for Research, Technology, and Higher Education.
Abbreviations
- CHL
chloroplastic lipocalin
- PDS
phytoenedesaturase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TIL
temperature-induced lipocalin
- VIGS
virus-induced gene silencing
References
- Abo-Ogiala A, Carsjens C, Diekmann H, Fayyaz P, Herrfurth C, Feussner I, Polle A (2014) Temperature-induced lipocalin (TIL) is translocated under salt stress and protects chloroplasts from ion toxicity. Plant Physiol 171: 250–259 [DOI] [PubMed] [Google Scholar]
- Altuhaish AAK, Miftahudin, Trikoesoemaningtyas, Yahya S (2014) Field adaptation of some introduced wheat (Triticum aestivum L.) genotypes in two altitudes of tropical agro-ecosystem environment of Indonesia. Hayati J Biosci 21: 31–38 [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Boca S, Koestler F, Ksas B, Chevalier A, Leymarie S, Fekete A, Mueller MJ, Havaux M (2014) Arabidopsis lipocalins AtCHL and AtTIL have distinct but overlapping functions essential for lipid protection and seed longevity. Plant Cell Environ 37: 368–381 [DOI] [PubMed] [Google Scholar]
- Buchner O, Karadar M, Bauer I, Neuner G (2013) A novel system for in situ determination of heat tolerance of plants first results on alpine dwarf shrubs. Plant Methods 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Wang J, Dong X, Han Y, Ma Q, Ding Y, Zhao F, Zhang J, Chen H, Xu Q, et al. (2015) Carotenoid accumulation affects redox status, starch metabolism, and flavonoid/anthocyanin accumulation in citrus. Plant Biol 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron JBF, Breton G, Badawi M, Sarhan F (2002) Molecular and structural analyses of a novel temperature stress-induced lipocalin from wheat and Arabidopsis. FEBS Lett 517: 129–132 [DOI] [PubMed] [Google Scholar]
- Charron JBF, Ouellet F, Pelletier M, Danyluk J, Chauve C, Sarhan F (2005) Identification, expression, and evolutionary analyses of plant lipocalins. Plant Physiol 139: 2017–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K, Yu J, Cheng Y, Ruan M, Wang R, Ye Q, Zhou G, Li Z, Yao Z, Yang Y, et al. (2016) The SOD gene family in tomato: Identification, phylogenetic relationships, and expression patterns. Front Plant Sci 7: 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48: 909–930 [DOI] [PubMed] [Google Scholar]
- He X, Sambe MAN, Zhuo C, Tu Q, Guo Z (2015) A temperature induced lipocalin gene from Medicago falcata (MfTIL1) confers tolerance to cold and oxidative stress. Plant Mol Biol 87: 645–654 [DOI] [PubMed] [Google Scholar]
- Jaspers P, Kangasjarvi J (2010) Reactive oxygen species in abiotic stress signaling. Physiol Plant 138: 405–413 [DOI] [PubMed] [Google Scholar]
- Kaur N, Dhawanm M, Sharma I, Pati PK (2016) Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol 16: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque-Tremblay G, Havaux M, Ouellet F (2009) The chloroplastic lipocalin AtCHL prevents lipid peroxidation and protects Arabidopsis against oxidative stress. Plant J 60: 691–702 [DOI] [PubMed] [Google Scholar]
- Lipiec J, Doussan C, Nosalewicz A, Kondracka K (2013) Effect of drought and heat stresses on plant growth and yield: A review. Int Agrophys 27: 463–477 [Google Scholar]
- Munne Bosch S, Penuelas J (2003) Photo and antioxidative protection during summer leaf senescence in Pistacialentiscus L. grown under Mediterranean field conditions. Ann Bot 92: 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5: 388–395 [DOI] [PubMed] [Google Scholar]
- Pucciariello C, Banti V, Perata P (2012) ROS signaling as common element in low oxygen and heat stresses. Plant Physiol Biochem 59: 3–10 [DOI] [PubMed] [Google Scholar]
- Shimizu N, Goto M, Miki W (1996) Carotenoids as singlet oxygen quenchers in marine organisms. Fish Sci 62: 134–137 [Google Scholar]
- Suzuki M, Takahashi S, Kondo T, Dohra H, Ito Y, Kiriiwa Y, Hayashi M, Kamiya S, Kato M, Fujiwara M, et al. (2015) Plastid proteomic analysis in tomato fruit development. PLoS One 10: e0137266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) Ros and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35: 259–270 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant 126: 45–51 [Google Scholar]
- Wahyudi A, Ariyani D, Gang M, Inaba R, Fukasawa C, Nakano R, Motohashi R (2018) Functional analyses of lipocalin proteins in tomato. Plant Biotechnol 35: 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahyudi A, Fukasawa C, Motohashi R (2019) Cytological analyses of over-expressed SlTILs and SlCHL in tomato. AIP Conf Proc 2202: 020080 [Google Scholar]
- Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I (1998) Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl. Biochem Pharmacol 56: 213–222 [DOI] [PubMed] [Google Scholar]