Abstract
Camelina sativa is a Brassicaceae oilseed plant used as a biotechnology platform for biofuel and healthy vegetable oil. As Camelina is closely related to the model plant Arabidopsis, the genetic tools of Arabidopsis are considered useful when applied to Camelina. Myosin XI-2 is one of the major motive forces driving cytoplasmic streaming in Arabidopsis. In our previous study, high-speed chimeric myosin XI-2, a myosin XI-2 artificially modified by genetically exchanging the motor domain of Arabidopsis myosin XI-2 with the faster Chara myosin XI, was shown to accelerate cytoplasmic streaming and promote plant growth in Arabidopsis. Here, we heterologously transformed this high-speed Chara-Arabidopsis chimeric myosin XI-2 gene in Camelina. The transgenic plants exhibited not only enhancement of leaf development and main stem elongation but also early flowering and seed setting, indicating that the high-speed chimeric myosin XI-2 can improve plant growth in Camelina. Interestingly, total seed yield was significantly increased in the transgenic plants as the total seed number increased. Our results suggest that the high-speed myosin XI system might also be effective to improve the growth of other closely related plant species.
Keywords: Camelina, heterologous transformation, high-speed chimeric myosin XI-2, plant growth, seed yield
Introduction
Cytoplasmic streaming, an active cytoplasmic flow occurring in a broad range of plant cells, is generated by the sliding of motor protein myosin XI associated with organelles along the actin cytoskeleton (Shimmen 2007). In Arabidopsis thaliana, there are 13 isotypes of myosin XI: XI-1, XI-2, XI-A, XI-B, XI-C, XI-D, XI-E, XI-F, XI-G, XI-H, XI-I, XI-J, and XI-K (Tominaga and Nakano 2012). Previous gene knockout studies revealed that several Arabidopsis myosins XI (XI-1, XI-2, XI-B, XI-I, and XI-K) are responsible for the movement of organelles, such as the endoplasmic reticulum, Golgi stacks, peroxisomes, and mitochondria (Peremyslov et al. 2010; Prokhnevsky et al. 2008; Ueda et al. 2010). Among these myosins XI, XI-2 and XI-K are considered to be the major motive forces for generating cytoplasmic streaming because only xi-2 and xi-k single knockouts exhibited defects in organelle trafficking and root hair growth (Peremyslov et al. 2008). Furthermore, triple and quadruple knockouts (among xi-1, xi-2, xi-b, xi-i, and xi-k) displayed additive defects in organelle movement and plant development (Ojangu et al. 2012; Peremyslov et al. 2010). These results suggest that cytoplasmic streaming plays an essential role in plant development.
The predicted molecular structure of Arabidopsis myosin XI is composed of a conserved motor domain with ATPase and actin-binding activities, a neck domain comprising six tandem repeats of IQ motifs, a coiled-coil domain for dimerization, and a globular tail domain that binds cargo (Tominaga and Nakano 2012). Using the motor domain, myosin covert chemical energy via ATP hydrolysis into physical movement along actin filaments (Ito et al. 2007, 2009). Thus, the velocity of myosin is mainly determined by enzymatic properties of its motor domains. To reveal the physiological role of cytoplasmic streaming velocity in plant development, we developed high-speed chimeric myosin XI-2 by genetically exchanging the motor domains of Arabidopsis myosin XI-2 with those of faster Chara myosin XI. Interestingly, cytoplasmic streaming velocity and plant size were found to be increased in plants with the high-speed chimeric myosin XI-2. These results suggest that cytoplasmic streaming is a key regulator determining plant size (Tominaga et al. 2013). Because cytoplasmic streaming is a common phenomenon from algae to angiosperm, we consider that this high-speed myosin XI system might also be effective to improve the growth of other plant species and could be applied to enhancing the growth of plant resources related to crops and biomass energy.
Camelina sativa is an oilseed crop of the Brassicaceae family used for both food and non-food applications. Camelina oil is a potential healthy vegetable oil for food because of its high level of alpha-linolenic acid, which is an essential omega-3 fatty acid (Abramovic and Abram 2005). Thus, it has been recommended that Camelina oil be included in the diet for its cardiovascular benefits (Gebauer et al. 2006). On the other hand, Camelina oil has also been exploited for soap and varnish (Putnam et al. 1993; Zubr 1997). In recent years, Camelina has also attracted a lot of attention because its oil can be efficiently processed into high-quality renewable fuels such as biodiesel as well as jet fuel (Li and Mupondwa 2014). Thus, improving the seed yield of Camelina should enhance oil production, generating potential commercial value. Camelina is acceptable to Agrobacterium-mediated transformation by floral dip infiltration under vacuum, which is similar with a procedure commonly used in Arabidopsis (Lu and Kang 2008). As the close relationship between Camelina and Arabidopsis suggests good competency, the heterologous transformation of Arabidopsis genes in Camelina is considered to be a useful tool. Here, we heterologously transformed the Arabidopsis high-speed chimeric myosin XI-2 in Camelina plants to examine the effect on plant growth and seed yield.
Materials and methods
Plant materials and growth conditions
Camelina sativa (Linicola strain) was used in all experiments. Seeds were sown on peat moss (SUPERMIX-A; Sakata Seed Corporation, Yokohama, Japan) and vermiculite (NITTAI, Japan) mixture (1 : 1) irrigated with water and chilled for 48 h at 4°C in the dark. Plants were grown at 23°C with 30% relative humidity under light conditions (90 µmol m−2 s−1 photon flux) on a 16-h day/8-h night cycle.
Camelina transformation
GFP-fused Chara-Arabidopsis high-speed chimeric myosin XI-2 driven by myosin XI-2 promoter (ProXI-2: sGFP: high-speed chimeric myosin XI-2) in pGWB501 was transformed into the Agrobacterium tumefaciens strain GV3101::pMP90 by electroporation using a Gene Pulser (Bio-Rad, Tokyo, Japan), as described previously (Tominaga et al. 2013). It was then introduced into wild-type Camelina plants by the floral dipping method, via a slightly modified version of a previously described procedure (Lu and Kang 2008). Camelina inflorescences were immersed in Agrobacterium-containing solution for 30 s. The immersed flowers were then exposed to a vacuum at about 85 kPa for 5 min.
PCR analysis of transgenic Camelina plants
DNA was extracted from cotyledons using QuickGene Mini80 system and QuickGene DNA Tissue Kit S (Kurabo, Osaka, Japan), in accordance with the manufacturer’s instructions. Transgenic plants were identified by PCR using the following primers for ProXI-2: sGFP: high-speed myosin XI-2 (5′-TTC TCC CAC ATG CAC ATG-3′ and 5′-GTC GTG CTG CTT CAT GTG GTC-3′).
RNA extraction and RT-PCR
Seven-day-old seedlings were frozen in liquid nitrogen. The frozen samples were first pulverized, and total RNA was extracted from the frozen leaves using the RNeasy Plant Mini kit (Qiagen, Tokyo, Japan). cDNA was reverse transcribed from the total RNA using SuperScript IV reverse transcriptase (Invitrogen Corporation, Japan). RT-PCR was carried out with following gene-specific primers: Camelina ACTIN11 gene: 5′-ACA ATT TCC CGC TCT GCT GTT GTG-3′ and 5′-AGG GTT TCT CTC TTC CAC ATG CCA-3′, sGFP: high-speed myosin XI-2 gene: 5′-ATC ACT CAC GGC ATG GAC G-3′ and 5′-GTA TTC CAC CTG TCC TGC AT-3′.
Seed productivity measurements
Under the growth conditions applied in this study, the life cycle of the Camelina plants from seeds (planting) to seeds (harvest of desiccated seeds) lasted ∼90 days. All desiccated seeds were harvested using a paper envelope. For seed size measurements, Camelina seeds were photographed under a bright-field stereomicroscope (SZX10; Olympus, Japan). Seed size is quantified by surface area of seeds using ImageJ software (NIH). Two independent transgenic Camelina lines were grown for analyses and confirmation of the results.
Results
Generation of high-speed chimeric myosin XI-2 transgenic Camelina plants
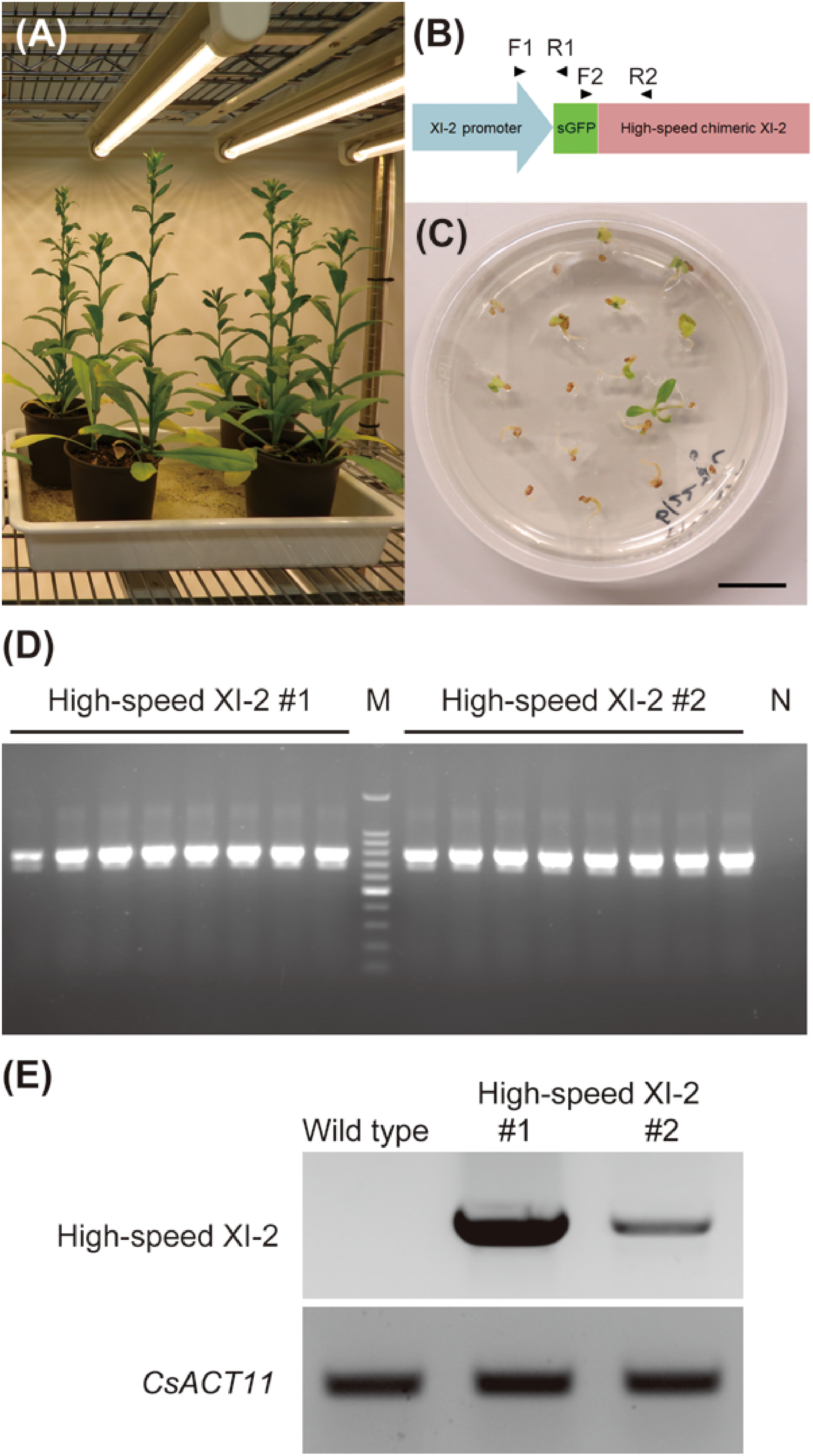
When wild-type Camelina plants had been planted and grown for 33 days, we clipped their primary bolts about 15 cm high at the early flowering stage (Figure 1A). The second bolts were then induced and used for transformation by the floral dipping method (Lu and Kang 2008). A binary plasmid containing high-speed chimeric myosin XI-2 constructed previously (Tominaga et al. 2013) was used in this study (Figure 1B). Transformed Camelina seeds of the first generation (T1) with resistance to hygromycin were selected (Figure 1C). Hygromycin resistance also segregated with the T2 transgenic lines with a single T-DNA insertion because of the 3 : 1 segregation ratio. We then obtained the homozygous T3 or T4 seeds and performed PCR analysis of the homozygous transgenic plants using primers specific for high-speed chimeric myosin XI-2. The amplification results confirmed homozygous insertion of the high-speed chimeric myosin XI-2 gene in two independent transgenic lines (high-speed XI-2 #1 and #2) (Figure 1D). Next, the expression of high-speed chimeric myosin XI-2 was confirmed by RT-PCR in these homozygous lines. Transcript of high-speed chimeric myosin XI-2 was detected in both high-speed XI-2 #1 and #2 (Figure 1E). Additionally, the transcript level of high-speed chimeric myosin XI-2 in high-speed XI-2 #1 was significantly higher than that in high-speed XI-2 #2. These results showed that heterologous transformation of high-speed chimeric myosin XI-2 in Camelina was successful.
Figure 1. Generation of transgenic Camelina expressing high-speed chimeric myosin XI-2. (A) Wild-type Camelina plants for transformation. (B) Schematic diagram of GFP-fused high-speed chimeric myosin XI-2 construct. F1 and R1, forward and reverse primers for PCR analysis of the homozygous transgenic plants. F2 and R2, forward and reverse primers for RT-PCR to confirm the transcript of GFP-fused high-speed chimeric myosin XI-2 gene. (C) Selection of T1 generation seeds using Murashige and Skoog medium containing 30 µM hygromycin and 250 µM claforan. Bar=1 cm. (D) PCR analysis of two independent homozygous transgenic plants. M, 100 bp ladder; N, non-transgenic plant. (E) RT-PCR analysis of two independent homozygous transgenic plants. Camelina ACTIN11 (CsACT11) was used as an internal control.
Effects of high-speed chimeric myosin XI-2 on Camelina
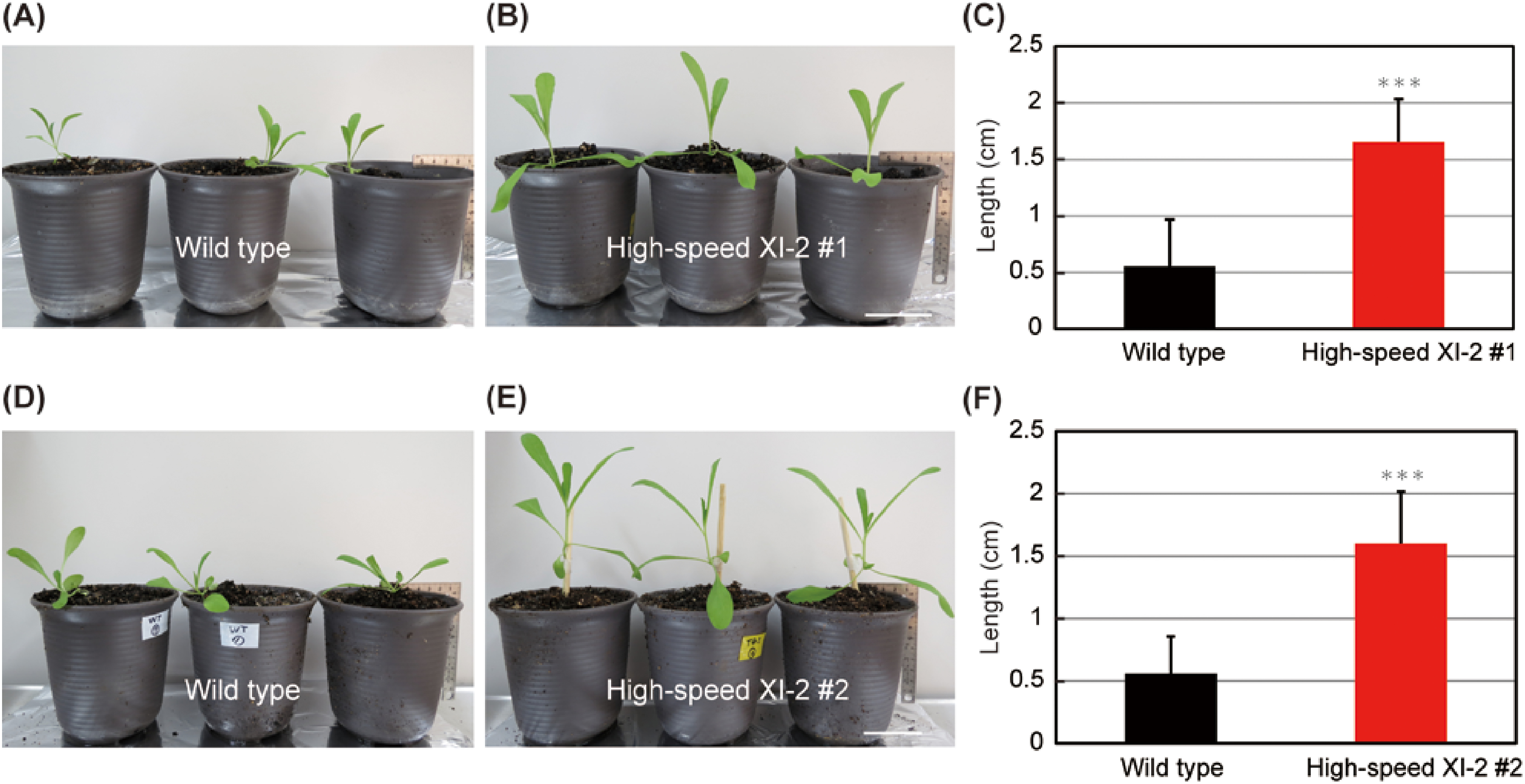
We examined the effects of high-speed chimeric myosin XI-2 on Camelina in two independent lines that exhibit gene expression. At 18 days, visually leaf size and stem length in high-speed XI-2 #1 were bigger than those in the wild-type plants (Figure 2A, B). We measured the length of the first internodes (between the nodes of the first and third leaves) to quantify growth of these Camelina plants. The length of the first internodes in high-speed XI-2 #1 was approximately 2-fold longer than that in wild-type plants (Figure 2C). Furthermore, similar effects of the high-speed chimeric myosin XI-2 were confirmed in high-speed XI-2 #2 (Figure 2D–F). These results indicated that high-speed chimeric myosin XI-2 can promote plant growth in Camelina.
Figure 2. Effects of high-speed chimeric myosin XI-2 on Camelina. Phenotypes of the wild type (A) and high-speed XI-2 line (B) at 18 days. (C) The first internode length of 18-d-old plants in wild-type and high-speed XI-2 #1 plants (mean±SE, n=11). Phenotypes of the wild type (D) and another independent high-speed XI-2 line (E) at 19 days. (F) The first internode length of 19-d-old plants in wild-type and high-speed XI-2 #2 plants (mean±SE, n=12). Bars=5 cm. *** p<0.001 by Student’s t test compared with the wild type.
Enhancement of leaf development and main stem elongation in high-speed XI-2 plants
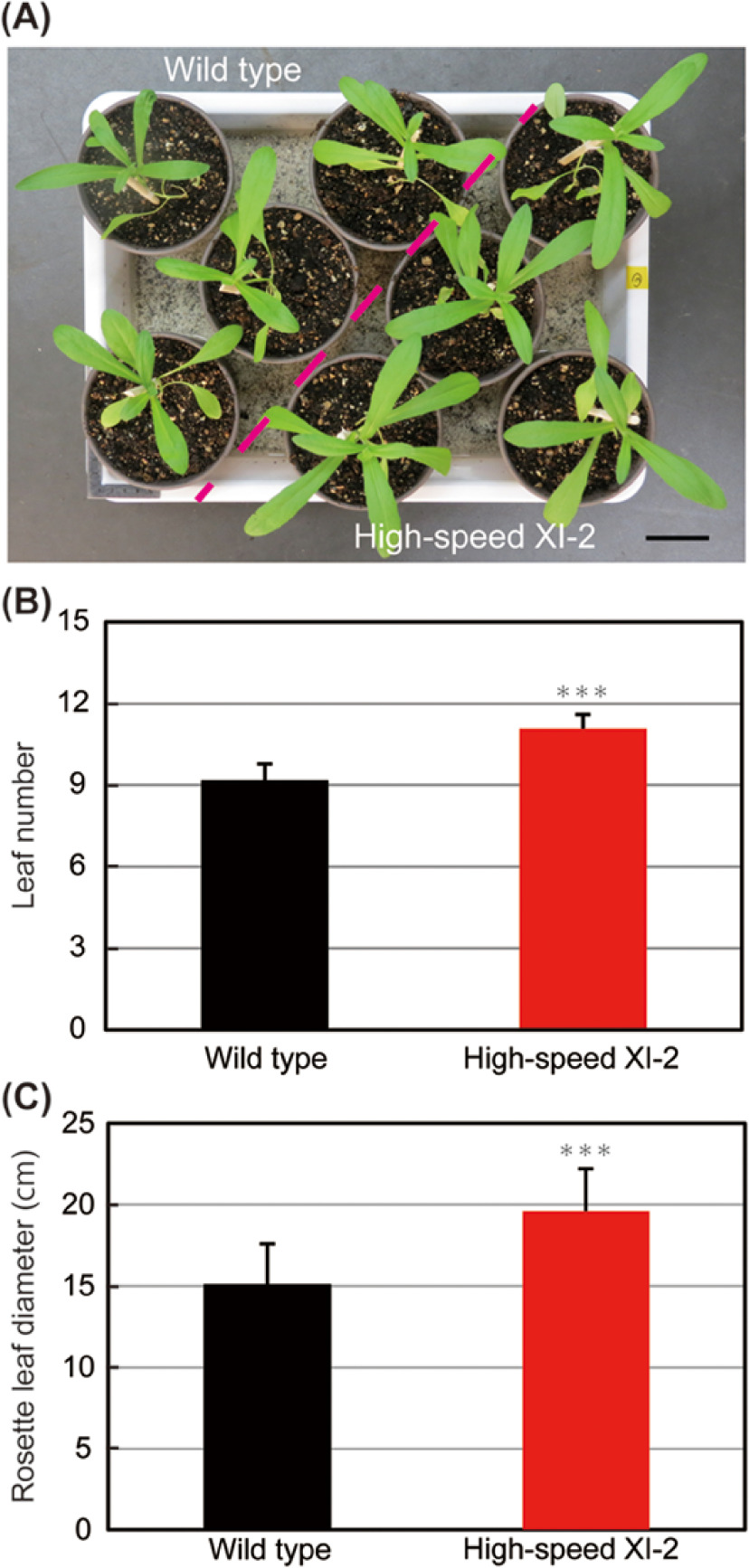
Although the effect of high-speed chimeric myosin XI-2 on Camelina was similar between high-speed XI-2 #1 and #2, high-speed XI-2 #1, the line exhibited higher transcript level of high-speed chimeric myosin XI-2, was used for further analyses. Camelina sativa develops pair of true leaves on the first node after cotyledonary stage. A single leaf is continuously developed on each additional node later on (Martinelli and Galasso 2010). To determine whether high-speed chimeric myosin XI-2 promotes leaf development, we analyzed leaf number and rosette leaf diameter in wild-type and high-speed myosin XI-2 plants at 27 days before inflorescence emergence. Leaf number in high-speed XI-2 plants was increased compared with that in wild-type plants. Compared with the 9.2 leaves per plant in the wild type, high-speed XI-2 developed up to 11.1 leaves per plant (Figure 3A, B). Rosette diameter was measured as the greatest distance between the apices of two opposite leaves (Peremyslov et al. 2010). The rosette leaf diameter in high-speed XI-2 plants was significantly increased, approximately 30% longer than that in wild-type plants (Figure 3A, C). The results indicated that leaf development of Camelina is enhanced by high-speed chimeric myosin XI-2.
Figure 3. Effects of high-speed chimeric myosin XI-2 on leaf development. (A) Phenotype of 27-day-old wild-type and high-speed XI-2 Camelina. (B) Leaf number of 27-day-old plants (mean±SE, n=11). (C) Mean rosette leaf diameters of 27-day-old plants (mean±SE, n=11). Bar=5 cm. *** p<0.001 by Student’s t test compared with the wild type.
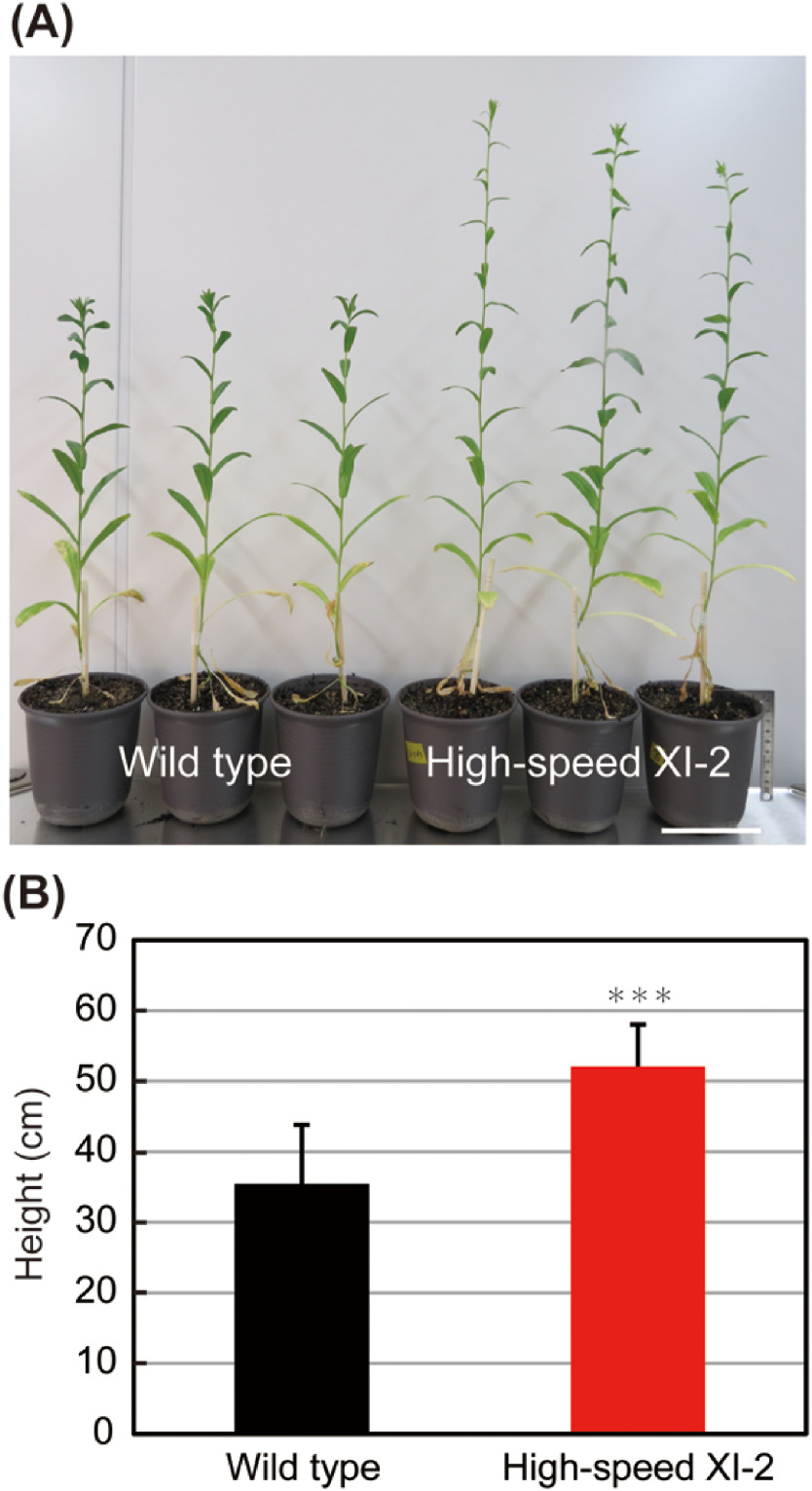
In Camelina sativa, main stem elongation usually takes place concomitantly with leaf development (Martinelli and Galasso 2010). Here, we also measured the plant height of wild-type and high-speed XI-2 at 42 days before flower opening. Plant height of high-speed XI-2 was 46% higher than that of the wild type (Figure 4A, B), indicating that main stem elongation of Camelina was enhanced by high-speed chimeric myosin XI-2.
Figure 4. Effects of high-speed chimeric myosin XI-2 on main stem development. (A) Phenotype of wild-type and high-speed XI-2 plants. (B) Plant height of 42-d-old plants (mean±SE, n=11). Bar=10 cm. *** p<0.001 by Student’s t test compared with the wild type. Bar=10 cm.
Promotion of flowering and seed setting in high-speed XI-2 plants
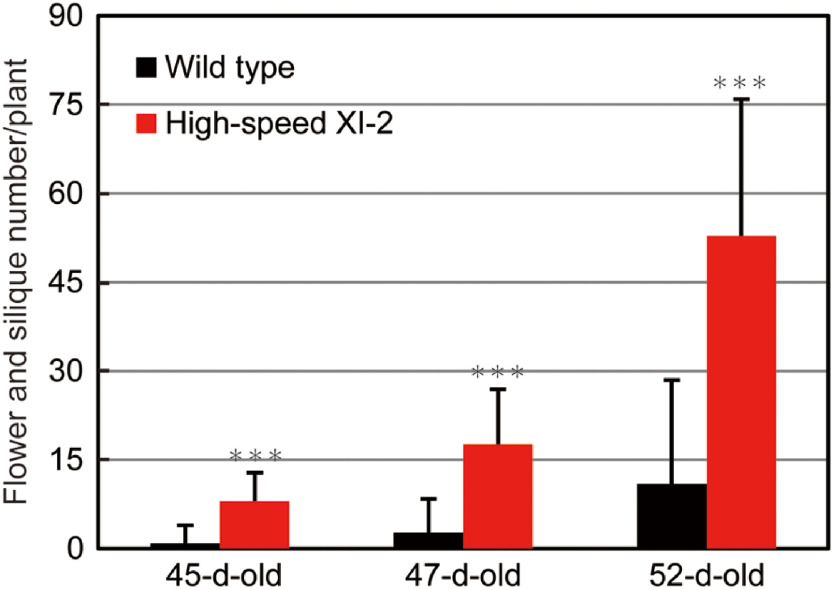
In Camelina sativa, flowering stage begins when the first flower opened (Martinelli and Galasso 2010). To investigate the effect of high-speed chimeric myosin XI-2 on flowering and seed setting, the numbers of fully opened flowers and siliques were determined (Figure 5). At 45 days, there were 8.0 flowers per plant in high-speed XI-2, whereas opened flowers hardly developed in the wild type. At 47 days, high-speed XI-2 developed 17.6 flowers and siliques, whereas the wild type developed 2.7 flowers per plant but no siliques. At 52 days, high-speed XI-2 developed 52.8 flowers and siliques per plant, which was approximately 3.8-fold greater than the number in the wild type (11.0 flowers and siliques per plant). These results indicated that high-speed chimeric myosin XI-2 leads to early flowering and seed setting in Camelina.
Figure 5. Effects of high-speed chimeric myosin XI-2 on flowering and seed setting. Flower and silique number time course for wild-type and high-speed XI-2 Camelina from 45 to 52 days. *** p<0.001 by Student’s t test compared with the wild type.
Seed yield increase in high-speed myosin XI-2 plants
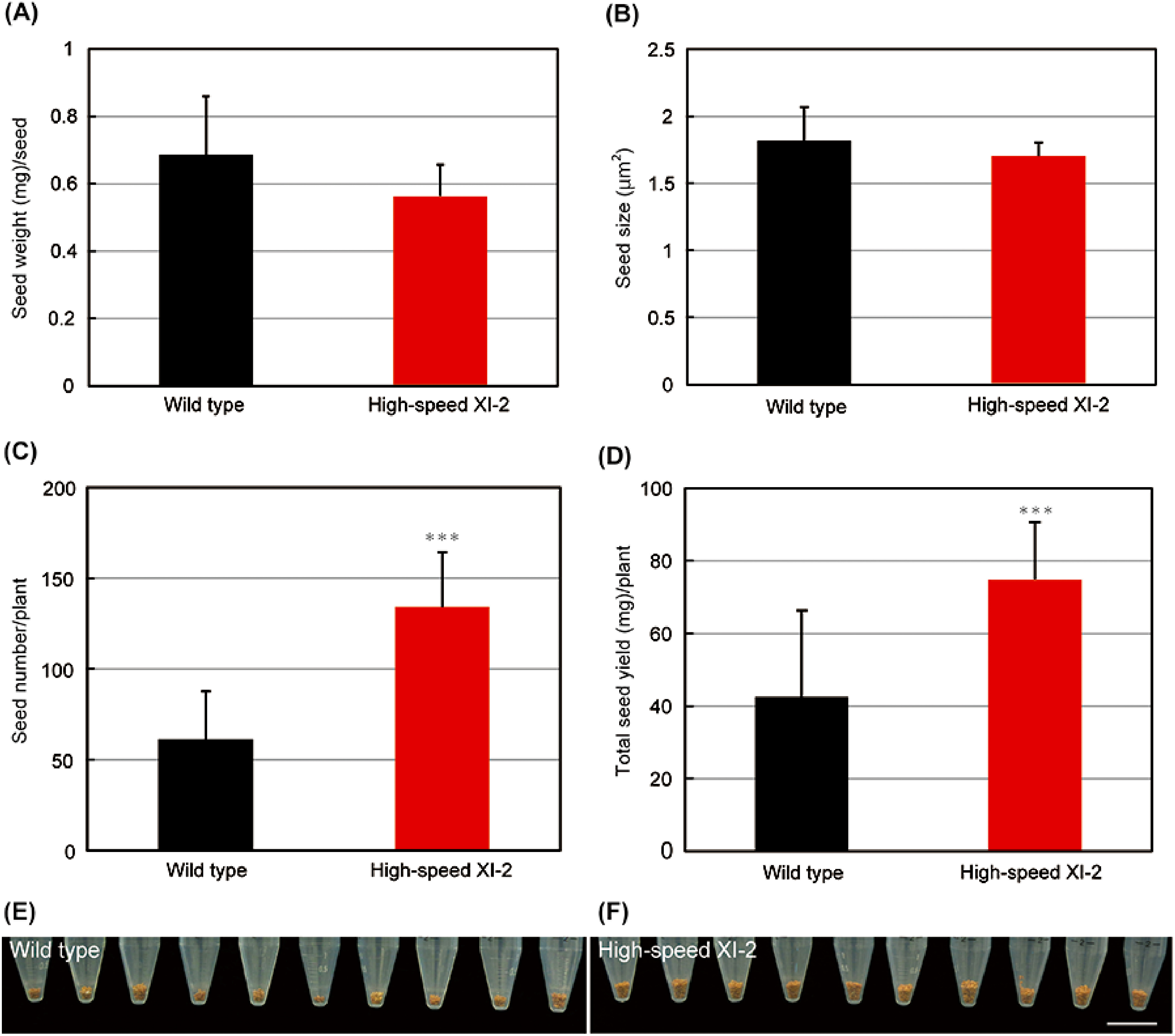
When analyzing seed yield characteristics, the weight and size per seed in high-speed XI-2 plants were almost unchanged compared with those in wild-type plants (Figure 6A, B). Nevertheless, seed number per plant in high-speed XI-2 was approximately 2-fold greater than that in the wild type (Figure 6C). As a consequence, total seed yield in high-speed XI-2 was also significantly increased compared with that in the wild type, with a ratio of increase similar to that in seed number (Figure 6D–F). These results indicated that the effect of high-speed chimeric myosin XI-2 on the improved seed number led to a significant increase in total seed yield in Camelina.
Figure 6. Effects of high-speed chimeric myosin XI-2 on seed production. (A) Seed weight per seed. (B) Seed size per seed. (C) Seed number per plant. (D) Total seed yield per plant (mean±SE, n=11). *** p<0.001 by Student’s t test compared with the wild type. (E) Total seed yield of representative wild-type plants. (F) Total seed yield of representative high-speed XI-2 plants. Bar=2 cm.
Discussion
Camelina is closely related to the model plant Arabidopsis, which suggests good transferability of genetic and genomic tools developed in Arabidopsis so far (Hutcheon et al. 2010). A previous study reported that an Arabidopsis gene for drought resistance in Camelina was successfully expressed and led to drought resistance through cuticular wax (Lee et al. 2014). The results confirmed the transferability of heterologous expression of Arabidopsis genes in Camelina. In our previous study, expression of a high-speed chimeric myosin XI-2 gene promoted cytoplasmic streaming and plant growth in Arabidopsis (Tominaga et al. 2013). In the present study, we heterologously transformed the high-speed chimeric myosin XI-2 and attempted to improve plant growth in Camelina. As the results we summarized in Table 1, the high-speed chimeric myosin XI-2 promoted plant growth at different stages, such as by increasing rosette diameter, leaf number, and plant height. These results indicated the physiological competency of the high-speed chimeric myosin XI-2 in Camelina. However, we can’t observe the cytoplasmic streaming in Camelina using bright-field microscopy because of technical restrictions. It would be needed to visualize the fluorescent marker for organelles to confirm the intracellular transport in Camelina.
Table 1. All parameters about Camelina phenotypes measured in this study.
| Wild type | High-speed XI-2 | |
|---|---|---|
| The first internode length at 18-day-old (cm) (mean±SE, n=11) | 0.5±0.4 | 1.7±0.4 |
| Leaf number at 27-day-old (mean±SE, n=11) | 15.1±2.5 | 19.6±2.6 |
| Rosette leaf diameters at 27-day-old (cm) (mean±SE, n=11) | 9.2±0.6 | 11.1±0.5 |
| Plant height at 42-day-old (cm) (mean±SE, n=11) | 35.5±8.3 | 52.1±6.0 |
| Flower number at 45-day-old (mean±SE, n=11) | 1.0±3.0 | 8.0±4.8 |
| Flower and silique number at 45-day-old (mean±SE, n=11) | 2.7±5.7 | 17.6±9.3 |
| Flower and silique number at 52-day-old (mean±SE, n=11) | 11.0±17.5 | 52.8±32.1 |
| Seed weight per seed (mg) (mean±SE, n=11) | 0.7±0.2 | 0.6±0.1 |
| Seed size per seed (µm2) (mean±SE, n=11) | 1.8±0.2 | 1.7±0.1 |
| Seed number per plant (mean±SE, n=11) | 61±27 | 135±30 |
| Total seed yield per plant (mg) (mean±SE, n=11) | 42.5±23.8 | 75.0±15.7 |
Interestingly, the high-speed XI-2 Camelina exhibited early flowering and seed setting, suggesting that the high-speed chimeric myosin XI-2 is a useful tool for crop production. For example, the early flowering effect of high-speed chimeric myosin XI-2 in Camelina could shorten the harvest cycle time and would be suitable for cultivation within a shorter period with favorable temperatures to avoid environmental stress. Surprisingly, the enhancement of plant growth in the high-speed XI-2 Camelina finally led to significant increases in seed number and total seed yield (Figure 6, Table 1). Camelina seeds possess high oil content (triacylglycerol: TAG) ranging from 28 to 40% of the seed weight (Putnam et al. 1993). If oil content per seed in the high-speed XI-2 Camelina is higher or comparable compared with that in wild type, the increased seed yield in the high-speed XI-2 Camelina would be equally effective in TAG production. Therefore, there is a need to investigate the TAG level in high-speed XI-2 Camelina seeds in future studies.
In several previous studies using genetic engineering on Camelina, transgenic plants were successfully generated and were applied to obtain Camelina oil with a high level of omega-3 fatty acids (Horn et al. 2013; Mansour et al. 2014; Petrie et al. 2014; Ruiz-Lopez et al. 2014). Because the high-speed XI-2 Camelina significantly improved seed yield, the combination of high-speed XI-2 and altered fatty acid composition in Camelina is anticipated to generate plants with high commercial value given their high-quantity and -quality oil production.
Acknowledgments
We thank Kae Yoshino and Seiko Takagi for technical assistance with the experiments. This work was supported by a grant from the Japan Science and Technology Agency, ALCA [JPMJAL1401 to Z.D., K.I. and M.T.].
References
- Abramovic H, Abram V (2005) Physico-chemical properties, composition and oxidative stability of Camelina sativa oil. Food Technol Biotechnol 43: 63–70 [Google Scholar]
- Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM (2006) n-3 fattyacid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr 83: 1526–1535 [DOI] [PubMed] [Google Scholar]
- Horn PJ, Silva JE, Anderson D, Fuchs J, Borisjuk L, Nazarenus TJ, Shulaev V, Cahoon EB, Chapman KD (2013) Imaging heterogeneity of membrane and storage lipids in transgenic Camelina sativa seeds with altered fatty acid profiles. Plant J 76: 138–150 [DOI] [PubMed] [Google Scholar]
- Hutcheon C, Ditt RF, Beilstein M, Comai L, Schroeder J, Goldstein E, Shewmaker CK, Nguyen T, De Rocher J, Kiser J (2010) Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol 10: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Ikebe M, Kashiyama T, Mogami T, Kon T, Yamamoto K (2007) Kinetic mechanism of the fastest motor protein, Chara myosin. J Biol Chem 282: 19534–19545 [DOI] [PubMed] [Google Scholar]
- Ito K, Yamaguchi Y, Yanase K, Ichikawa Y, Yamamoto K (2009) Unique charge distribution in surface loops confers high velocity on the fast motor protein Chara myosin. Proc Natl Acad Sci USA 106: 21585–21590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SB, Kim H, Kim RJ, Suh MC (2014) Overexpression of Arabidopsis MYB96 confers drought resistance in Camelina sativa via cuticular wax accumulation. Plant Cell Rep 33: 1535–1546 [DOI] [PubMed] [Google Scholar]
- Li X, Mupondwa E (2014) Life cycle assessment of camelina oil derived biodiesel and jet fuel in the Canadian Prairies. Sci Total Environ 481: 17–26 [DOI] [PubMed] [Google Scholar]
- Lu C, Kang J (2008) Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep 27: 273–278 [DOI] [PubMed] [Google Scholar]
- Mansour MP, Shrestha P, Belide S, Petrie JR, Nichols PD, Singh SP (2014) Characterization of oilseed lipids from “DHA-producing Camelina sativa”: A new transformed land plant containing long-chain omega-3 oils. Nutrients 6: 776–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli T, Galasso I (2010) Phenoligical growth stages of Camelina sativa according to the extended BBCH scales. Ann Appl Biol 158: 87–94 [Google Scholar]
- Ojangu EL, Tanner K, Pata P, Jarve K, Holweg CL, Truve E, Paves H (2012) Myosins XI-K, XI-1, and XI-2 are required for development of pavement cells, trichomes, and stigmatic papillae in Arabidopsis. BMC Plant Biol 12: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peremyslov VV, Prokhnevsky AI, Dolja VV (2010) Class XI myosins are required for development, cell expansion, and F-Actin organization in Arabidopsis. Plant Cell 22: 1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie JR, Shrestha P, Belide S, Kennedy Y, Lester G, Liu Q, Divi UK, Mulder RJ, Mansour MP, Nichols PD, et al. (2014) Metabolic engineering Camelina sativa with fish oil-like levels of DHA. PLoS One 9: e85061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhnevsky AI, Peremyslov VV, Dolja VV (2008) Overlapping functions of the four class XI myosins in Arabidopsis growth, root hair elongation, and organelle motility. Proc Natl Acad Sci USA 105: 19744–19749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam DH, Budin JT, Field LA, Breene WM (1993) Camelina: A promising low-input oilseed. In: Hanick J, Simon JE (eds) New Crops. Wiley, New York
- Ruiz-Lopez N, Haslam RP, Napier JA, Sayanova O (2014) Successful high-level accumulation of fish oil omega-3 long-chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J 77: 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmen T (2007) The sliding theory of cytoplasmic streaming: Fifty years of progress. J Plant Res 120: 31–43 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kimura A, Yokota E, Haraguchi T, Shimmen T, Yamamoto K, Nakano A, Ito K (2013) Cytoplasmic streaming velocity as a plant size determinant. Dev Cell 27: 345–352 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kojima H, Yokota E, Nakamori R, Anson M, Shimmen T, Oiwa K (2012) Calcium-induced mechanical change in the neck domain alters the activity of plant myosin XI. J Biol Chem 287: 30711–30718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Nakano A (2012) Plant-specific Myosin XI, a molecular perspective. Front Plant Sci 3: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I (2010) Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci USA 107: 6894–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubr J (1997) Oil-seed crop: Camelina sativa. Ind Crops Prod 6: 113–119 [Google Scholar]