Abstract
The plant-specific NAC transcription factor VASCULAR-RELATED NAC-DOMAIN 7 (VND7) functions in xylem vessel cell differentiation in Arabidopsis thaliana. To identify novel factors regulating xylem vessel cell differentiation, we previously performed ethyl methanesulfonate mutagenesis of a transgenic 35S::VND7-VP16-GR line in which VND7 activity can be induced post-translationally by glucocorticoid treatment. We successfully isolated mutants that fail to form ectopic xylem vessel cells named seiv (suppressor of ectopic vessel cell differentiation induced by VND7) mutants. Here, we isolated eight novel dominant seiv mutants: seiv2 to seiv9. In these seiv mutants, ectopic xylem vessel cell differentiation was inhibited in aboveground but not underground tissues. Specifically, the shoot apices of the mutants, containing shoot apical meristems and leaf primordia, completely lacked ectopic xylem vessel cells. In these mutants, the VND7-induced upregulation of downstream genes was reduced, especially in shoots compared to roots. However, endogenous xylem vessel cell formation was not affected in the seiv mutants. Therefore, the seiv mutations identified in this study have repressive effects on cell differentiation in shoot meristematic regions, resulting in inhibited ectopic xylem vessel cell differentiation.
Keywords: Arabidopsis, seiv mutants, VND7, xylem vessel cell
Introduction
Xylem vessels are highly specialized tissues that function in long-distance water conduction in vascular plants. Xylem vessel formation occurs via two processes: patterned secondary cell wall (SCW) deposition and programmed cell death (PCD) (Fukuda 2004; Oda and Fukuda 2012; Ohtani et al. 2016; Schuetz et al. 2013). The SCW in xylem vessel cells contains polysaccharides, cellulose and hemicellulose, and the phenolic polymer lignin. The rigid, lignified SCW provides mechanical support to prevent xylem vessel cells from collapsing during high-pressure water uptake, as well as providing waterproofing (Fukuda 2004; Turner et al. 2007). During PCD, all intracellular contents, including the nucleus and cytoplasm, are removed from the cell, leading to the formation of a hollow tube structure to create an effective path for water uptake (Fukuda 2004; Turner et al. 2007).
Transcriptome analysis using an in vitro induction system for xylem vessel cell differentiation from Arabidopsis thaliana suspension cells identified the plant-specific NAM/ATAF/CUC (NAC) domain transcription factors VASCULAR-RELATED NAC-DOMAIN 1 (VND1) to VND7 as possible key factors for xylem vessel cell differentiation. VND1 to VND7 are upregulated during the early stages of xylem vessel cell differentiation (Kubo et al. 2005). All VND genes are preferentially expressed in developing vascular tissues (Kubo et al. 2005; Yamaguchi et al. 2008). Overexpressing these genes induced the transdifferentiation of nonvascular cells into xylem vessel cells with patterned SCWs (Endo et al. 2014; Kubo et al. 2005; Yamaguchi et al. 2010a; Zhou et al. 2014). Conversely, the functional repression of VND6 and VND7 severely affected the formation of metaxylem and protoxylem vessel cells, respectively (Kubo et al. 2005; Yamaguchi et al. 2010b). These findings suggest that VND family proteins are master regulators of xylem vessel cell differentiation (Kubo et al. 2005; Nakano et al. 2015; Ohtani et al. 2011; Ohtani and Demura 2019; Schuetz et al. 2013; Yamaguchi et al. 2008, 2011; Xu et al. 2014).
To obtain novel insight into the regulatory mechanisms of xylem vessel cell differentiation, we previous isolated suppressor mutants of VND7-induced xylem vessel cell differentiation named as suppressor of ectopic xylem vessel cell differentiation induced by VND7 (seiv) mutants (Kawabe et al. 2018; Ohtani et al. 2018). Specifically, we performed ethyl methanesulfonate (EMS)-mutagenesis of transgenic Arabidopsis line VND7-VP16-GR in which transdifferentiation into protoxylem-like vessel cells is induced by the post-translational activation of VND7 via glucocorticoid treatment (Yamaguchi et al. 2010a) and isolated seiv mutants. In the recessive seiv1 mutant, a single nucleic acid substitution (G to A) leading to an amino acid substitution (E36K) is present in the gene encoding S-NITROSOGLUTATHIONE REDUCTASE 1 (GSNOR1), which regulates the turnover of the natural nitric oxide (NO) donor S-nitrosoglutathione (Kawabe et al. 2018). GSNOR1 is an important regulator of the S-nitrosylation of proteins, i.e., the covalent binding of NO-related species to a cysteine residue, strongly suggesting that this process plays crucial roles in xylem vessel cell differentiation (Kawabe et al. 2018; Ohtani et al. 2018).
In the present study, we isolated eight novel, dominant seiv mutants: seiv2 to seiv9. Transdifferentiation was significantly repressed in the aboveground tissues of these mutants, especially in the shoot apical region. Consistent with this finding, the VND7-induced expression of downstream genes was strongly inhibited in the shoots, but not roots, of the seiv mutants, suggesting that these seiv mutations affect VND7 activity in a tissue-specific manner. However, endogenous xylem vessel cell differentiation was not affected in the seiv mutants. Taken together, these findings suggest that the repression of cell differentiation in the shoot meristematic region is enhanced in the seiv mutants. The seiv mutants represent unique materials for further analyzing the molecular mechanisms underlying xylem vessel cell differentiation.
Materials and methods
Plant materials and growth conditions
The Arabidopsis (Arabidopsis thaliana) VASCULAR-RELATED NAC-DOMAIN 7 inducible line VND7-VP16-GR and the vector control line were described in Yamaguchi et al. (2010a). The suppressor of ectopic xylem vessel cell differentiation induced by VND7 (seiv) mutants were isolated from EMS-mutagenized VND7-VP16-GR pools as described in Kawabe et al. (2018). Briefly, after the two-round screening of EMS-mutagenized plants, the isolated mutant candidates were backcrossed with VND7-VP16-GR. The segregation rates of F1 plants were calculated to determine whether the mutations responsible were inherited as Mendelian characteristics, and if they were dominant or recessive. The mutant lines were established by two-round backcross with VND7-VP16-GR, and confirmed that no mutation was found in the VND7-VP16-GR cassette by sequencing of their genome (Kawabe et al. 2018). The seeds were surface-sterilized using 0.2% (v/v) PPM solution (Plant Preservative Mixture; Plant Cell Technology, Inc.) and sown on Murashige and Skoog (MS) medium with 0.6% gellan gum containing 1% (w/v) sucrose (pH 5.7). The plates were incubated at 4°C in the dark for 2–3 day and transferred to a growth chamber under continuous light at 22°C. To propagate seeds, the plants were grown on plates for 2–3 weeks, transferred to soil, and cultured in a growth chamber at 22°C under a 16-h-light photoperiod.
Dexamethasone treatment
For dexamethasone (DEX) treatment, 7-day-old seedlings grown on MS medium were soaked in sterile water containing 10 µM DEX and incubated in a growth chamber under continuous light at 22°C (Yamaguchi et al. 2010a). The treated seedlings were collected after 1 day and 4 day of DEX treatment for quantitative RT-PCR and microscopy, respectively.
Microscopy
To observe ectopic xylem vessel cells, DEX-treated seedlings were fixed in 90% (v/v) acetone for more than 1 week at −30°C. The samples were cleared and mounted in a few drops of chloral hydrate solution (an 8 : 1 : 2 mixture of chloral hydrate : water : glycerol [w/v/v]). Images were captured under a microscope (BX53; Olympus) equipped with differential interference contrast (DIC) optics and a digital camera (DP72; Olympus).
Quantitative RT-PCR analysis
Total RNA samples were isolated separately from shoots and roots using an RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. The RNA was treated with RQ1 RNase-Free DNase (Promega) to remove genomic DNA contamination, and 1 µg of DNase-treated RNA was reverse-transcribed to synthesize first-strand cDNA using Transcriptor Reverse Transcriptase with anchored oligo (dT)18 primer (Roche) following the manufacturer’s instructions. Quantitative RT-PCR (qRT-PCR) was carried out using the LightCycler 96 system with LightCycler 480 SYBR Green I Master (Roche). The UBQ10 gene was used as an internal control. The relative expression levels of the mRNAs were manually calculated using the comparative CT method, 2-∆∆ CT. All qRT-PCR was performed in three independent biological replicates with technical replicates. The primers used for qRT-PCR are shown in Supplementary Table S1.
Chromosome mapping
The seiv mutants were crossed with wild-type Landsberg erecta (Ler), and the resulting F3 progeny were treated with DEX to obtain homozygous lines of each seiv mutant. The homozygous seiv F3 plants (∼30 plants) were subjected to chromosome mapping by examining simple sequence length polymorphism (SSLP) markers (Bell and Ecker 1994; The Arabidopsis Information Resource, https://www.arabidopsis.org). The percentage of Ler alleles showing polymorphism (recombination ratio) was calculated for each mapping marker, and when the recombination ratio was below 50%, a chi-square test was performed to check the significance of differences in the recombination ratios of Ler alleles (p<0.05). The primers used for chromosome mapping are shown in Supplementary Table S1.
Results
Isolation of dominant seiv mutants with defects in VND7-induced ectopic xylem vessel cell differentiation
A glucocorticoid-mediated posttranslational induction system for VDN7 expression (VND7-VP16-GR) can be used to induce ectopic xylem vessel cell differentiation (Yamaguchi et al. 2010a). To identify novel factors related to xylem vessel cell differentiation, a forward genetic screening of ethyl methanesulfonate (EMS)-mutagenized VND7-VP16-GR plants was previously used to identify lines with altered xylem vessel cell differentiation (Kawabe et al 2018; Ohtani et al. 2018). This screening successfully identified suppressor mutants of VND7-induced xylem vessel cell differentiation named seiv mutants, including the recessive mutant seiv1 (Kawabe et al. 2018; Ohtani et al. 2018). In the current study, using a similar approach, we isolated the dominant mutants seiv2 to seiv9 in the VND7-VP16-GR background (Figure 1).
Figure 1. The seiv mutants show inhibited xylem vessel cell differentiation caused by effects on DEX-induced VND7 activity. Photographs of the dominant suppressor mutants seiv2–9, the parental line VND7-VP16-GR, and the vector control. Seven-day-old seedlings were treated without (−DEX, in A) or with (+DEX, in B) DEX for 4 day. Bar=1 cm.
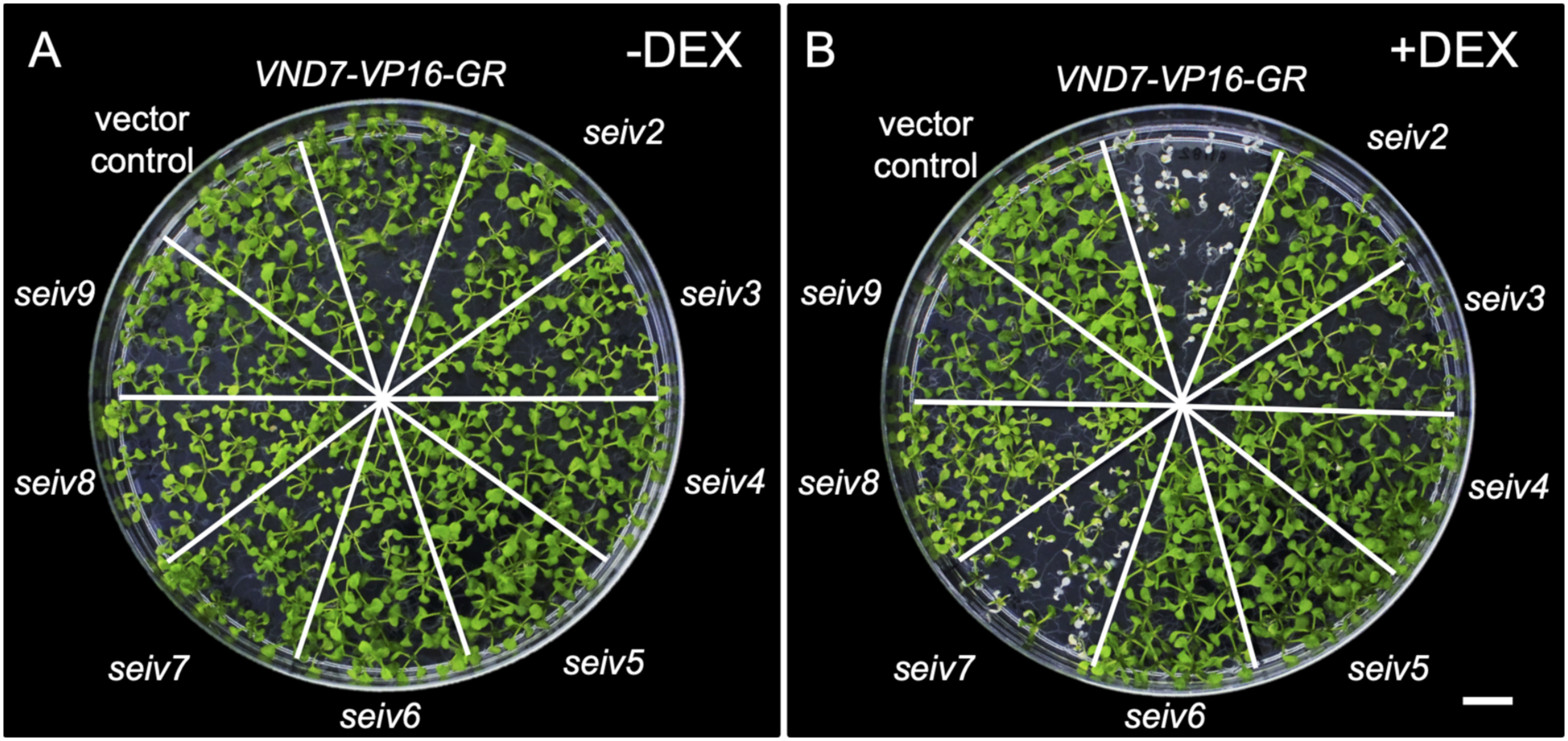
We treated seven-day-old seiv2 to seiv9 seedlings with dexamethasone (DEX) for 4 days and examined them for ectopic xylem vessel cell differentiation. Under control (mock treatment) conditions, VND7-VP16-GR and seiv seedling did not show any severe visible differences compared to the vector control (Figure 1A). Following DEX treatment, almost all VND7-VP16-GR seedlings were severely discolored and eventually died, except for a few seedlings that survived by generating new true leaves (Figure 1B). By contrast, almost all seiv seedlings survived despite DEX treatment and continued their organogenesis (Figure 1B). Some cotyledons of seiv seedlings were pale green compared to the vector control (Figure 1B). Among the seiv mutants, seiv7 displayed the weakest suppression of the VND7-VP16-GR phenotype, as the cotyledons were sometimes completely white, like those of the parental line VND7-VP16-GR (Figure 1B). We found that a part of seiv7 seedlings showed the delay of growth like VND7-VP16-GR (Figure 1A), suggesting the possibility that the defective growth at early stages of seedling development would affect the strength of inhibition for ectopic xylem vessel cell differentiation in seiv7.
Significant defects in xylem vessel cell differentiation are present in the shoot but not root regions of seiv
We further examined the defects in ectopic xylem vessel cell differentiation in seiv2 to seiv9 by microscopy. No ectopic differentiation of xylem vessel cells was detected in any parts of vector control seedlings (Table 1). Conversely, all tissues of VND7-VP16-GR, except the root apical meristem regions, exhibited a high frequency of ectopic xylem vessel cell differentiation, which was characterized based on patterned SCW thickening (Table 1) as described previously (Yamaguchi et al. 2010a).
Table 1. Frequency of ectopic xylem vessel cell differentiation in the seiv mutants.
| Underground tissues | Aboveground tissues | |||||||
|---|---|---|---|---|---|---|---|---|
| RAM | Primary root | Lateral root | Hypocotyl | Cotyledon | Leaf | Leaf Primordium | SAM | |
| Vector control | — | — | — | — | — | — | — | — |
| VND7-VP16-GR | — | ++++ | +++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| seiv2 | — | ++++ | +++ | + | ++ | + | — | — |
| seiv3 | — | ++++ | +++ | + | ++ | + | — | — |
| seiv4 | — | ++++ | +++ | + | ++++ | + | — | — |
| seiv5 | — | ++++ | +++ | — | + | ++ | — | — |
| seiv6 | — | ++++ | +++ | — | + | + | — | — |
| seiv7 | — | ++++ | +++ | ++ | +++ | ++ | — | — |
| seiv8 | — | ++++ | +++ | — | ++ | +++ | — | — |
| seiv9 | — | ++++ | +++ | + | ++ | ++ | — | — |
“—”; no ectopic xylem vessel cells detected. “+”; ectopic xylem vessel cell differentiation detected. The number of “+” symbols reflects the relative frequency in each mutant compared to VND7-VP16-GR. RAM, root apical meristem; SAM, shoot apical meristem.
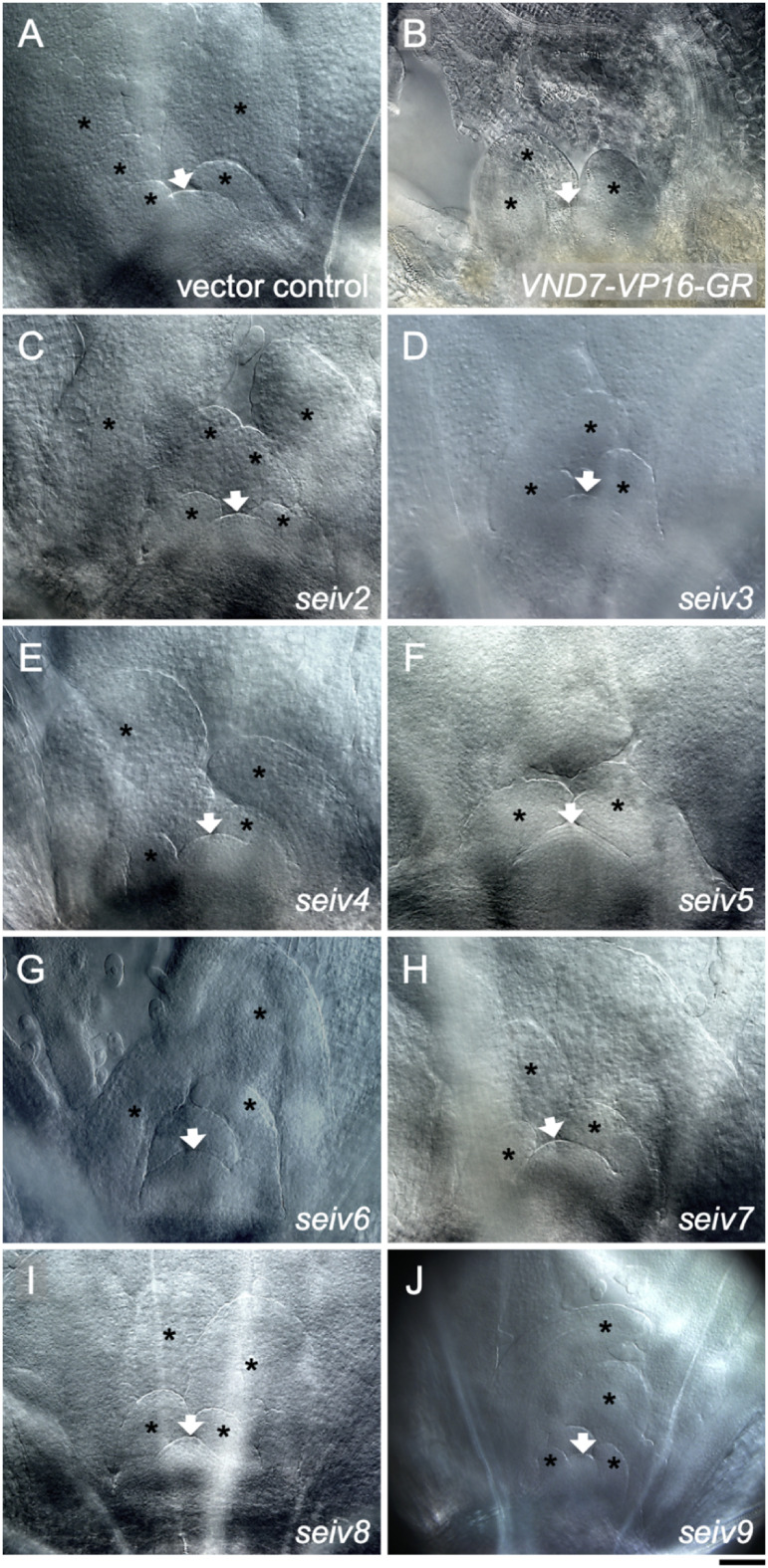
Ectopic xylem vessel cells were detected in the cotyledons of the seiv mutants, although at a lower frequency (in all seiv mutants except seiv4) compared to VND7-VP16-GR (Figure 2, Table 1). Decreased differentiation frequencies were also observed in seiv hypocotyls; specifically, ectopic SCW formation was not detected in the hypocotyls of seiv5, seiv6, or seiv8 (Supplementary Figure S1, Table 1). These results suggest that each mutation has tissue-specific effects on ectopic xylem vessel cell differentiation. Notably, although ectopic SCW deposits were clearly observed in shoot apical regions of VND7-VP16-GR (Figure 3B), such deposits were not observed in any of the seiv mutants (Figure 3C–J). Thus, the survival of the seiv mutants following DEX treatment can primarily be attributed to the suppressed transdifferentiation of the shoot apical region (especially the shoot apical meristem) into xylem vessel cell differentiation.
Figure 2. Effects of the seiv mutations on ectopic xylem vessel cell differentiation in cotyledons. Seven-day-old seedlings were treated with 10 µM DEX for 4 day and subjected to microscopic observation. Cotyledons of the vector control (A), VND7-VP16-GR (B), seiv2 (C), seiv3 (D), seiv4 (E), seiv5 (F), seiv6 (G), seiv7 (H), seiv8 (I), and seiv9 (J) are shown. Bar=200 µm.
Figure 3. Effect of the seiv mutations on ectopic xylem vessel cell differentiation in the shoot apical region. Seven-day-old seedlings were treated with 10 µM DEX for 4 day and subjected to microscopic observation. The shoot apical regions of the vector control (A), VND7-VP16-GR (B), seiv2 (C), seiv3 (D), seiv4 (E), seiv5 (F), seiv6 (G), seiv7 (H), seiv8 (I), and seiv9 (J) are shown. Arrows and asterisks indicate shoot apical meristems and leaf primordia, respectively. Bar=50 µm.
In the seiv mutants, we detected ectopic xylem vessel cell differentiation in root tissues, which also occurred in VND7-VP16-GR (Supplementary Figure S2, Table 1). This observation indicates that these seiv mutations only affect ectopic xylem vessel cell differentiation in aboveground tissues and not in underground tissues.
Endogenous xylem vessel cells are normal in the seiv mutants
To examine the effects of the seiv mutations on endogenous xylem vessel cell differentiation, we examined xylem vessels in primary roots of the seiv mutants in the absence of DEX treatment. No obvious difference was detected in xylem vessel cells between VND7-VP16-GR and the seiv mutants: the number of vessels, timing of xylem vessel cell differentiation in roots, and SCW deposition patterns appeared normal in the mutants (Supplementary Figure S3). Therefore, the seiv mutations do not disturb endogenous xylem vessel cell differentiation, but they inhibit the ectopic induction of xylem vessel cell differentiation in the shoot apical meristem region.
VND7-induced downstream gene expression is affected in the seiv mutants
VND7 positively regulates xylem vessel cell differentiation by activating genes associated SCW biosynthesis as well as PCD (Ohashi-Ito et al. 2010; Yamaguchi et al. 2011; Zhong et al. 2010). To determine whether the seiv mutations affect VND7-induced target gene expression, we performed quantitative RT-PCR (qRT-PCR) to measure the expression levels of SCW-related genes, including cellulose synthase subunit A (CesA) genes (CesA4/IRX5, CesA7/IRX3, CesA8/IRX1; Turner and Somerville 1997), hemicellulose biosynthetic genes (IRX8 and IRX10; Peña et al. 2007; Wu et al. 2009), a cysteine protease gene (XCP1; Avci et al. 2008), and transcription factor genes MYB46 and LBD30/ASL19 (Zhong et al. 2007; Soyano et al. 2008). Since the seiv mutations have different effects on ectopic xylem vessel cell differentiation in aboveground vs. underground tissues (Table 1), we subjected the shoot and root regions of the seedlings to separate qRT-PCR analyses after 1 day of DEX treatment.
As shown in Figure 4, DEX treatment upregulated the expression of VND7 target genes in all seiv shoots, although at significantly lower levels compared to VND7-VP16-GR (~10-fold lower levels in all seiv shoots; Figure 4). The lowest expression levels of SCW-related and transcription factor genes were detected in seiv6, in which almost no induction of CesA8 and IRX10 was observed (Figure 5). This result is in accordance with the finding that seiv6 cotyledons showed the lowest ectopic xylem vessel cell differentiation frequency among lines (Figure 2, Table 1). By contrast, in the root region, only a limited reduction in the upregulation of VND7 target genes was observed (Figure 5). Several seiv mutants showed similar levels of upregulation of CesA7 and CesA8 in response to DEX treatment, and the induction of CesA4 was not affected in any of the seiv mutants (Figure 5). These results are well in accordance with the observation that the frequency of ectopic xylem vessel cell differentiation in roots was not affected by the seiv mutations (Supplementary Figure S2, Table 1).
Figure 4. qRT-PCR analysis of the expression of genes downstream of VND7 in shoot regions of the seiv mutants. Seven-day-old vector control, VND7-VP16-GR, and seiv seedlings were treated with 10 µM DEX for 1 day, separated into shoot and root regions, and subjected to qRT-PCR analysis. The UBQ10 gene was used as an internal control. The expression levels of each gene are shown relative to the vector controls (set to 1). The results are means±SE (n=3). Statistically significant differences between VND7-VP16-GR and each seiv mutant are indicated by asterisks (Student’s t test, * p<0.05).
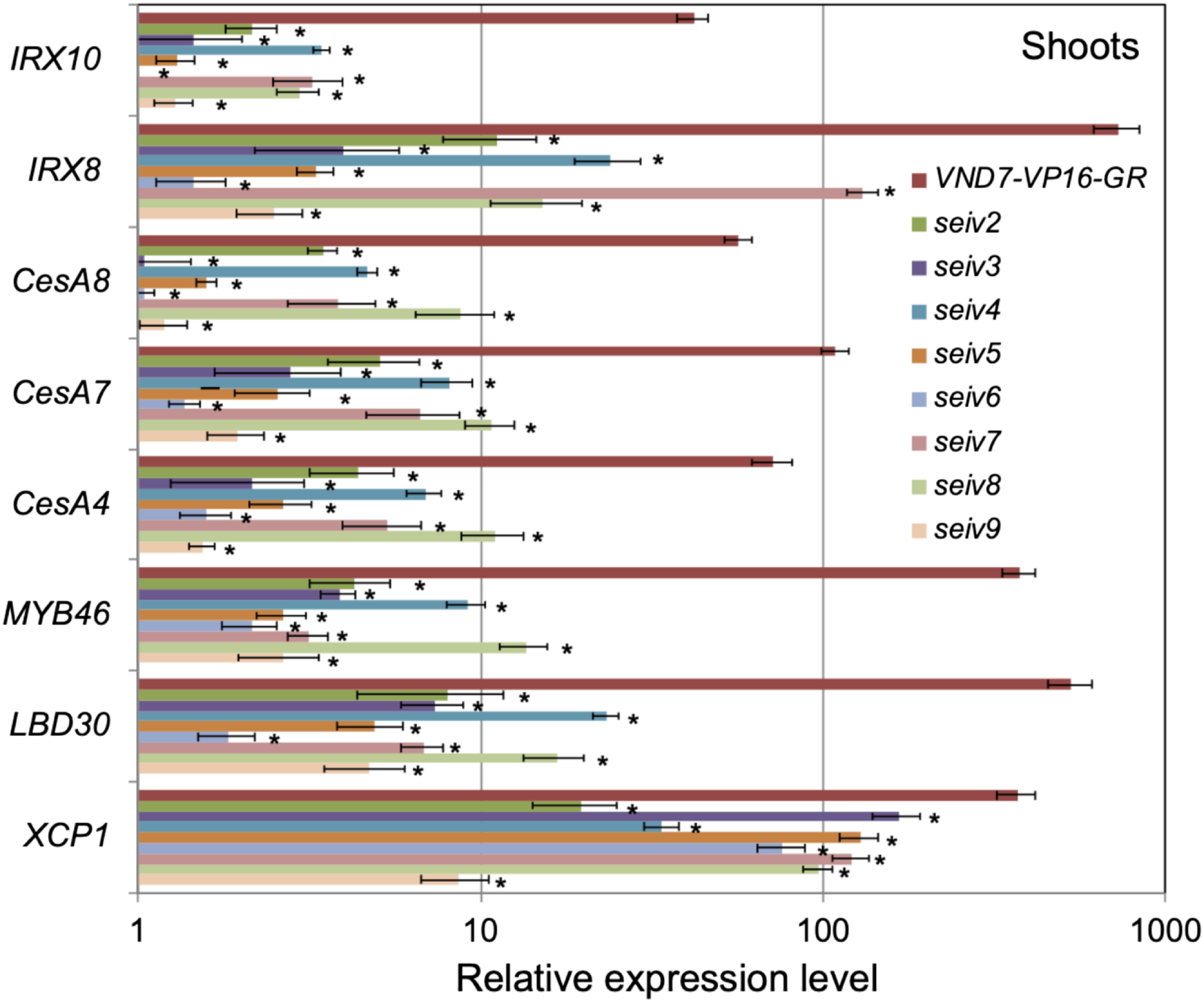
Figure 5. qRT-PCR analysis of the expression of genes downstream of VND7 in the root regions of the seiv mutants. Seven-day-old vector control, VND7-VP16-GR, and seiv seedlings were treated with 10 µM DEX for 1 day, separated into shoot and root regions, and subjected to qRT-PCR analysis. The UBQ10 gene was used as an internal control. The expression levels of each gene are shown as relative values compared to the vector controls (set at 1). The results are means±SE (n=3). Statistically significant differences between VND7-VP16-GR and each seiv mutant are indicated by asterisks (Student’s t test, * p<0.05).

Chromosome mapping of seiv mutant loci
Finally, we performed rough chromosome mapping of the seiv mutant loci. Unfortunately, we did not obtain a sufficient number of homozygous F3 progenies from the cross between seiv9 and the wild type (Ler), but for the other seiv mutants, ∼30 homozygous F3 progenies were generated. In the parental line, the 35S-VND7-VP16-GR cassette was inserted into the bottom of chromosome I (i.e., the intergenic region between At1g69830 and At1g69840). Not surprisingly, in all seiv mutants, the recombination ratios (i.e., percentage of Ler-type polymorphism) were significantly lower for markers localized within the lower arm of chromosome I compared to the other positions (Supplementary Table S2). The gene loci corresponding to seiv2 and seiv3 were not mapped to single position by this chromosome mapping analysis. However, the other seiv mutations were mapped to single loci: seiv4 was mapped onto chromosome 4, seiv5, seiv6, and seiv7 were mapped onto chromosome 5, and seiv8 was mapped onto chromosome 3. These results suggest that the seiv mutants analyzed in this study contain at least three independent gene loci responsible for the inhibition of ectopic xylem vessel cell differentiation.
Discussion
VND7 is a master transcriptional regulator of xylem vessel cell differentiation in Arabidopsis (Kubo et al. 2005; Nakano et al. 2015; Ohtani and Demura 2019; Yamaguchi et al. 2008, 2011). VND7 induces the expression of a group of target genes involved in SCW thickening and PCD, in addition to transcription factor and signal transduction-related genes (Ohashi-Ito et al. 2010; Yamaguchi et al. 2011; Zhong et al. 2010). In the present study, we identified eight dominant seiv mutants, which were isolated as suppressors of VND7-based ectopic xylem vessel cell differentiation (Kawabe et al. 2018; Ohtani et al. 2018). seiv seedlings survived even after the activation of VND7 activity by DEX treatment (Figure 1). No obvious changes in morphology or growth were detected in the seiv2 to seiv9 mutants under normal growth conditions (Figure 1), and endogenous xylem vessel cell differentiation appeared to be normal (Supplementary Figure S3). By contrast, under normal conditions, the recessive seiv1 mutant shows severe growth inhibition and abnormal vein formation (Kawabe et al. 2018; Ohtani et al. 2018). The gene responsible for the seiv1 mutation is S-NITROSOGLUTATHIONE REDUCTASE 1 (GSNOR1), encoding a critical factor regulating NO metabolism in plant cells (Kawabe et al. 2018; Ohtani et al. 2018). Therefore, unlike seiv1, the genes responsible for seiv2 to seiv9 might be involved in specific aspects of the VND7-based induction of xylem vessel cell differentiation, but not in a broad range of cell differentiation processes. The chromosome mapping analysis suggested that seiv2 to seiv9 mutations would be attributed to at least three independent loci at chromosome 3, 4, and 5 (Supplementary Table S2). Moreover, the seiv5, seiv6, and seiv7 mutations, which were all mapped to chromosome 5, showed the different inhibitory effects on the transdifferentiation (Figures 1, 2, 4, 5), suggesting the possibility that the seiv5, seiv6, and seiv7 mutations can be localized in different gene loci. Thus, we expect that the seiv2 to seiv9 mutants could provide more than 3 independent gene loci contributing to the regulation of VND7-based induction of xylem vessel cell differentiation.
Detailed observations of ectopic xylem vessel cell differentiation in seiv2 to seiv9 showed that ectopic xylem vessel cell differentiation was significantly inhibited in aboveground tissues, but not in underground tissues (Figures 2, 3, Table 1). In the shoot apical regions, the seiv mutations had strong inhibitory effects on xylem vessel cell differentiation (Figure 3). Consistently, the upregulation of VND7 target genes was substantially reduced in the shoots of the seiv2 to seiv9 mutants, with less of an effect in roots (Figures 4, 5). Interestingly, even in the parental line VND7-VP16-GR, organogenesis from the shoot apex was rarely observed after DEX treatment (Figure 1B). Microscopic observation of VND7-VP16-GR revealed that some shoot meristematic cells lacked SCW deposits (Figure 3, Table 1). Based on these observations, we speculate that meristematic cells possess stronger native activity to inhibit transdifferentiation into xylem vessel cells compared to non-meristematic cells. In the seiv2 to seiv9 mutants, such inhibitory activity would be enhanced, particularly in the shoot apex, leading to the maintenance of meristem activity for continuous organogenesis. Considering the fact that the seiv2 to seiv9 mutants are dominant, it is possible that gain-of-function mutations on negative regulators for cell differentiation would be involved in the seiv phenotypes; for example, the dominant seiv mutations could influence the function, stability, binding, and subcellular localization of negative regulators (Meinke 2013).
Vascular plants typically contain three types of meristems: the shoot apical meristem, root apical meristem, and vascular meristem (or cambium). The positions and structures of these meristems differ, but interestingly, the regulatory factors that maintain meristem cells are partially overlapping. In all types of meristems, the secreted peptides CLAVATA3/EMBRYO SURROUNDING REGION-related (CLE) family members and their receptors LEUCINE-RICH REPEAT RECEPTOR-LIKE KINASE (LRR-RLK) and the homeobox gene WUSCHEL and its homologs WUSCHEL-RELATED HOMEOBOX (WOX) genes play central roles in regulating meristem size (Groß-Hardt and Laux 2003; Miyashima et al. 2013; Pierre-Jerome et al. 2018). In addition to these factors, class III homeodomain-leucine zipper (HD-ZIP III) transcription factors function in both the shoot apical meristem and vascular meristem (Byrne 2006; Miyashima et al. 2013). Notably, in both cases, HD-ZIP III transcription factors are involved not only in regulating meristem size, but also in tissue/organ development: HD-ZIP III transcription factors determine leaf polarity in shoots and promote xylem formation (Baima et al. 2001; Byrne 2006; Carlsbecker et al. 2010; McConnell and Barton 1998; Miyashima et al. 2013).
These findings suggest that the phenotypes of seiv shoot apical regions, i.e., the lack of ectopic xylem vessel cell differentiation (Figure 3, Table 1), can be attributed to the mutations of genes encoding common regulators shared by the shoot apical meristem and vascular meristem and/or regulators of meristem maintenance and cellular differentiation. Further analysis of the seiv mutants should provide novel insights into molecular factors that regulate xylem vessel cell differentiation, which could function at the intersection between meristem cell regulation and cell differentiation.
Acknowledgments
We thank Dr. Ko Kato, Dr. Minoru Kubo, Dr. Masaaki Umeda and Dr. Toshiro Ito (NAIST) for fruitful discussions, and Ms. Ayumi Ihara, Ms. Ryoko Hiroyama, Ms. Tomoko Matsumoto, Ms. Akiko Sato, Ms. Toshie Kita, and Ms. Kayo Kitaura (RIKEN) and Ms. Shizuka Nishida, Ms. Yuki Mitsubayasi, and Ms. Rieko Tanaka (NAIST) for their excellent technical assistance. This work was supported in part by RIKEN Center for Sustainable Resource Science, the JSPS KAKENHI (grant numbers JP25291062 and JP18H02466 to T.D., and JP20H03271 to M.O.), the MEXT KAKENHI (grant numbers JP24114002 to T.D., JP25114520, JP15H01235, and JP 20H05405 to M.O., JP18H05484 and JP18H05489 to M.O. and T.D.), and the ERATO from JST (grant number JPMJER1602 to M.O.).
Abbreviations
- DEX
dexamethasone
- GSNOR1
S-NITROSOGLUTATHIONE REDUCTASE 1
- EMS
ethyl methanesulfonate
- IRX
IRREGULAR XYLEM
- NAC
NAM/ATAF/CUC
- NO
nitric oxide
- NST3
NAC SECONDARY WALL THICKENING PROMOTING 3
- PCD
programmed cell death
- RAM
root apical meristem
- SAM
shoot apical meristem
- SCW
secondary cell wall
- seiv
suppressor of ectopic xylem vessel cell differentiation induced by VND7
- SND1
SECONDARY WALL-ASSOCIATED NAC DOMAIN 1
- VND
VASCULAR-RELATED NAC-DOMAIN
Supplementary Data
References
- Avci U, Earl Petzold H, Ismail IO, Beers EP, Haigler CH (2008) Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J 56: 303–315 [DOI] [PubMed] [Google Scholar]
- Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, Ruberti I, Morelli G (2001) The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol 126: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137–144 [DOI] [PubMed] [Google Scholar]
- Bollhöner B, Zhang B, Stael S, Denancé N, Overmyer K, Goffner D, Van Breusegem F, Tuominen H (2013) Post mortem function of AtMC9 in xylem vessel elements. New Phytol 200: 498–510 [DOI] [PubMed] [Google Scholar]
- Byrne ME (2006) Shoot meristem function and leaf polarity: The role of class III HD-ZIP genes. PLoS Genet 2: e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vatén A, Thitamadee S, et al. (2010) Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Yamaguchi M, Tamura T, Nakano Y, Nishikubo N, Yoneda A, Kato K, Kubo M, Kajita S, Katayama Y, et al. (2014) Multiple classes of transcription factors regulate the expression of VASCULAR-RELATED NAC-DOMAIN7, a master switch of xylem vessel differentiation. Plant Cell Physiol 56: 242–254 [DOI] [PubMed] [Google Scholar]
- Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5: 379–391 [DOI] [PubMed] [Google Scholar]
- Groß-Hardt R, Laux T (2003) Stem cell regulation in the shoot meristem. J Cell Sci 116: 1659–1666 [DOI] [PubMed] [Google Scholar]
- Kawabe H, Ohtani M, Kurata T, Sakamoto T, Demura T (2018) Protein S-nitrosylation regulates xylem vessel cell differentiation in Arabidopsis. Plant Cell Physiol 59: 17–29 [DOI] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy RL, Zhong R, Ye ZH (2009) MYB83 is a direct target of SND1 and acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell Physiol 50: 1950–1964 [DOI] [PubMed] [Google Scholar]
- McConnell JR, Barton MK (1998) Leaf polarity and meristem formation in Arabidopsis. Development 125: 2935–2942 [DOI] [PubMed] [Google Scholar]
- Meinke DW (2013) A survey of dominant mutations in Arabidopsis thaliana. Trends Plant Sci 18: 84–91 [DOI] [PubMed] [Google Scholar]
- Miyashima S, Sebastian J, Lee JY, Helariutta Y (2013) Stem cell function during plant vascular development. EMBO J 32: 178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Yamaguchi M, Endo H, Rejab NA, Ohtani M (2015) NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front Plant Sci 6: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2012) Secondary cell wall patterning during xylem differentiation. Curr Opin Plant Biol 15: 38–44 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Oda Y, Fukuda H (2010) Arabidopsis VASCULAR-RELATED NAC-DOMAIN6 directly regulates the genes that govern programmed cell death and secondary wall formation during xylem differentiation. Plant Cell 22: 3461–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Akiyoshi N, Takenaka Y, Sano R, Demura T (2016) Evolution of plant conducting cells: Perspectives from key regulators of vascular cell differentiation. J Exp Bot 68: 17–26 [DOI] [PubMed] [Google Scholar]
- Ohtani M, Demura T (2019) The quest for transcriptional hubs of lignin biosynthesis: Beyond the NAC-MYB-gene regulatory network model. Curr Opin Biotechnol 56: 82–87 [DOI] [PubMed] [Google Scholar]
- Ohtani M, Kawabe H, Demura T (2018) Evidence that thiol-based redox state is critical for xylem vessel cell differentiation. Plant Signal Behav 13: e1428512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Nishikubo N, Xu B, Yamaguchi M, Mitsuda N, Goué N, Shi F, Ohme-Takagi M, Demura T (2011) A NAC domain protein family contributing to the regulation of wood formation in poplar. Plant J 67: 499–512 [DOI] [PubMed] [Google Scholar]
- Peña MJ, Zhong R, Zhou GK, Richardson EA, O’Neill MA, Darvill AG, York WS, Ye ZH (2007) Arabidopsis irregular xylem8 and irregular xylem9: Implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19: 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre-Jerome E, Drapek C, Benfey PN (2018) Regulation of division and differentiation of plant stem cells. Annu Rev Cell Dev Biol 34: 289–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz M, Smith R, Ellis B (2013) Xylem tissue specification, patterning, and differentiation mechanisms. J Exp Bot 64: 11–31 [DOI] [PubMed] [Google Scholar]
- Soyano T, Thitamadee S, Machida Y, Chua NH (2008) ASYMMETRIC LEAVES2-LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis. Plant Cell 20: 3359–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Gallois P, Brown D (2007) Tracheary element differentiation. Annu Rev Plant Biol 58: 407–433 [DOI] [PubMed] [Google Scholar]
- Turner SR, Somerville CR (1997) Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9: 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AM, Rihouey C, Seveno M, Hörnblad E, Singh SK, Matsunaga T, Ishii T, Lerouge P, Marchant A (2009) The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. Plant J 57: 718–731 [DOI] [PubMed] [Google Scholar]
- Xu B, Ohtani M, Yamaguchi M, Toyooka K, Wakazaki M, Sato M, Kubo M, Nakano Y, Sano R, Hiwatashi Y, et al. (2014) Contribution of NAC transcription factors to plant adaptation to land. Science 343: 1505–1508 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Goué N, Igarashi H, Ohtani M, Nakano Y, Mortimer JC, Nishikubo N, Kubo M, Katayama K, Dupree P, et al. (2010a) VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol 153: 906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Kubo M, Fukuda H, Demura T (2008) VASCULAR-RELATED NAC-DOMAIN7 is involved in the differentiation of all types of xylem vessels in Arabidopsis roots and shoots. Plant J 55: 652–664 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Ohtani M, Mitsuda N, Kubo M, Ohme-Takagi M, Fukuda H, Demura T (2010b) VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 22: 1249–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Mitsuda N, Ohtani M, Ohme-Takagi M, Kato K, Demura T (2011) VASCULAR-RELATED NAC-DOMAIN 7 directly regulates the expression of a broad range of genes for xylem vessel formation. Plant J 66: 579–590 [DOI] [PubMed] [Google Scholar]
- Zhong R, Lee C, Ye ZH (2010) Global analysis of direct targets of secondary wall NAC master switches in Arabidopsis. Mol Plant 3: 1087–1103 [DOI] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH (2007) The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 19: 2776–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhong R, Ye ZH (2014) Arabidopsis NAC domain proteins, VND1 to VND5, are transcriptional regulators of secondary wall biosynthesis in vessels. PLoS One 9: e105726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.