Abstract
Most leguminous plants produce (−)-type enantiomers of pterocarpans as the phytoalexin, but pea (Pisum sativum L.) produces the opposite stereoisomer of pterocarpan, (+)-pisatin. Biosynthesis of (−)-pterocarpan skeleton is completely characterized at the molecular level, and pterocarpan synthase (PTS), a dirigent (DIR) domain-containing protein, participates in the last dehydration reaction. Similarly, isoflav-3-ene, a precursor of (+)-pisatin, is likely to be biosynthesized by the DIR-mediated dehydration reaction; however the biosynthesis is still unknown. In the present study, we screened PTS homologs based on RNA-sequence data from (+)-pisatin-producing pea seedlings and demonstrated that one of the candidates encodes isoflav-3-ene synthase (I3S). Real-time PCR analysis revealed that transcripts of I3S, in addition to other genes involved in the (+)-pisatin pathway, transiently accumulated in pea upon elicitation prior to the maximum accumulation of (+)-pisatin. I3S orthologs were also found in soybean and Lotus japonicus that are not known to accumulate (+)-pterocarpan, and the catalytic function of gene products was verified to be I3S by the in vitro enzyme assay. Incubation of the crude extract of elicited soybean cells with isoflav-3-ene yielded coumestrol, suggesting that isoflav-3-ene is a precursor of coumestrol biosynthesis in soybean.
Keywords: coumestrol, isoflav-3-ene, phytoalexin, (+)-pisatin, Pisum sativum
Introduction
Phytoalexins are plant-producing antimicrobial compounds induced by both biotic and abiotic stresses, and leguminous plants mainly produce pterocarpan-based isoflavonoids as phytoalexins (Aoki et al. 2000; Ingham 1982). Pterocarpans contain two asymmetric carbons at C-6a and C-11a, but only two cis configurations are sterically possible and found in nature (Figure 1) (Dewick 1986). All levorotatory pterocarpans are widely accepted to have the (6aR,11aR) configuration, such as (−)-medicarpin (6a) and (−)-maackiain (6b), and dextrorotatory pterocarpans have the opposite configuration, such as (+)-maackiain (7a) and (+)-pisatin (7c) (Slade et al. 2005). Among the two enantiomers of pterocarpans, most legumes produce (−)-type enantiomers, and only a limited number of plant species, such as peanut (Arachis hypogaea), Japanese pagoda tree (Styphnolobium japonicum), and pea (Pisum sativum), produce (+)-pterocarpans (Ingham 1979; Strange et al. 1985; VanEtten et al. 1989). The stereochemistry of pterocarpan is important because it determines the antimicrobial activity against pathogens; that is, some plant pathogens can detoxify (−)-isomers but not (+)-isomers, and as a result, (+)-pterocarpans show higher activity than the (−)-isomers (Delserone et al. 1992). The entire biosynthesis of (−)-pterocarpan was revealed by identifying pterocarpan synthase (PTS) as the long-standing missing link (Uchida et al. 2017), but further studies are required to elucidate the biosynthesis of (+)-pterocarpan.
Figure 1. Biosynthesis of (+)-pisatin and related compounds. Names of skeletons and individual compounds are shown in bold and plain fonts, respectively. Isoflav-3-enes are shown inside the dotted-line frame. Biosynthesis of isoflavones and 2′-hydroxyisoflavones shown here constitutes a metabolic grid, and enzymes involved are shown in parentheses. Constituents of isoflavonoids of leguminous plants are as follows: (6a) Glycyrrhiza spp. and Medicago spp., (6b) Maackia spp., Cicer spp., and Trifollium spp., (7a) Styphnolobium japonicum, (7c) Pisum sativum, (8) Glycine max, Glycyrrhiza spp., Lotus japonicus, and Medicago spp., (9) Cicer spp., (10) Lespedeza homoloba, and (11) Vigna unguiculate. Abbreviations: IFS, 2-hydroxyisoflavanone synthase; HID, 2-hydroxyisoflavanone dehydratase; HI4′OMT, 2-hydroxyisoflavanone 4′-O-methyltransferase; I2′H, isoflavone 2′-hydroxylase; I3′H, isoflavone 3′-hydroxylase; I3S, isoflav-3-ene synthase; IFR, isoflavone reductase; I4R, 2′-hydroxyisoflavanone 4-reductase; PBS, pseudobaptigenin synthase; PTS, pterocarpan synthase; SOR, sophorol reductase; VR, vestitone reductase; 2′HF, 2′-hydroxyformononetin; DMD, 7,2′-dihydroxy-4′,5′-methylenedioxyisoflavone; DMI, 7,2′-dihydroxy-4′-methoxyisoflavanol; DMDI, 7,2′-dihydroxy-4′,5′-methylenedioxyisoflavanol; DMIF, 7,2′-dihydroxy-4′-methoxyisoflav-3-ene; DMDIF, 7,2′-dihydroxy-4′,5′-methylenedioxyisoflav-3-ene; THIF, 7,2′,4′-trihydroxyisoflav-3-ene.
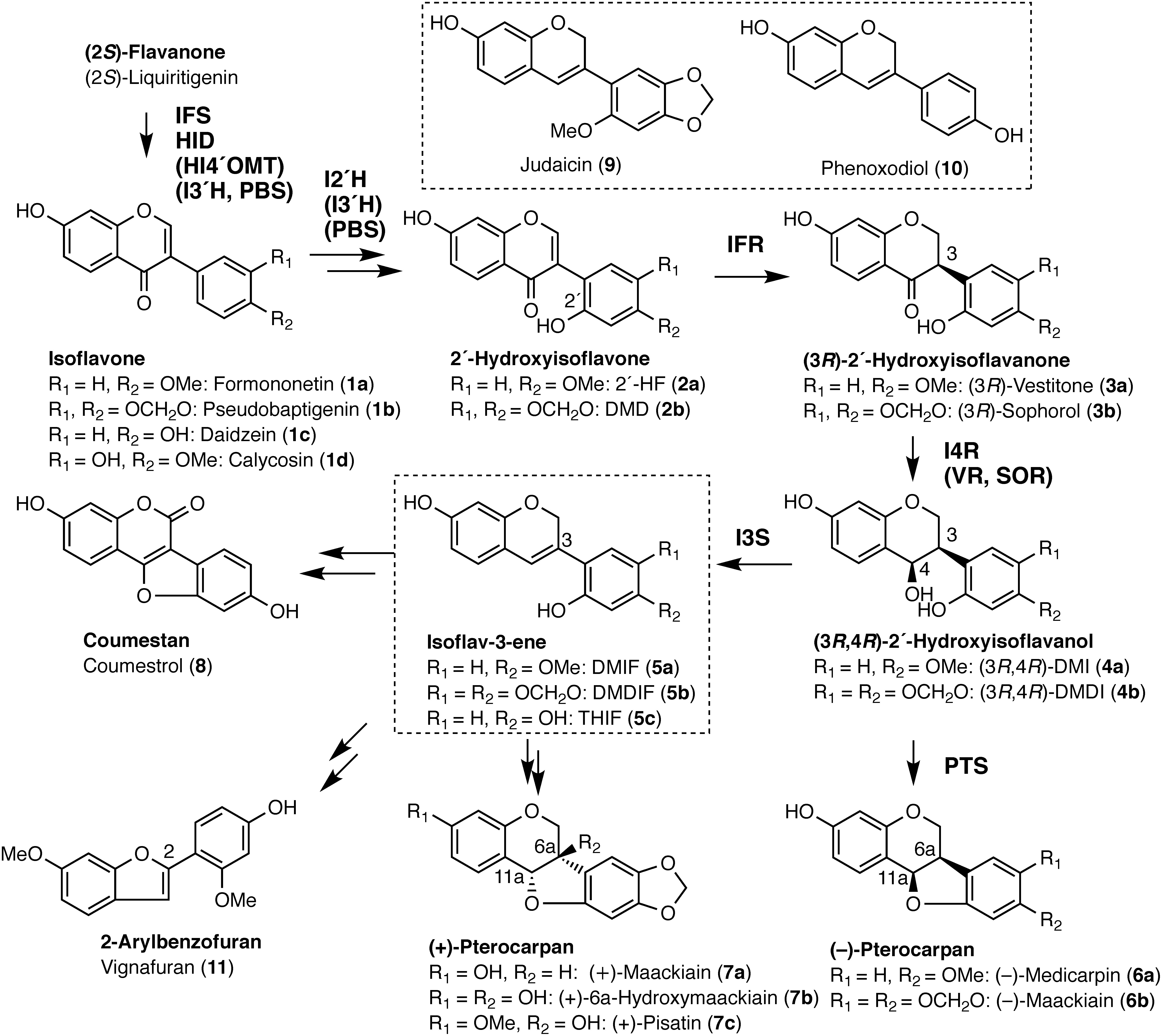
(+)-Pisatin (7c), which was the first chemically identified phytoalexin and is exclusively produced by pea (Cruickshank and Perrin 1960), is one of the best-studied (+)-pterocarpans but its biosynthesis has only been partially elucidated. The biosynthesis of (−)-pterocarpan has been found to involve two stereospecific intermediates, namely (3R)-2′-hydroxyisoflavanone and (3R,4R)-2′-hydroxyisoflavanol, the latter of which is converted to (−)-pterocarpan by PTS (Figure 1). Thus, clarifying the branching point to produce opposite stereoisomers is crucial for the elucidation of (+)-pterocarpan biosynthesis. As isoflavone reductase (IFR) catalyzes the first introduction step of chirality in pterocarpan biosynthesis, it was originally postulated to be the pivotal enzyme that determines the stereochemistry of the end product (Banks and Dewick 1982a, b). Indeed, several IFRs of the (−)-pterocarpan-producing legumes, such as soybean (Glycine max), alfalfa (Medicago sativa), and chickpea (Cicer arietinum), converted achiral 2′-hydroxyisoflavones into (3R)-2′-hydroxyisoflavanones (Fischer et al. 1990; Paiva et al. 1991; Tiemann et al. 1991; Uchida et al. 2017). An IFR isolated from (+)-pisatin-producing tissue of pea did not produce the expected (3S)-2′-hydroxyisoflavanone and yielded only (3R)-enantiomer (Paiva et al. 1994). The involvement of an epimerase, which converts (3R)-sophorol (3b) into (3S)-sophorol, has been previously suggested (Dewick 1986; Paiva et al. 1994). However, in a tracer experiment, 3H-labeled (3R)-sophorol (3b) was efficiently incorporated into (+)-pisatin (7c) compared with that from (3S)-sophorol (DiCenzo and VanEtten 2006), showing no evidence to support the hypothetical epimerase.
The next step to IFR is the conversion of (3R)-2′-hydroxyisoflavanone to (3R,4R)-2′-hydroxyisoflavanol, and 2′-hydroxyisoflavanone 4-reductase (I4R) mediates this reaction. I4R is also designated as a sophorol reductase (SOR) in pea (DiCenzo and VanEtten 2006) and vestitone reductase (VR) in alfalfa (Guo and Paiva 1995), and pea SOR specifically converts (3R)-sophorol (3b) to (3R,4R)-7,2′-dihydroxy-4′,5′-methylenedioxyisoflavanol (DMDI, 4b). The suppressed expression of the IFR and SOR genes by RNA-mediated genetic interference in the hairy roots of pea led to a decrease in the (+)-pisatin (7c) accumulation (Kaimoyo and VanEtten 2008). Taken together, these results indicate that (+)-pisatin (7c) is biosynthesized via (3R)-sophorol (3b) and (3R,4R)-DMDI (4b). More recently, in vitro enzyme assays using a cell-free extract of (+)-pisatin-producing pea seedlings have demonstrated the conversion of (3R,4R)-DMDI (4b) to 7,2′-dihydroxy-4′,5′-methylenedioxyisoflav-3-ene (DMDIF, 5b). Consequently, the achiral isoflav-3-ene was proposed to be an intermediate of (+)-pisatin biosynthesis (Celoy and VanEtten 2014). Thus, the molecular characterization of isoflav-3-ene synthase (I3S, also termed 2′-hydroxyisoflavanol 3,4-dehydratase) is essential to clarify the biosynthetic pathway of (+)-pisatin (7c).
To date, numerous studies have revealed the various biological activities of isoflav-3-enes. Based on chemical ecology, judaicin (9) of Cicer plants has antifeedant activity against the herbivorous pest insect, Helicoverpa armigera (Simmonds and Stevenson 2001). Furthermore, phenoxodiol (haginin E, 7,4′-dihydroxyisoflav-3-ene, 10) of Lespedeza homoloba induces apoptosis in chemoresistant ovarian cancer cells, thereby exerting significant antitumor activity (Kamsteeg et al. 2003). In isoflavonoid biosynthesis, isoflav-3-enes are predicted to be intermediates in the biosynthesis of coumestan (coumestrol, 8) and 2-arylbenzofuran (vignafuran, 11) (Kinoshita 1997; Martin and Dewick 1980). Isoflav-3-enes are labile compounds and their content in plant cells is very low; therefore, the identification and application of I3S would facilitate further studies on the biological activities of isoflav-3-ene-related isoflavonoids.
Recently, we biochemically identified PTS proteins of three leguminous plants and found that they belong to the b/d subfamily (DIR-b/d) of the dirigent (DIR) domain-containing protein (Uchida et al. 2017). They form a clade (PTS clade) with their orthologues with which they share >75% amino acid sequence identity. We also showed that a considerable number of PTS-like proteins from leguminous plants form a monophyletic group within a leguminous DIR-like clade. As I3S shares the substrate with PTS and catalyzes a similar dehydration reaction, suggesting structural similarity of I3S with the PTS clade. In the present study, we screened the RNA-sequence data of (+)-pisatin-producing pea seedlings for DIR protein-like transcripts and demonstrated that one of the candidates encodes I3S. Its orthologues from soybean and Lotus japonicus were also identified by homology-dependent gene isolation and biochemical characterization. Furthermore, an efficient synthesis of isoflav-3-enes from isoflavones was developed using co-cultured E. coli cells expressing several biosynthetic genes. Moreover, metabolic correlation between the consumption of isoflav-3-ene and production of coumestrol was also observed in the in vitro assay using crude extract of elicited soybean cells. The results achieved herein will offer a new perspective for the elucidation of stereoisomer-specific (+)-pterocarpan biosynthesis, mediated by DIR domain-containing proteins that produce isoflav-3-ene.
Materials and methods
Chemicals
Formononetin (1a) and coumestrol (8) were purchased from Tokyo Chemical Industry (Tokyo, Japan) and Sigma-Aldrich (St. Louis, MO, USA), respectively. (±)-Maackiain and (3R,4R)-DMDI (4b) were kindly donated by Dr. VanEtten HD (University of Arizona). DMDIF (5b) was prepared from maackiain according to a previous report (Martin and Dewick 1980). (3R,4R)-7,2′-Dihydroxy-4′-methoxyisoflavanol (DMI, 4a) was obtained from laboratory stock (Uchida et al. 2017).
(+)-Pisatin (7c) was isolated from elicited pea sprouts. Pea sprouts purchased from a grocery store were soaked in 10 mM CuCl2 for 1 h and rinsed with distilled water three times. After 24 h incubation at 25°C, leaves and stems were collected and soaked in hexane. Subsequently, the hexane extracts were evaporated, and (+)-pisatin (7c) was purified by successive silica-gel thin-layer chromatography (TLC, Silica-gel F254, Merck, Darmstadt, Germany) developed with the solvents, toluene : ethyl acetate=3 : 1 (v/v) and toluene : ethyl acetate : methanol : light petroleum (6 : 4 : 1 : 3, v/v/v/v). The nuclear magnetic resonance (NMR) spectra were recorded on an ECA-500 system (JEOL, Tokyo, Japan).
Plant materials and elicitation
Elicitation of pea seedlings was carried out according to a previous report with slight modifications (DiCenzo and VanEtten 2006). Briefly, surface-sterilized pea seeds (Pisum sativum cv. Usui, Takii Seed, Kyoto, Japan) were germinated on moist vermiculite and grown for 6 days in the dark. Cotyledons excised from seedlings were placed on filter paper soaked with 5 mM CuCl2 and periodically harvested. For the control, cotyledons were soaked with sterile distilled water. Elicited and control pea cotyledons were extracted with methanol, and the extracts were analyzed by HPLC using the conditions defined as ‘program 1’ (Supplementary Data S1). The concentration of (+)-pisatin (7c) was calculated from the area under the curve of the peak of the compound.
RNA-sequence and de novo assembly
Total RNAs were isolated from elicited (10 mM CuCl2 for 24 h) pea seedlings using the SV Total RNA Isolation System (Promega, Madison, WI, USA). RNA quality was evaluated on an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA). The cDNA library was prepared using the TruSeq RNA Sample Prep Kit v2 (Illumina, San Diego, CA, USA) and subjected to sequencing on the Illumina Miseq platform with 150 bp paired-end reads. Reads were de novo assembled with CLC Genomics Workbench ver 5.5 (CLC bio, Aarhus, Denmark) to obtain 47,799 contigs.
cDNA cloning and vector construction
cDNAs were synthesized from the total RNAs of elicited pea cotyledon using a SuperScript III First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA). The coding sequences of PsIFR (DDBJ accession no. AAB31368) and PsSOR (DDBJ accession no. AF107404), and N-terminus truncated PsI3S1, LjI3S1, LjI3S2, and GmI3S1 were amplified and cloned into the vectors (pCR8/GW/TOPO for PsIFR, PsSOR, and PsI3S1; pET46 Ek/Lic for LjI3S1, LjI3S2, and GmI3S1) using PrimeSTAR HS DNA polymerase (TaKaRa, Shiga, Japan), the primer sets (Supplementary Data S2), and the cDNA templates prepared from elicited pea cotyledons or L. japonicus and soybean cDNAs (Uchida et al. 2015). A multiple expression vector was constructed using the In-Fusion cloning system (TaKaRa). Briefly, coding regions of PsIFR, PsSOR, and N-terminus truncated PsI3S1 cDNAs were transferred into the pET-53-DEST vector using a Gateway System (Invitrogen). T7 promotor and coding region of PsSOR and PsI3S1 in pET-53-DEST were amplified by PCR, and the PCR products were cloned into a downstream region of PsIFR cDNA in pET-53-DEST.
The nucleotide sequence data reported herein have been deposited in the DDBJ, GenBank, and EMBL databases under the following accession numbers: PsI3S1, LC497416; GmI3S1, LC497417; LjI3S2, LC497419; LjI3S1, LC497418; and GmI4R, LC497420.
Heterologous expression and in vitro enzyme assay
The E. coli strain BL21-CodonPlus (DE3)-RIPL (Agilent Technologies) harboring an expression vector integrated with N-terminus truncated I3S (PsI3S1, LjI3S1, LjI3S2, and GmI3S1), were cultured overnight in 10 ml Luria Broth (LB) liquid medium containing 50 mg l−1 carbenicillin and 30 mg l−1 chloramphenicol. The cells were collected by centrifugation (9,000 g for 1 min at 4°C) and re-suspended in 200 ml terrific broth liquid medium containing 1% glucose, 2% ethanol, 50 mg l−1 carbenicillin, and 0.1 mM IPTG. After incubation for 24 h at 18°C, E. coli cells were harvested, and the recombinant I3S proteins were purified according to a previous report (Akashi et al. 2003). The enzyme assay was carried out in 0.1 M potassium phosphate buffer (pH 6.5) at 30°C in a total volume of 300 µl. For the initial assay, the purified PsI3S1 protein (ca. 2 µg) and the crude protein extract of E. coli cells (ca. 200 µg) expressing GmI3S1, LjI3S1, and LjI3S2 were incubated for 5 min and 30 min, respectively. To determine the relative activity, 50 µM (3R,4R)-DMI (4a) was incubated with purified I3S proteins (PsI3S1, 80 ng; GmI3S, 15 ng; LjI3S1 and LjI3S2, 12 ng) for 10 min. Reaction mixtures were extracted with ethyl acetate and analyzed by HPLC using the conditions defined as ‘program 2’ (Supplementary Data S1).
Phylogenetic analysis
Phylogenetic analysis was performed using MEGA6 software (Tamura et al. 2013). Amino acid sequences were aligned using ClustalW, and the phylogenetic tree was constructed with the default settings of neighbor-joining method with 1,000 bootstrap replicates.
Real-time PCR analysis
Total RNAs were isolated as described above, and cDNAs were synthesized using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). Real-time PCR was carried out as described previously (Uchida et al. 2017) using primer sets (Supplementary Data S2).
One-pot synthesis of isoflav-3-ene from isoflavone
Recombinant E. coli C41 (DE3) cells harboring LjCPR1 cDNA and codon-optimized LjI2′H gene (Uchida et al. 2015) or harboring PsIFR, PsSOR, and PsI3S1 cDNAs were pre-cultured separately in 50 ml of LB liquid medium containing 50 mg l−l carbenicillin overnight at 37°C. The cultures were combined in a 2,000 ml baffle flask. Thereafter, 1,000 ml of the LB medium was added with carbenicillin and 30 mg formononetin (1) dissolved in dimethyl sulfoxide : tween 80 : ethanol=1 : 1 : 1 (v/v/v), and supplements optimized for P450 expression (Uchida et al. 2015). Co-cultured E. coli cells were grown at 25°C for 72 h. E. coli cells were removed by centrifugation (9,000 g for 1 min at 4°C), and the supernatant was extracted three times with ethyl acetate. The ethyl acetate extracts of the reaction mixtures were analyzed by HPLC with the conditions defined as ‘program 3’ (Supplementary Data S1). For NMR analysis, the extract was concentrated in vacuo and applied to silica-gel TLC with the solvent toluene : ethyl acetate : methanol : light petroleum (6 : 4 : 1 : 3, v/v/v/v) and the product (Rf value=0.49) was subsequently collected.
Results
RNA-sequence analysis and selection of the I3S candidate gene
RNA-sequence and de novo assemble generated 47,799 contigs. When TBLASTN was employed using GePTS1 as the query, 5 contigs that share >50% identity were obtained (Supplementary Figure S1). Among them, Pisum21_contig00001700 had the highest reads per kilobase of exon model per million mapped reads (RPKM) value and seemed to contain the full-length coding sequence. The corresponding cDNA was cloned by RT-PCR and tentatively referred to as PsI3S1 for the high structural similarity (56% amino acid identity) to GePTS1.
Biochemical analysis of PsI3S1
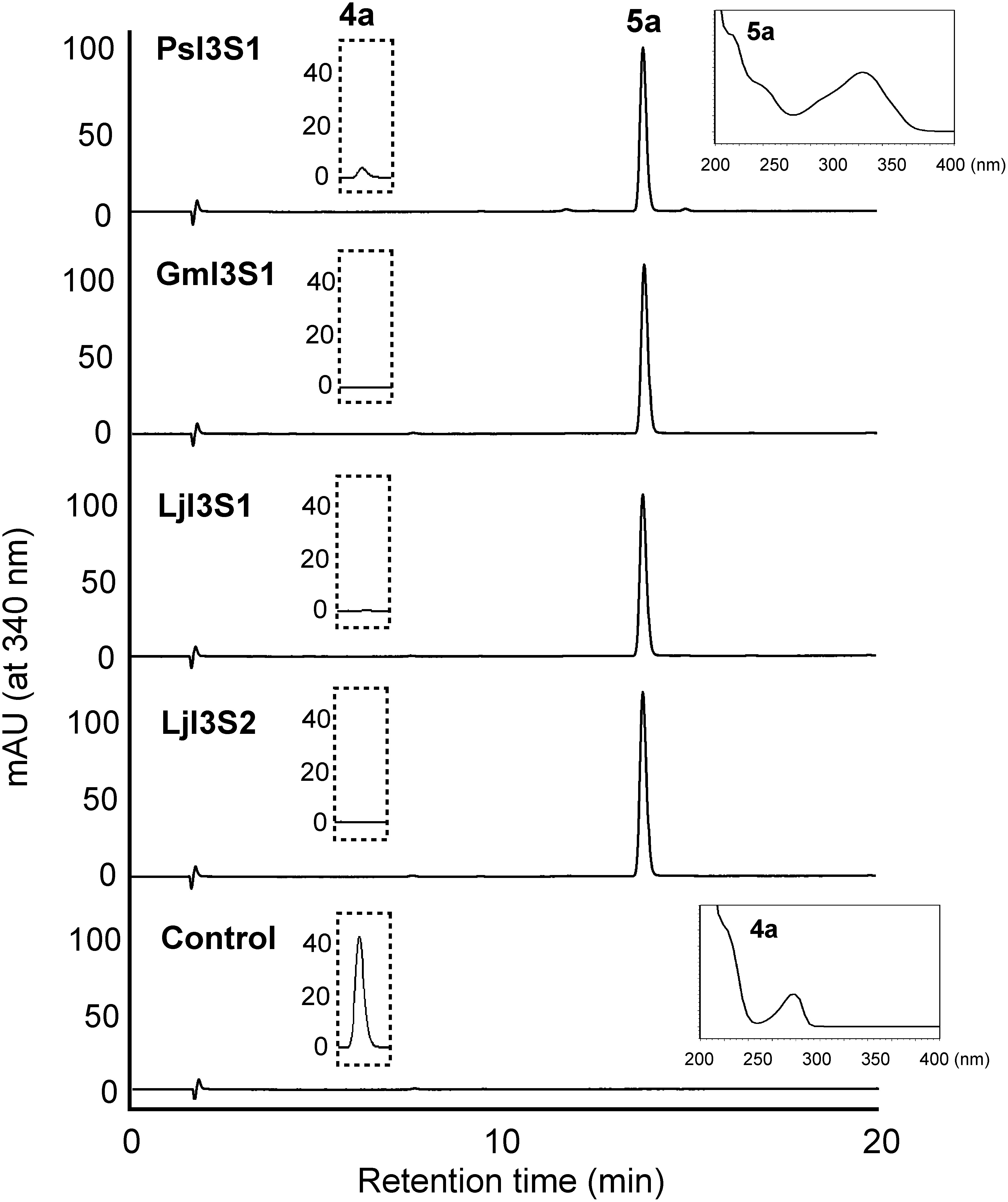
PsI3S1 was predicted to have the coding sequence of 657 bp and encode 218 amino acids. Most of the DIR domain-containing proteins have been reported to contain a putative signal peptide sequence at the N-terminus and processed into mature active forms after truncation of the signal peptide (Kim et al. 2002). The prediction of subcellular localization using WoLFPSORT (http://wolfpsort.org/) indicated that PsI3S1 is extracellular and/or vacuolar localized as well as GePTS1 (Uchida et al. 2017). Therefore, for biochemical analysis of PsI3S1, recombinant proteins were expressed as signal peptide-truncated and histidine-tagged forms in E. coli cells. Enzymatic properties were examined after affinity purification, and the assay was carried out with (3R,4R)-DMDI (4b) and (3R,4R)-DMI (4a) as substrates. The substrates are unstable and rapidly convert to pterocarpans under acidified conditions; therefore, 0.1 M potassium phosphate buffer (pH 6.5), which is the same buffer as in the PTS reaction, was used for the enzyme assay (Uchida et al. 2017). The incubation mixture of PsI3S1 with (3R,4R)-DMDI (4b) or (3R,4R)-DMI (4a) gave a single product on HPLC, and the products were identified as DMDIF (5b) and 7,2′-dihydroxy-4′-methoxyisoflav-3-ene (DMIF, 5a), respectively, by comparing UV, mass, or 1H-NMR spectra (Figure 2, Supplementary Figure S2, Data S3). These results show that PsI3S1 possesses isoflav-3-ene synthetic activity in vitro.
Figure 2. HPLC elution profiles of the products of recombinant I3S reactions. Affinity purified PsI3S1 and crude extracts of E. coli expressing I3S proteins of soybean (GmI3S1) and L. japonicus (LjI3S1 and LjI3S2) were reacted with (3R,4R)-DMI (4a), and ethyl acetate extracts of the reaction mixtures were analyzed. Because the maximum absorbances of the substrate (λmax 280 nm, retention time 5.6 min) and product (5a, λmax 340 nm, retention time 13.8 min) are largely different, the substrate was not detected in this chromatogram. The ordinate scales of the HPLC charts are equal. The eluates (retention times 5.0 to 6.0 min) monitored at 280 nm are shown in the dotted line frame, and the ordinate scales represent mAU. UV spectra of the product (5a) by PsI3S1 reaction and the substrate (4a) are shown in the line frame. A crude extract of E. coli transformed with pET-21a was used as the control.
Distribution of I3S in leguminous plants and phylogenetic analysis
To examine the distribution of the I3S protein in leguminous plants, I3S-like cDNAs were cloned from soybean and L. japonicus by RT-PCR. N-terminus truncated proteins were expressed in E. coli, and catalytic activity was tested using crude protein extracts with (3R,4R)-DMI (4a) as the substrate. As a result, the I3S activity of three orthologues, GmPTS-L4 from soybean and LjPTS-L1 and LjPTS-L2 from L. japonicus, were confirmed (designated as GmI3S1, LjI3S2, and LjI3S1, respectively) (Figure 2). The specific activity of I3S proteins toward (3R,4R)-DMI (4a) was compared using the purified recombinant proteins. GmI3S1 showed the highest specific activity (151.6±0.8 µmol min−1 mg−1; relative activity, 100%). PsI3S1, LjI3S1, and LjI3S2 showed 5%, 46%, and 53% of the activity of GmI3S1, respectively. The pH dependence of I3S activity was also examined using GmI3S1 and (3R,4R)-DMI. The optimum pH range was pH 6.0–8.0, and activity decreased rapidly outside this range (Supplementary Figure S3).
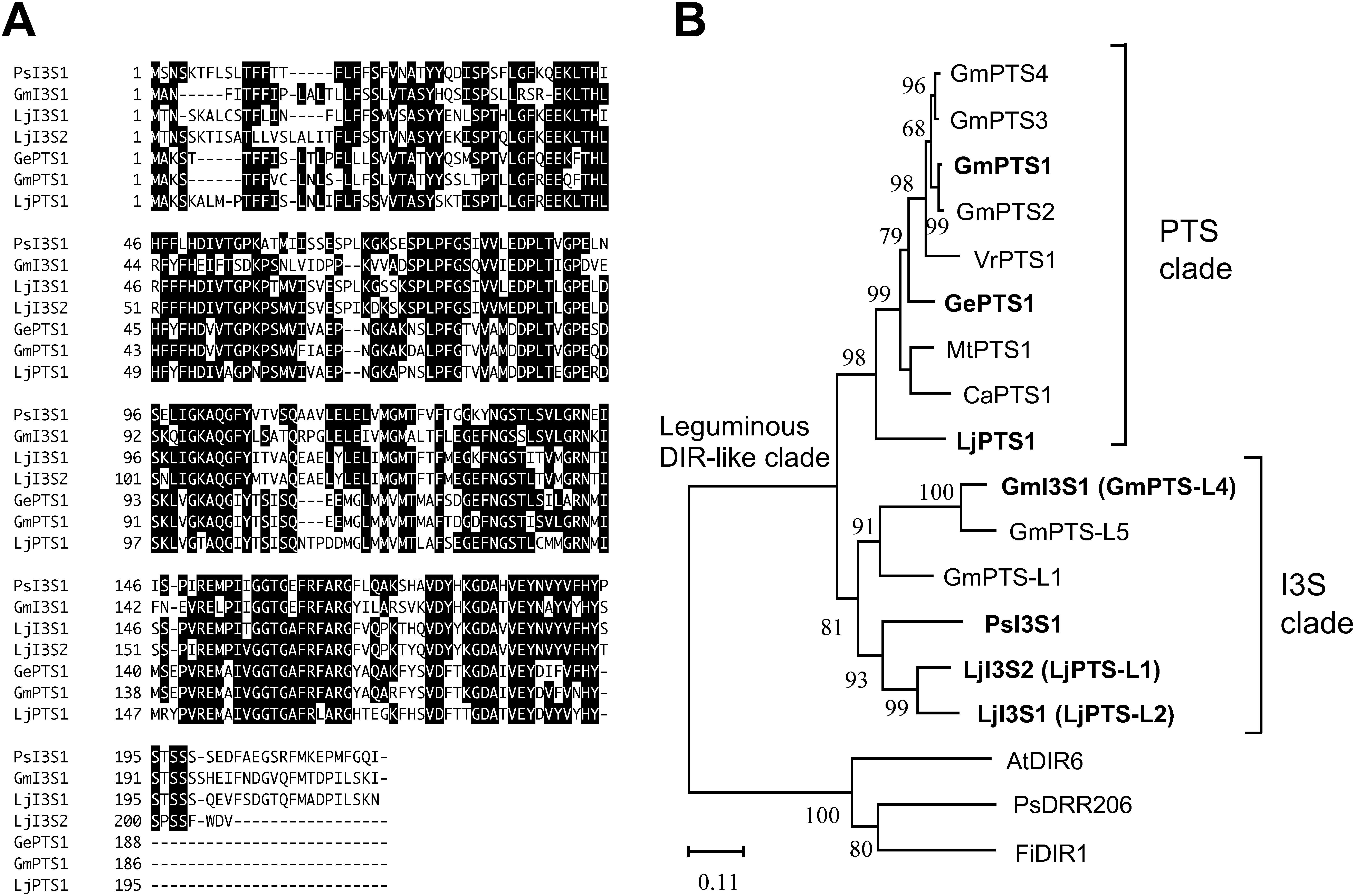
The identity of amino acid sequences among I3S proteins was 55–87% (Supplementary Data S4). Alignment of the amino acid sequence of the function-confirmed PTS and I3S showed that the I3S proteins have a C-terminus region that is 9–25 aa longer than that of PTS proteins (Figure 3A). As the postulated substrate and hypothetical reaction mechanism of I3S were the same as or very similar to those of PTS, a phylogenetic tree was constructed using the amino acid sequence of DIR domain-containing proteins, in particular the DIR-b/d subfamily (Uchida et al. 2017). Phylogenetic analysis showed that the I3S proteins constituted a distinct clade from that of the PTS (Figure 3B).
Figure 3. Alignment of amino acid sequences and the phylogenetic relationships among I3S, PTS, and the related DIR domain-containing proteins. (A) The amino acid residues with at least four identical sequences are in the reverse type. Gaps (−) are inserted to optimize alignment. (B) Evolutionary history was inferred using the Neighbor-Joining method. The percentage of replicate trees where the associated taxa are clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. Accession numbers of proteins are listed in Supplementary Data S6.
Time courses of (+)-pisatin-accumulation and transcript levels of the biosynthetic genes in elicited pea cotyledon
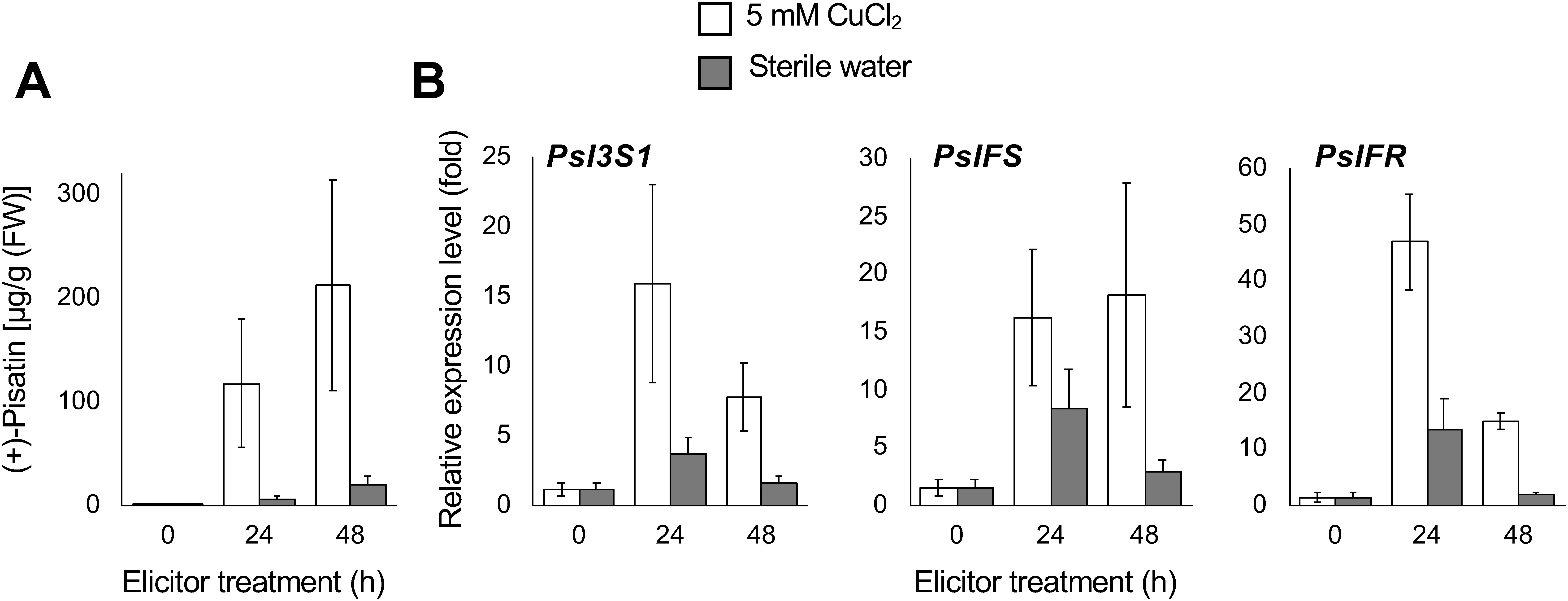
To verify whether PsI3S1 participates in (+)-pisatin biosynthesis, the accumulation of (+)-pisatin (7c) and the expression of its biosynthetic genes were analyzed. An abiotic stress agent, CuCl2, activates isoflavonoid metabolism in leguminous plants (Dewick 1986). (+)-Pisatin (7c) production was induced by 5 mM CuCl2 treatment, and its maximum content was observed at 24 h and 48 h after elicitation (Figure 4A). Although (+)-pisatin (7c) was also induced by the mock treatment, its amount was more than 10-fold lower than that of the CuCl2-treated cotyledon. The transcript levels of PsI3S1 and (+)-pisatin biosynthetic genes (PsIFS and PsIFR) were markedly increased by the elicitation, and the levels were 4.8–7.5-fold higher than the mock-treated cotyledon (Figure 4B).
Figure 4. Time course of (+)-pisatin accumulation (A) and transcript levels of the biosynthetic genes (B) in pea cotyledon upon CuCl2 treatment. Data are expressed as mean±SE (n=3 biological replicates). Transcript levels were analyzed using the ΔΔCt method. β-Tubulin was used as the internal standard. Transcript levels were normalized to those of non-treated cotyledons (at 0 h).
One-pot synthesis of isoflav-3-ene from isoflavone using a co-cultured recombinant E. coli system and biosynthesis of coumestrol in elicited soybean cells
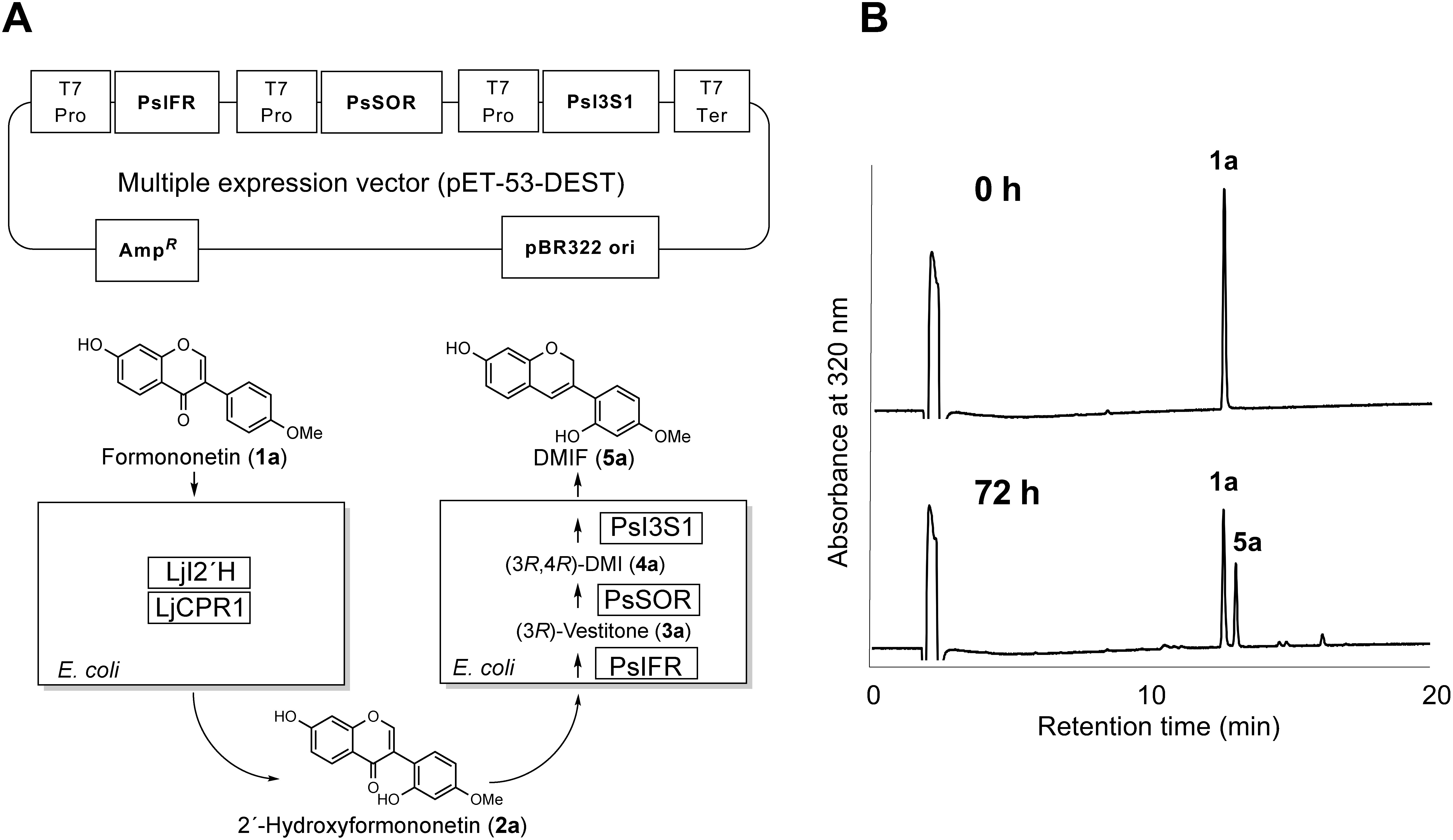
Isoflav-3-enes are considered to be precursors in some of the isoflavonoid pathways such as (+)-pterocarpan, coumestan, and 2-arylbenzofuran. An effective method for isoflav-3-ene production is thus important to elucidate the biosynthetic mechanism. For the biotechnological production of isoflav-3-ene, recombinant E. coli cells co-expressing isoflavone 2′-hydroxylase (I2′H), cytochrome P450 reductase (CPR), IFR, I4R, and I3S were tested for their in vivo metabolism (bioconversion) from exogenously supplied isoflavone. First, we constructed a multiple expression vector by introducing coding sequences of PsIFR, PsSOR, and PsI3S1 of pea (Figure 5A). Recombinant E. coli cells harboring LjCPR1 and codon-optimized LjI2′H (Uchida et al. 2015) of L. japonicus or harboring PsIFR, PsSOR, and PsI3S1 were pre-cultured separately in LB liquid medium, and then both cultures were combined in one flask and fermented for 72 h in the presence of the substrate formononetin (1). As shown in Figure 5B, a new peak was observed by HPLC, but no other biosynthetic intermediates were detected in this condition. The chemical structure of the product was confirmed by 1H-NMR to be DMIF (5a). The amount of DMIF (5a) calculated from the peak area on HPLC was ca. 15 mg l−1 after 72 h of fermentation. The stability of DMIF (5a) in the neutral buffer (pH 6.5 at 30°C) was also examined. The compound was decreased by a first-order reaction, and the half-life period under this condition was 136 h.
Figure 5. One-pot synthesis of isoflav-3-ene from isoflavone. Multiple expression vector and biosynthetic scheme (A) and HPLC chromatogram (B) are shown. Upper chromatogram and lower chromatogram show 0 h and 72 h after incubation with 1a, respectively (1a: formononetin, 5a: DMIF). T7 Pro, T7 promoter; T7 Ter, T7 terminator; AmpR, ampicillin resistance gene.
To examine the role of I3S proteins in soybean and L. japonicus, the metabolism of isoflav-3-ene in soybean was analyzed by an in vitro enzyme assay. The substrate, 7,2′,4′-trihydroxyisoflav-3-ene (THIF, 5c), was prepared using co-cultured E. coli cells expressing I2′H (CYP81E18), IFR, I4R, and GmI3S1 of soybean and LjCPR1 of L. japonicus, with daidzein (1c) as the precursor (Supplementary Figure S4 and Data S5). Then, THIF (5c) was incubated with crude extract of elicited soybean cells, and the reaction mixture was analyzed by HPLC. As the substrate was labile, a contaminant emerged during the incubation of both the control and crude extract of soybean, but two new peaks appeared in the crude extract of soybean (Supplementary Figure S5). Based on the retention times and UV spectra, one of the peaks was identified to be coumestrol (8).
Discussion
Isoflav-3-enes have been of interest because of their characteristic biological activities, and considerably attracted attention as a key intermediate in (+)-pisatin biosynthesis of pea. In the present study, we identified I3S cDNAs from pea (PsI3S1), soybean (GmI3S1), and L. japonicus (LjI3S1 and LjI3S2). It was predicted that all I3S proteins contained a putative signal peptide sequence at the N-terminus and were localized to extracellular region or vacuole. Although their subcellular localizations were not experimentally verified, the signal peptide-truncated proteins were found to produce only isoflav-3-enes from (3R,4R)-2′-hydroxyisoflavanols and functionally distinct from PTS proteins. The specific activities of the soybean and L. japonicus I3S proteins for (3R,4R)-DMI (4a) were roughly at the same level as that of recombinant PTS proteins, but PsI3S1 showed lower activity than the other I3S proteins (Uchida et al. 2017). (+)-Pisatin (7c) has a methylenedioxy ring, and the bridge formation is thought to occur at the early stage of biosynthesis because 14C-labeled methylenedioxylated isoflavone (pseudobaptigenin, 1b) was efficiently incorporated into pisatin (Banks and Dewick 1982a) (Figure 1). Thus, it is assumed that the natural substrate of PsI3S1 is (3R,4R)-DMDI (4b) (Celoy and VanEtten 2014), and the low activity of PsI3S1 toward (3R,4R)-DMI (4a) is attributable to the substrate preference of the protein. In general, the biosynthetic genes of phytoalexins are induced by biotic and abiotic stresses (e.g., attack by pathogens, herbivory, and elicitor treatment), and are considerably upregulated prior to the increased accumulation of phytoalexins. Therefore, comparing the time courses of (+)-pisatin (7c) accumulation and transcript levels is helpful to estimate the relevant biosynthetic pathway. In the present study, transient upregulation of PsI3S1, PsIFS, and PsIFR transcripts was clearly observed, compared to non-elicited, in pea cotyledon prior to (+)-pisatin (7c) accumulation (Figure 4). These results strongly support the possibility that PsI3S1 is involved in (+)-pisatin biosynthesis. The external stimuli, such as mechanical wounding, induce phytoalexins accumulation. (+)-Pisatin (7c) production was also induced by water (mock) treatment, even though the amount was over 10-fold lower than that of elicitor-treated cotyledons. This result indicates that cutting cotyledons from seedlings promoted (+)-pisatin (7c) accumulation.
Generally, structures of phytoalexin are lineage-specific, and (+)-pisatin (7c) is a characteristic product (namely, specialized metabolite) in pea. Most leguminous plants such as soybean and L. japonicus produce (−)-pterocarpans and their derivatives by stress responses, whereas the biosynthesis of (+)-pterocarpans remains unknown, and biosynthetic mechanisms of coumestan and 2-arylbenzofuran have also been a matter of debate (Kinoshita 1997; Martin and Dewick 1980). Considering that coumestrol and its derivatives are widely distributed among leguminous plants, and soybean cells produce a prenylated coumestrol by elicitation (Yoneyama et al. 2016), our data suggest that the soybean and L. japonicus I3S proteins participate in coumestan biosynthesis in planta. In fact, the activity of the conversion of THIF (5c) to coumestrol (8) was found in the crude extract of elicited soybean cells, also supporting our conclusion (Supplementary Figure S5). Oxidation at C-2 and ring closure reactions of isoflav-3-ene are necessary for the formation of coumestan skeleton, and indeed, coumestrol (8) production in enzyme preparation was accompanied by the formation of an unknown compound. These results imply that other enzymes, which are yet to be identified, are involved in the temporally coordinated biosynthesis with I3S.
Recently, we identified PTS as the first DIR domain-containing protein that possesses enzymatic activity. PTS has been assumed to catalyze the dehydration between the hydroxy groups at C-4 and C-2′ via a quinone methide intermediate. Accordingly, the 4R configuration of the substrate should be essential for the reaction, and configuration at C-3 could determine the stereochemistry of pterocarpan (Supplementary Figure S6) (Uchida et al. 2017). The I3S produces the achiral product, isoflav-3-ene, by losing the hydrogen at C-3 and the hydroxy group at C-4 of (3R,4R)-2′-hydroxyisoflavanol, contrasting the PTS-mediated reaction, which produces the chiral product (−)-pterocarpan from the same substrate (Uchida et al. 2017). A previous report showed that the crude extract of elicited pea tissues converts the cis-(−)-DMDI [(3R,4R)-isomer (4b)] into isoflav-3-ene; however, trans-(−)-DMDI (probably (3R,4S)-isomer) was not metabolized (Celoy and VanEtten 2014). Thus, the configuration at the C-4 position of the substrate is likely to be critical for both the I3S and PTS reactions, and the abstraction of a hydrogen at C-2′ hydroxy or C-3 of the quinone methide intermediate is expected to determine the product structure as catalytic outcome. As the amino acid sequences of both I3S and PTS proteins and the crystal structures of AtDIR6 and PsDRR206 proteins in the lignan pathway (Gasper et al. 2016; Kim et al. 2015) are now available, future structural studies based on homology modeling and mutagenesis would lead to the elucidation of key amino acid residues determining their distinct catalysis.
Several important biosynthetic steps of (+)-pisatin (7c) are yet to be resolved. The introduction of a methylenedioxy ring into the isoflavone skeleton, that is, the conversion of calycosin (1d) into pseudobaptigenin (1b), was detected in the microsomal fraction of elicited chickpea cells and demonstrated to be a cytochrome P450-dependent reaction (Clemens and Barz 1996). The reaction is consistent with different cytochrome P450s, CYP719A, and CYP81Q, which specifically catalyze the methylenedioxy ring formation for different plant-specialized metabolites isoquinoline alkaloids, and lignan (Ikezawa et al. 2007; Ono et al. 2006). An enzyme similar to chickpea cytochrome P450 should be involved in (+)-pisatin biosynthesis in pea. Furthermore, of particular interest is the introduction of (+)-chirality into the pterocarpan skeleton. The current model for the late steps of (+)-pisatin biosynthesis employs (+)-6a-hydroxymaackiain (7b) as a direct precursor of (+)-pisatin (7c). Although the biochemical basis for the conversion of achiral DMDIF (5b) to (+)-6a-hydroxymaackiain (7b) is yet to be elucidated, the oxygen of the C-6a hydroxy group of (+)-pisatin (7c) is shown to be derived from the H2O molecule and not the O2 molecule (Matthews et al. 1987).
Finally, many phytochemicals are of great interest because of their biological activities; however, their low availability in plant tissues is often the bottleneck for further investigation. Bioconversion using recombinant microbes is a potential solution for obtaining plant metabolites of interest, and using a co-culture system is an emerging approach for producing a variety of biochemicals (Hori et al. 2016; Zhang et al. 2015). In fact, isoflavones were efficiently converted into the end product isoflav-3-enes (Figure 5). DMIF (5a) could be recovered at a relatively high yield (ca. 80%) by silica-gel TLC, whereas THIF (5c) was unstable and easily decomposed on silica-gel TLC (recovery, ca. 20%). In the present study, TLC-purified THIF (5c) was used for the biosynthetic analysis of coumestrol, but we recently found that the degradation and recovery (ca. 70%) of the compound can be improved by using C18 octadecylsilyl column chromatography with aqueous methanol solution as the solvent (data not shown). Preparation of isoflav-3-ene by this system will therefore accelerate the elucidation of the biosynthetic mechanism introducing (+)-chirality in pea as well as coumestrol biosynthesis in soybean.
Acknowledgments
Dr. Toshio Aoki, a professor at Nihon University and a member of the editorial board of JSPCMB, passed away on March 20, 2019, after about two years of fighting against his illness. We dedicate this paper to the memory of Professor Aoki (1961–2019). Professor Hans D. VanEtten (University of Arizona), a pioneer of pisatin biosynthetic studies and a former collaborator of one of the present authors (T. Akashi), has also deceased in 2015. We thank the late Dr. VanEtten for authentic samples and express a deep regret on his passing. We are grateful for the cDNA clone (GMFL02-07-L14) provided by the National Bioresource Project–Lotus/Glycine (https://www.legumebase.brc.miyazaki-u.ac.jp/). Finally, the technical assistance of Saori Takemoto and Takahide Misaki of Nihon University is also gratefully acknowledged.
Abbreviations
- DIR
dirigent
- DMDI
7,2′-dihydroxy-4′,5′-methylenedioxyisoflavanol
- DMDIF
7,2′-dihydroxy-4′,5′-methylenedioxyisoflav-3-ene
- DMI
7,2′-Dihydroxy-4′-methoxyisoflavanol
- DMIF
7,2′-dihydroxy-4′-methoxyisoflav-3-ene
- HPLC
high performance liquid chromatography
- I2′H
isoflavone 2′-hydroxylase
- I3S
isoflav-3-ene synthase
- I4R
2′-hydroxyisoflavanone 4-reductase
- IFR
isoflavone reductase
- LB
Luria Broth
- NMR
nuclear magnetic resonance
- PTS
pterocarpan synthase
- RPKM
reads per kilobase of exon model per million mapped reads
- RT-PCR
reverse transcription polymerase chain reaction
- SOR
sophorol reductase
- THIF
7,2′,4′-trihydroxyisoflav-3-ene
- TLC
thin-layer chromatography
- VR
vestitone reductase
Supplementary Data
References
- Akashi T, Sawada Y, Shimada N, Sakurai N, Aoki T, Ayabe S (2003) cDNA cloning and biochemical characterization of S-adenosyl-L-methionine: 2,7,4′-trihydroxyisoflavanone 4′-O-methyltransferase, a critical enzyme of the legume isoflavonoid phytoalexin pathway. Plant Cell Physiol 44: 103–112 [DOI] [PubMed] [Google Scholar]
- Aoki T, Akashi T, Ayabe S (2000) Flavonoids of leguminous plants: Structure, biological activity, and biosynthesis. J Plant Res 113: 475–488 [Google Scholar]
- Banks SW, Dewick PM (1982a) Biosynthesis of the 6a-hydroxypterocarpan phytoalexin pisatin in Pisum sativum. Phytochemistry 21: 2235–2242 [Google Scholar]
- Banks SW, Dewick PM (1982b) (−)-Pisatin, an induced pterocarpan metabolite of abnormal configuration from Pisum sativum. Phytochemistry 21: 1605–1608 [Google Scholar]
- Celoy RM, VanEtten HD (2014) (+)-Pisatin biosynthesis: From (−) enantiomeric intermediates via an achiral 7,2′-dihydroxy-4′,5′-methylenedioxyisoflav-3-ene. Phytochemistry 98: 120–127 [DOI] [PubMed] [Google Scholar]
- Clemens S, Barz W (1996) Cytochrome P450-dependent methylenedioxy bridge formation in Cicer arietinum. Phytochemistry 41: 457–460 [Google Scholar]
- Cruickshank IA, Perrin DR (1960) Isolation of a phytoalexin from Pisum sativum L. Nature 187: 799–800 [DOI] [PubMed] [Google Scholar]
- Delserone LM, Matthews DE, Vanetten HD (1992) Differential toxicity of enantiomers of maackiain and pisatin to phytopathogenic fungi. Phytochemistry 31: 3813–3819 [Google Scholar]
- Dewick PM (1986) Isoflavonoids. In: Harborne JB (ed) The Flavonoids: Advances in Research Since 1980. Chapman & Hall, London, pp 125–209
- DiCenzo GL, VanEtten HD (2006) Studies on the late steps of (+) pisatin biosynthesis: Evidence for (−) enantiomeric intermediates. Phytochemistry 67: 675–683 [DOI] [PubMed] [Google Scholar]
- Fischer D, Ebenau-Jehle C, Grisebach H (1990) Purification and characterization of pterocarpan synthase from elicitor-challenged soybean cell cultures. Phytochemistry 29: 2879–2882 [DOI] [PubMed] [Google Scholar]
- Gasper R, Effenberger I, Kolesinski P, Terlecka B, Hofmann E, Schaller A (2016) Dirigent protein mode of action revealed by the crystal structure of AtDIR6. Plant Physiol 172: 2165–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Paiva NL (1995) Molecular cloning and expression of alfalfa (Medicago sativa L.) vestitone reductase, the penultimate enzyme in medicarpin biosynthesis. Arch Biochem Biophys 320: 353–360 [DOI] [PubMed] [Google Scholar]
- Hori K, Okano S, Sato F (2016) Efficient microbial production of stylopine using a Pichia pastoris expression system. Sci Rep 6: 22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa N, Iwasa K, Sato F (2007) Molecular cloning and characterization of methylenedioxy bridge-forming enzymes involved in stylopine biosynthesis in Eschscholzia californica. FEBS J 274: 1019–1035 [DOI] [PubMed] [Google Scholar]
- Ingham JL (1979) Phytoalexin production by flowers of garden pea (Pisum sativum). Z Naturforsch C 34: 296–298 [Google Scholar]
- Ingham JL (1982) Phytoalexins from the Leguminosae. In: Bailey JA, Mansfield JW (eds) Phytoalexins. Wiley, New York, pp 21–80
- Kaimoyo E, VanEtten HD (2008) Inactivation of pea genes by RNAi supports the involvement of two similar O-methyltransferases in the biosynthesis of (+)-pisatin and of chiral intermediates with a configuration opposite that found in (+)-pisatin. Phytochemistry 69: 76–87 [DOI] [PubMed] [Google Scholar]
- Kamsteeg M, Rutherford T, Sapi E, Hanczaruk B, Shahabi S, Flick M, Brown D, Mor G (2003) Phenoxodiol—an isoflavone analog—induces apoptosis in chemoresistant ovarian cancer cells. Oncogene 22: 2611–2620 [DOI] [PubMed] [Google Scholar]
- Kim KW, Smith CA, Daily MD, Cort JR, Davin LB, Lewis NG (2015) Trimeric structure of (+)-pinoresinol-forming dirigent protein at 1.95 A resolution with three isolated active sites. J Biol Chem 290: 1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Jeon JH, Fujita M, Davin LB, Lewis NG (2002) The western red cedar (Thuja plicata) 8-8′ DIRIGENT family displays diverse expression patterns and conserved monolignol coupling specificity. Plant Mol Biol 49: 199–214 [DOI] [PubMed] [Google Scholar]
- Kinoshita T (1997) A plausible chemical analogy for biosynthesis of 2-arylbenzofuran of isoflavonoid origin and its application to synthesis of vignafuran. Tetrahedron Lett 38: 259–262 [Google Scholar]
- Martin M, Dewick PM (1980) Biosynthesis of pterocarpan, isoflavan and coumestan metabolites of Medicago sativa: The role of an isoflav-3-ene. Phytochemistry 19: 2341–2346 [Google Scholar]
- Matthews DE, Weiner EJ, Matthews PS, Vanetten HD (1987) Role of oxygenases in pisatin biosynthesis and in the fungal degradation of maackiain. Plant Physiol 83: 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E, Nakai M, Fukui Y, Tomimori N, Fukuchi-Mizutani M, Saito M, Satake H, Tanaka T, Katsuta M, Umezawa T, et al. (2006) Formation of two methylenedioxy bridges by a Sesamum CYP81Q protein yielding a furofuran lignan, (+)-sesamin. Proc Natl Acad Sci USA 103: 10116–10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva NL, Edwards R, Sun YJ, Hrazdina G, Dixon RA (1991) Stress responses in alfalfa (Medicago sativa L.) 11. Molecular cloning and expression of alfalfa isoflavone reductase, a key enzyme of isoflavonoid phytoalexin biosynthesis. Plant Mol Biol 17: 653–667 [DOI] [PubMed] [Google Scholar]
- Paiva NL, Sun Y, Dixon RA, VanEtten HD, Hrazdina G (1994) Molecular cloning of isoflavone reductase from pea (Pisum sativum L.): Evidence for a 3R-isoflavanone intermediate in (+)-pisatin biosynthesis. Arch Biochem Biophys 312: 501–510 [DOI] [PubMed] [Google Scholar]
- Simmonds MSJ, Stevenson PC (2001) Effects of isoflavonoids from Cicer on larvae of Helicoverpa armigera. J Chem Ecol 27: 965–977 [DOI] [PubMed] [Google Scholar]
- Slade D, Ferreira D, Marais JP (2005) Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 66: 2177–2215 [DOI] [PubMed] [Google Scholar]
- Strange RN, Ingham JL, Cole DL, Cavill ME, Edwards C, Cooksey CJ, Garrattd PJ (1985) Isolation of the phytoalexin medicarpin from leaflets of Arachis hypogaea and related species of the tribe Aeschynomeneae. Z Naturforsch C 40: 313–316 [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann K, Inzé D, Van Montagu M, Barz W (1991) Pterocarpan phytoalexin biosynthesis in elicitor-challenged chickpea (Cicer arietinum L.) cell cultures. Purification, characterization and cDNA cloning of NADPH: Isoflavone oxidoreductase. Eur J Biochem 200: 751–757 [DOI] [PubMed] [Google Scholar]
- Uchida K, Akashi T, Aoki T (2015) Functional expression of cytochrome P450 in Escherichia coli: An approach to functional analysis of uncharacterized enzymes for flavonoid biosynthesis. Plant Biotechnol 32: 205–213 [Google Scholar]
- Uchida K, Akashi T, Aoki T (2017) The missing link in leguminous pterocarpan biosynthesis is a dirigent domain-containing protein with isoflavanol dehydratase activity. Plant Cell Physiol 58: 398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEtten HD, Matthews DE, Matthews PS (1989) Phytoalexin detoxification: Importance for pathogenicity and practical implications. Annu Rev Phytopathol 27: 143–164 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Akashi T, Aoki T (2016) Molecular characterization of soybean pterocarpan 2-dimethylallyltransferase in glyceollin biosynthesis: Local gene and whole-genome duplications of prenyltransferase genes led to the structural diversity of soybean prenylated isoflavonoids. Plant Cell Physiol 57: 2497–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pereira B, Li Z, Stephanopoulos G (2015) Engineering Escherichia coli coculture systems for the production of biochemical products. Proc Natl Acad Sci USA 112: 8266–8271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


