Abstract
Introduction:
The COVID-19 pandemic is rapidly evolving with the number of cases exponentially rising. The research scientific community has reacted promptly as evidenced by an outstanding number of COVID-19 related publications. As the number of scientific publications rapidly rises, there is a need to dissect the factors that lead to highly impactful publications. To that end, the present paper summarizes the characteristics of the top 50 cited COVID-19-related publications that emerged early during the pandemic.
Methods:
A systematic search of the Web of Science, Scopus, and Google Scholar was performed, using keywords related to COVID-19 and SARS-CoV-19. Two independent authors reviewed all the search results, screening for the top 50 cited COVID-19-related articles. Inclusion criteria comprised any publication on COVID-19 or the SARS-CoV-2 virus. Data extracted included the type of study, journal, number of citations, number of authors, country of publication, and study content.
Results:
As of May 29th, the top 50 cited articles were cited 63849 times during the last 4 months. On average, 14 authors contributed to each publication. Over half of the identified articles were published in only 3 journals. Furthermore, 42% and 26% of the identified articles were retrospective case series and correspondence/viewpoints, respectively, while only 1 article was a randomized controlled trial. In terms of content, almost half (48%) of the identified publications reported clinical/radiological findings while only 7 out of the 50 articles investigated potential treatments.
Conclusion:
By highlighting the characteristics of the top 50 cited COVID-19-related articles, the authors hope to disseminate information that could assist researchers to identify the important topics, study characteristics, and gaps in the literature.
Keywords: COVID-19, Bibliometric analysis, Evidence-based research
Introduction
December 2019 witnessed the first presentation of pneumonia-like illness that was soon after attributed to the novel SARS-CoV-2 virus.1 The Corona Virus Disease (COVID-19) aggressively spread throughout China in the following several weeks and became a world-wide pandemic affecting almost every country in the world within the subsequent 4 months.2 More worryingly, recent evidence shows that almost 20% of COVID-19 patients will require hospitalization, causing an enormous burden on health care systems across the world.3 As of May 3rd 2020, over 3.5 million cases tested positive with the Corona Virus Disease (COVID-19) of which over 250 000 had a fatal outcome.2
While the numbers continue to rise, the scientific research community has promptly responded, with over 500 COVID-19-related clinical trials currently underway.4 Research and scientific inquisitions are of paramount importance in the face of pandemics such as COVID-19. The absence of clinically proven curative treatments or vaccines coupled with a paucity in our understanding of the disease and long term clinical sequalae fuels the need for further research to help us better combat the current pandemic.
Bibliometric analyses assess the current status and trends in a specific research domain. This allows us to identify areas of importance and potential gaps in the literature which helps provide ideas and directions for future research. Such studies have been conducted in many different surgical and non-surgical domains; however, none have been performed on COVID-19 related publications.5-8 With respect to the COVID-19 pandemic, previous bibliometric analyses have been conducted to investigate research activity in different counties.9-12 However, none were conducted with the goal of highlighting the most highly cited COVID-19 early research publications. With the exponential growth of COVID-19-related literature, a bibliometric analysis of the top 50 cited COVID-19 related research is warranted.
To that end, the goal of this study was to present a bibliometric analysis to identify and dissect the characteristics of the top 50 cited COVID-19-related articles published early on following the outbreak. This will provide a deeper understanding of the current COVID-19 research milieu while highlighting potential patterns that could assist future researchers in their scientific pursuits.
Materials and Methods
Search strategy
A systematic search of the Web of Science, Scopus, and Google Scholar was performed, using keywords related to COVID-19 and SARS-CoV-19. The search strategy included the following key terms (2019-nCoV) OR (SARS-CoV-2) OR (corona virus) OR (COVID-19) and their associated variations. Search results were limited to 2019-2020. The search was conducted on, and included all articles up until May 29th 2020. The initial search yielded a total of 35 042 results.
Data collection and synthesis
The results from each database were sorted according to the citation count. The top 200 most cited publications from each search engine were exported to Microsoft Excel. Duplicate entries were manually identified and extracted by 2 independent authors. The top 50 most cited non-duplicate COVID-19 related publications were then identified. Inclusion criteria comprised any publication on COVID-19 or the SARS-CoV-2 virus. Publications in all languages were included. The only exclusion criteria were publications on other coronavirus diseases such as SARS. Data extracted included the type of study, journal, number of citations, number of authors, country of publication, and study content. All descriptive statistics were performed using Microsoft Excel functions.
Results
As of May 29th 2020, the top 50 cited COVID-19 related articles were cited a total of 63 849 times, with an average of 1277 (SD: 1084) per article.2,13-61 Over a third of the articles (n = 19) had more than 1000 citations and 18% (n = 9) had more than 2000 citations. The highest cited article was published in The Lancet and was cited 5897 times in a span of less than 5 months.26 As one of the earliest cohort studies on COVID-19’s clinical presentations, this study reported the clinical findings as well as patient characteristics of 41 COVID-19 patients in Wuhan.
The top 50 cited publications were published in 22 journals. Over half (n = 26) were published in only 3 journals: The Lancet, the New England Journal of Medicine, and the Journal of American Medical Association (JAMA). More interestingly, these 26 articles were cited a total of 44117 (69.1% of the total number of citations). The number of authors on each article varied from 2 to 65 authors with a mean of 14 (SD:12.2) authors. Furthermore, the most common types of articles were retrospective case series (42%) and correspondences/viewpoints (26%) while only one publication was a randomized controlled trial (RCT).
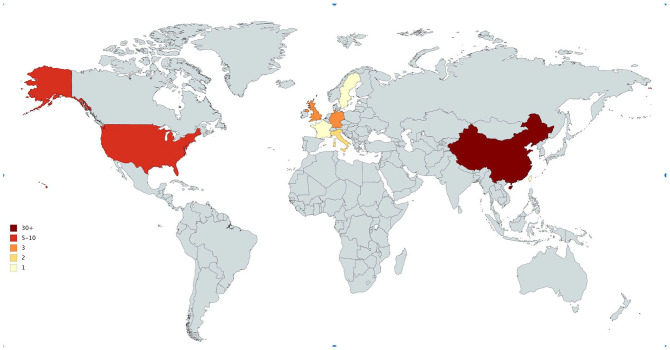
The top 50 cited articles were published out of 8 counties. China and the United States contributed to the majority of the publication (n = 31 and n = 8, respectively). Germany and the United Kingdom contributed to 3 publications each. Italy contributed to 2 publications while the remaining countries all contributed to 1 publication each (Figure 1). In terms of content, 48% of the articles (n = 24) reported clinical/radiological findings, 18% (n = 9) discussed basic science/genomic characterizations of the virus; and only 14% (n = 7) discussed treatment options (Table 1).
Figure 1.
Top 50 cited COVID-19 related articles’ country of origin.
Table 1.
Characteristics of the top 50 cited COVID-19-related publications.
| Journal | Number of publications | Total citations | Number of each publication type | Publication content | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective/case series | Correspondence/letter to the editor*** | Trials* | Review | Forecasting/modeling | Clinical findings | Public health/epidemiology | Transmission | Basic science/genomic characterization | Treatment | Other | |||
| The Lancet | 12 | 20 508 | 6 | 3 | 0 | 1 | 2 | 5 | 3 | 1 | 1 | 1 | 1 |
| New England Journal of Medicine | 8 | 15 177 | 4 | 3 | 1 | 0 | 0 | 5 | 0 | 2 | 0 | 1 | 0 |
| Lancet Associated Journals* | 5 | 4167 | 3 | 2 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 |
| JAMA/JAMA Internal Medicine | 6 | 8432 | 3 | 3 | 0 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 0 |
| Nature/Nature Reviews | 3 | 3986 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| International Journal of Antimicrobial Agents | 2 | 1590 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Science | 2 | 1470 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 |
| Others** | 12 | 8519 | 3 | 2 | 0 | 2 | 5 | 4 | 0 | 1 | 3 | 4 | 0 |
| Total | 50 | 63 849 | 21 | 13 | 2 | 5 | 9 | 24 | 5 | 4 | 9 | 7 | 1 |
Lancet associated journals include Lancet Respiratory Medicine, Lancet Infectious Disease and Lancet Global Health.
Other journals include American Journal of Obstetrics and Gynecology, Annals of Internal Medicine, Bioscience Trends, Eurosurveillance, Intensive Care Medicine, International Journal of Infectious Disease, Journal of Autoimmunity, Journal of Korean Medical Science, Journal of Travel Medicine, Journal of Virology, Science, Radiology.
Correspondence/letter to the editor additionally include viewpoints, editorials and comments.
Discussion
The current paper presents a bibliometric analysis of the top 50 cited articles related to COVID-19. Our analysis assesses the current status and trends of early COVID-19 research and highlights several interesting themes.
The first of which is that the scientific community’s response to the current COVID-19 pandemic was prompt and rigorous as evidenced by the outstanding number of citations that the identified articles received over the last 4 months. The open access policy that many journals have implemented with regards to COVID-19 publications has potentially contributed to quick dissemination of information and the exponential growth of publications in a short period of time.
The top 50 articles were published in 22 journals. Among these journals are the highest 7 out of 10 (including the top 2) impact factor journals across all domains. Approximately a quarter (n = 12) of the top 50 studies were published in 1 journal (The Lancet) and over half (n = 26) were published in 3 journals (The Lancet, New England Journal of Medicine, and JAMA). The distribution of the publications among these journals is consistent with Bradford’s law which stipulates that if you sort the journals by the number of articles they publish into 4 groups, each with about a quarter of the number of publications, then the number of journals in each group will be proportional to 1:2:22:23.62
The majority of the highly cited research assessed COVID-19’s clinical presentation and disease description while only 7 papers discussed potential treatment. This could be explained by the current limitation in our understanding of the SARS-CoV-2 virus and the need to better understand its associated disease before being able to investigate potential treatments. However, as the medical domain gets more accustomed to COVID-19, future studies should examine the efficacy of various treatments and vaccines.
Our analysis showed that on average each research article was conducted by 14 authors, which demonstrates the value of collaboration especially in high acuity situations such as pandemics. In addition, the results of this report demonstrate that the majority of highly cited COVID-19-related research were retrospective case series or commentaries/correspondences. While randomized controlled trials remain the gold standard of scientific research, due to the rapidly evolving nature of the situation and the short time period since the inception of the pandemic, lower grade evidence from retrospective research is of great value and can provide important information to help us better characterize the disease and its clinical presentation. The authors believe that higher-level evidence from controlled trials is still warranted to further our understanding of this disease.
This study has some limitations. First, the authors did not search all scientific databases. However, to address this limitation and ensure maximal inclusivity, the authors searched 3 of the most commonly used databases. Moreover, a limitation to any bibliometric analysis is the fact that citation frequency is affected by multiple factors such as journal and institution reputation and therefore is not a perfect reflection for academic influence. Furthermore, due to the short period of time since the inception of the pandemic and the quickly evolving nature of COVID-19 research, the number of citations will change over time. While this limitation is present with any bibliometric analysis, the main goal of this study was to highlight the characteristics of the highly cited research articles early during the COVID-19 pandemic and the dynamic nature of citation count should not diminish the value of the information presented here.
Conclusion
The current study presents a concise bibliometric analysis of the current COVID-19 research milieu. Our study shows that the scientific community has been very rigorous and active in publishing COVID-19-related articles as evidenced by the enormous number of citations the top 50 articles received, within less than half a year following the emergence of this disease. The majority of these papers are published among the most reputable journals world-wide. With the majority of the top 50 articles assessing COVID-19’s clinical/radiological findings, there remains a paucity in articles investigating treatments/vaccines.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interest:The authors declare that there is no conflict of interest.
Author Contributions: HE and AS contributed to the idea conception. All authors contributed to the data analysis, manuscript writing, and editing.
References
- 1. Lescure FX, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series [published online ahead of print March 27, 2020]. Lancet Infect Dis. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kinross P, Suetens C, Dias JG, et al. Rapidly increasing cumulative incidence of coronavirus disease (COVID-19) in the European Union/European Economic Area and the United Kingdom, 1 January to 15 March 2020. Euro Surveill. 2020;25:2000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. COVID-19 Clinical Research Coalition. Global coalition to accelerate COVID-19 clinical research in resource-limited settings. Lancet. 2020;395:1322-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandt JS, Hadaya O, Schuster M, Rosen T, Sauer MV, Ananth CV. A bibliometric analysis of top-cited journal articles in obstetrics and gynecology. JAMA Netw Open. 2019;2:e1918007-e1918007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreutzer JS, Agyemang AA, Weedon D, et al. The top 100 cited neurorehabilitation papers. NeuroRehabilitation. 2017;40:163-174. [DOI] [PubMed] [Google Scholar]
- 7. Landreneau JP, Weaver M, Delaney CP, et al. The 100 most cited papers in the history of the American Surgical Association. Ann Surg. 2020;271:663-670. [DOI] [PubMed] [Google Scholar]
- 8. Wang CY, Li BH, Ma LL, et al. The top-100 highly cited original articles on drug therapy for ventilator-associated pneumonia. Front Pharmacol. 2019;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chahrour M, Assi S, Bejjani M, et al. A bibliometric analysis of COVID-19 research activity: a call for increased output. Cureus. 2020;12:e7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tao Z, Zhou S, Yao R, et al. COVID-19 will stimulate a new coronavirus research breakthrough: a 20-year bibliometric analysis. Ann Transl Med. 2020;8:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dehghanbanadaki H, Seif F, Vahidi Y, et al. Bibliometric analysis of global scientific research on Coronavirus (COVID-19). Med J Islam Repub Iran. 2020;34:354-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gómez-Ríos D, López-Agudelo VA, Ramírez-Malule H. Repurposing antivirals as potential treatments for SARS-CoV-2: from SARS to COVID-19. J Appl Pharm Sci. 2020;10:1-9. [Google Scholar]
- 13. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32-E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. [DOI] [PubMed] [Google Scholar]
- 23. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science. 2020;368:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27:taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [DOI] [PubMed] [Google Scholar]
- 36. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281-292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [DOI] [PubMed] [Google Scholar]
- 53. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71:732-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [DOI] [PubMed] [Google Scholar]
- 57. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bradford SC. Sources of information on specific subjects. Engineering. 1934;137:85-86. [Google Scholar]