Abstract
Aim:
The prognostic value of multifocality (Mu) in papillary thyroid cancer (PTC) remains controversial. The present study aimed to investigate this issue and test the possible prognostic significance of the sum of the diameters of single foci (SDSF), the total number of foci (TNF), and primary tumor size (PTS) in multifocal PTC.
Methods:
We retrospectively analyzed a single-center consecutive series of 370 PTCs. For multifocal cases we analyzed bilaterality occurrence, SDSF, TNF, and PTS.
Results:
Mu was observed in 41.1% PTCs, and bilaterality in 30%. Mu was associated with an advanced T-category. In bilateral multifocal PTC, the PTS was larger, and microPTC was less frequent, while T-categories were higher. Mu and bilaterality per se had no impact on prognosis. At univariate analysis, PTS, SDSF, vascular invasion, lymph node metastases, distant metastases, T-categories, Initial Risk Stratification System score, second treatment and TERT promoter mutation correlated with persistence/recurrence or death in the multifocal PTC group. On multivariate Cox proportional hazards regression analyses, SDSF again independently predicted persistence/recurrence or death in multifocal PTCs. We found that a cut-off for SDSF less than 40 mm was able to identify multifocal PTC patients with a very low risk of persistence/recurrence (negative predictive value 96.9%). Disease-free survival was significantly shorter in patients with multifocal PTCs and SDSF ⩾40 mm.
Conclusions:
Mu and bilaterality per se were not prognostically significant. SDSF emerged as a new independent prognostic factor for persistence/recurrence of multifocal PTC. SDSF might better represent the tumor burden in multifocal PTC, with SDSF < 40 mm identifying multifocal PTC patients with a good prognosis.
Keywords: bilaterality, multifocality, papillary thyroid cancer, sum of diameters of single foci, tumor burden
Introduction
Well-differentiated thyroid cancer includes papillary and follicular histotypes, and accounts for the vast majority (>90%) of all thyroid cancers (TCs).1 In the United States (US), the incidence of TC has tripled in recent times, from 4.9 per 100,000 in 1975 to 14.3 per 100,000 in 2009.2 Papillary thyroid cancer (PTC), the main culprit responsible for the constantly rising incidence of TC, has an excellent prognosis, with survival rates exceeding 97% at 5 years, and >90% at 10 years.3,4 Given this low mortality rate for PTC, clinical and pathological factors affecting disease-free survival – in other words, disease persistence and recurrence – rather than disease-related death are the key elements to consider at diagnosis and during patient follow up in every day clinical practice.
So, while the AJCC/UICC staging system (8th edition) is the most powerful tool for predicting the mortality risk,5–8 a combination of tumor size and gross extrathyroidal extension, more or less aggressive histologies, the burden of metastatic lymph nodes, incomplete tumor resections, and particular molecular profiles have all emerged as significant factors influencing the risk of recurrent structural disease.
On the other hand, vascular invasion, capsular invasion, and multifocality (Mu) are less clear prognostic determinants, and any role they may have is still being debated.9
PTC can occur as single or multifocal tumors (involving two or more anatomically separate foci). The prevalence of Mu in PTC ranges from 32% to 39%.10,11 Mu most often presents as multiple microPTC (maximum tumor size <10 mm), and only occasionally with lesions visible on ultrasound.12 The latest guidelines issued by the American and European Thyroid Associations (ATA and ETA) place patients with multifocal PTC in the category at low risk of persistence/recurrence.13,14 Recent studies have found, however, that Mu is associated with lymph node metastases at diagnosis, and with persistent/recurrent disease during follow-up.10,11,15,16 Mu in PTC is also often empirically interpreted by clinicians as a high-risk factor, and this prompts a more aggressive treatment.17
In the literature, Mu in PTC is associated with older age,15,18 male sex,18,19 and extrathyroidal extension.11,15,17 It is only in some studies that Mu seemed to be associated with vascular invasion, lymph node metastases, more advanced disease and higher Initial Risk Stratification System (IRSS) scores.16,18 Nevertheless, the real prognostic significance of Mu in contributing to PTC persistence/recurrence, or even mortality, remains controversial. As an example, two recent meta-analyses reported opposite findings. The one conducted by Guo et al. included 7048 patients from 13 studies, and found Mu per se of no value in predicting recurrent disease.19 The other meta-analysis, by Joseph et al., concerned 178,550 patients with PTC from five predominantly registry-based studies, found that Mu had an impact on disease recurrence with a hazard ratio (HR) of 2.81; no data on mortality were considered.15 An interesting paper by Qu et al. analyzed whether the number of single tumor foci affected the prognosis: Mu emerged as a risk factor for recurrence, with a strong linear effect: the more numerous the foci, the higher the risk of recurrence.20
In a very recent study by Geron et al., on 1039 consecutive PTC patients, Mu confirmed its association with a more aggressive disease in terms of baseline characteristics, intensity of treatment, persistence/recurrence rates, and mortality. That said, after adjusting for confounding variables using a propensity score matching, Mu was no longer significantly associated with recurrence, long-term outcome, and mortality rates. The authors thus concluded that Mu in PTC is a marker of more extensive disease on presentation, but not an independent prognostic factor of disease outcome.18
As far as bilaterality is concerned, multifocal PTC may be bilateral in 13–71% of cases,11 and the prognostic significance of this characteristic is still debated. A study by Kim et al. found Mu, but not bilaterality, associated with disease persistence/recurrence, and the authors judged that the number of tumor foci was of greater prognostic value than their location.11 Another retrospective study analyzing 496 PTC patients found instead that bilaterality, as opposed to unilateral Mu, was an independent risk factor for neck recurrence (HR = 4.052), distant metastasis (HR = 3.860), and cancer-related death (HR = 7.252).21
In the present study, we investigated the prognostic value of Mu and bilaterality in PTC in a large consecutive series of patients treated at a single center. We also made an effort to fully characterize multifocal PTC from a clinical, histopathological and molecular point of view. Our aim was ultimately to establish whether the sum of the diameters of single foci (SDSF), the total number of foci (TNF), and primary tumor size (PTS) are of prognostic significance in multifocal PTC.
Materials and methods
Patients
We conducted a retrospective analysis on a consecutive series of 370 adult patients at a single center with thyroid nodules found malignant or suspect for TC (TIR 4–5 according to the SIAPEC 2014 consensus statement)22 on fine-needle aspiration cytology (FNAC). Between 2007 and 2017, all patients underwent total thyroidectomy and were histologically confirmed as cases of PTC.23 Decisions regarding the extent of initial surgery in these patients (total thyroidectomy with or without prophylactic neck compartment dissection), 131I treatment and follow-up modalities have been reported elsewhere.23
The histological variants of these cases of PTC were identified in accordance with World Health Organization (WHO) criteria.24 Histological diagnostics and staging were managed according to the AJCC/UICC system (8th edition), and on the grounds of the first whole-body scan after 131I remnant ablation.5–8 The maximum diameter of the largest tumor focus was used to define primary tumor size (PTS) in each case. MicroPTC was diagnosed when the largest tumor was ⩽1.0 cm in diameter. The methodology adopted for the molecular analysis to identify somatic TERT promoter and BRAF mutations has been described elsewhere.23
Patient outcome was defined as an excellent response (ER), indeterminate response (IR), biochemically persistent disease (BPD), or structurally-persistent disease (SPD), according to the ATA guidelines for patients given 131I therapy,13 and the criteria proposed by Momesso et al. for non-radioiodine-treated patients.25
Considering the low mortality rate in our series, patients were divided for the purposes of our study into three possible outcome groups: “BPD + SPD + TC-related death (TCD)” or “IR” or “ER”. The median follow-up period was 69 months (IQR: 42–92 months).
All studies were conducted in accordance with the guidelines of the Declaration of Helsinki. The present study was approved by the ethics committee of Azienda Ospedaliera di Padova (code N° AOP1303). All participants gave their written informed consent before enrolling for the study.
Unifocality, multifocality, bilaterality, and Mu-related variables: definitions
Two experienced pathologists (F.G. and G.P.) reviewed all pathology specimens to confirm all diagnoses of PTC, their anatomopathological features, cases of Mu, the number of tumor foci, and the diameter of each one. Unifocality was defined as a solitary focus of PTC, and Mu as the presence of two or more tumor foci in the pathological specimen of thyroid. Bilaterality was defined as the presence of tumor foci in both thyroid lobes.
In cases of multifocal PTC, the Mu-related variables of interest were defined as follows: (1) the SDSF was the sum of the largest diameters of all tumor foci; (2) the TNF was the total number of tumor foci; and (3) the PTS corresponded to the size of the PTC focus with the largest diameter.
Statistical analysis
The Kolmogorov–Smirnov test was used to assess the normal distribution of each variable. All data were expressed as mean ± standard deviation for normally distributed variables, and as median with interquartile range (IQR) for those not normally distributed. Mann–Whitney, and chi-square tests were used to compare clinical and pathological features, molecular mutational status, and oncological outcomes between the unifocal and multifocal PTC groups, as appropriate. When a dichotomized oncological outcome was needed for statistical purposes [multivariate Cox proportional hazards regression analyses, receiver operating characteristic (ROC) curves and Kaplan–Meier analysis], we pooled ER and IR in a “good outcome” group, and BPD, SPD, TCD in a “worse outcome” group. The same statistical analyses were used to compare the unilateral multifocal PTC, and bilateral-multifocal PTC groups.
To examine the prognostic factors on univariate analysis in the subset of multifocal PTCs, the Mann–Whitney and Kruskal–Wallis tests (for nonparametric variables), and Student’s t-test and one-way analysis of variance (for parametric variables) were used to correlate continuous variables with final oncological outcomes, as appropriate; and categorical variables were compared with outcome using the Chi-square test. Multivariate Cox proportional hazards regression analyses was used to identify the independent prognostic factors associated with outcome in the subset of multifocal PTCs, using a backward stepwise selection procedure with all clinically relevant variable. Disease-free survival (DFS) data were also analyzed using the Kaplan–Meier method. A p value < 0.05 was considered statistically significant.
Results
Patient characteristics
This study included 370 patients with histologically confirmed PTC who underwent total thyroidectomy, 282 (76.2%) females and 88 (23.8%) males, with a median age of 47 years (IQR 38–57 years). The median follow-up period was 69 months (IQR: 42–92 months).
In detail, the PTCs were histologically classified as follows: 248/370 (67%) classical variant; 24/370 (6.5%) follicular variant; 31/370 (8.4%) oxyphilic variant; and 67/370 (18.1%) were aggressive variants of PTC. Mu was seen in 152/370 PTCs (41.1%), and bilaterality in 111/370 (30%). Of the 152 multifocal PTCs, 41 (27%) were unilateral, and 111/152 (73%) were bilateral. SDSF and TNF descriptive characteristics of our population are shown in Table 1.
Table 1.
Descriptive characteristics of multifocality in patients with PTC.
| n | % | |
|---|---|---|
| TNF | ||
| 2 | 80/152 | 52.6% |
| 3 | 26/152 | 17.1% |
| 4 | 14/152 | 9.2% |
| 5 | 12/152 | 7.9% |
| 6–10 | 10/152 | 6.6% |
| 11–20 | 3/152 | 2% |
| 21–30 | 6/152 | 4% |
| 31–50 | 1/152 | 0.7% |
| SDSF (mm) | ||
| 5–10 | 12/152 | 7.9% |
| 11–20 | 54/152 | 35.5% |
| 21–30 | 44/152 | 29% |
| 31–40 | 19/152 | 12.5% |
| 41–50 | 8/152 | 5.2% |
| 51–70 | 10/152 | 6.6% |
| 71–116 | 5/152 | 3.3% |
PTC, papillary thyroid cancer; SDSF, sum of diameters of single foci, TNF, total number of foci.
The median PTS in the study population as a whole was 14 mm (IQR 11–20 mm); 89/370 patients (24%) had microPTCs. Histology showed vascular invasion in 123/196 PTCs (62.2%). Cervical lymph node metastases (globally N1a and N1b) were identified in 160/341 patients (46.9%). In particular, level VI lymph node metastases (N1a) were confirmed histologically in 99/341 patients (29%), and lateral neck lymph node metastases were present in 61/341 patients (17.9%). Distant metastases were identified in 8/370 patients (2.2%).
According to the 8th edition of the TNM classification system (AJCC/UICC5–8), 314/370 patients (84.9%) with PTC were classified as stage I, 50/370 (13.5%) in stage II, 1/370 (0.3%) in stage III, and 5/370 (1.4%) in stage IV at diagnosis. As concerns T-categories, 90/370 patients (24.3%) were in T1a, 181/370 (48.9%) in T1b, 69/370 (18.6%) in T2, 17/370 (4.6%) in T3a, 6/370 (1.6%) in T3b, 7/370 (1.9%) in T4a, and none in T4b.
BRAF mutations were identified in 232/368 (63%) PTCs, and TERT promoter mutations in 18/370 (4.9%).
According to the ATA guidelines, patients were IRSS scored as follows: 116/370 (31.4%) were at low risk; 234/370 (63.2%) were at intermediate risk; and 20/370 (5.4%) were at high risk.
At the end of follow up, 310/370 patients (83.8%) had an ER, 37/370 (10%) had an IR, 11/370 (3.0%) had a BPD, 9/370 (2.4%) had a SPD, and 3/370 (0.8%) had died of their disease (TCD).
For the purposes of our study, patients’ final oncological outcomes were grouped as follows: 310/370 (83.8%) patients had an ER, 37/370 (10%) patients an IR and 23/370 (6.2%) a BPD or SPD or TCD.
Comparing unifocal with multifocal PTC
The unifocal and multifocal PTC groups did not differ significantly in terms of age at diagnosis, sex, histological variants, PTS, microPTC, vascular invasion, cervical lymph node involvement (central and lateral neck), distant metastases, TNM stage, IRSS score, postoperative radioactive iodine (RAI) therapy, administered activity of RAI, second treatment, follow-up period or BRAF and TERT mutation status (Table 2). When the two groups were compared by T-categories, on the other hand, there was a statistically significant difference between the multifocal and unifocal PTC groups, with a moderately higher frequency of cases in T3a, T3b, and T4a in the multifocal PTC group (p = 0.04). At the end of the follow up, the multifocal and unifocal PTC groups did not differ in terms of final oncological outcome, however.
Table 2.
Comparison of characteristics of patients with unifocal versus multifocal PTC patients.
| Unifocal PTC n = 218 (%) | Multifocal PTC n = 152 (%) | p value | |
|---|---|---|---|
| Age at diagnosis [median; (IQR)] | 47 years old (38–56) | 46 years old (36.5–58) | NS |
| Gender | NS | ||
| Male | 46 (21.1%) | 42 (27.6%) | |
| Female | 172 (78.9%) | 110 (72.4%) | |
| PTC histological classification | NS | ||
| Classical variant | 146 (66.9%) | 102 (67.2%) | |
| Follicular variant | 15 (6.9%) | 9 (5.9%) | |
| Oxyphilic variant | 20 (9.2%) | 11 (7.2%) | |
| Aggressive Variants | 37 (17.0%) | 30 (19.7%) | |
| Primary tumor size [median; (IQR)] | 15 mm (11–20.25) | 13 mm (10–20) | NS |
| Primary tumor size | NS | ||
| ⩽10 mm (microPTC) | 45 (20.6%) | 44 (28.9%) | |
| >10 mm | 172 (79.4%) | 108 (71.1%) | |
| Vascular invasion | 55 (57.3%) | 68 (68%) | NS |
| Extrathyroidal extension | 115 (57.2%) | 85 (58.6%) | NS |
| Cervical lymph node involvement | 88 (43.8%) | 72 (51.4%) | NS |
| Cervical lymph node involvement | NS | ||
| Central, N1a | 57 (28.4%) | 42 (30%) | |
| Lateral, N1b | 31 (15.4%) | 30 (21.4%) | |
| Distant metastases | 6 (2.8%) | 2 (1.3%) | NS |
| TNM stage (8th edition) | NS | ||
| Stage I | 184 (84.4%) | 130 (85.5) | |
| Stage II | 29 (13.3%) | 21 (13.8%) | |
| Stage III | 0 | 1 (0.7%) | |
| Stage IV | 5 (2.3%) | 0 | |
| T-categories (TNM, 8th edition) | 0.04 | ||
| T1a | 47 (21.6%) | 43 (28.3%) | |
| T1b | 113 (51.8%) | 68 (44.7%) | |
| T2 | 47 (21.6%) | 22 (14.5%) | |
| T3a | 6 (2.8%) | 11 (7.2%) | |
| T3b | 2 (0.9%) | 4 (2.6%) | |
| T4a | 3 (1.4%) | 4 (2.6%) | |
| T4b | 0 | 0 | |
| Postoperative RAI therapy | NS | ||
| Yes | 208 (95.4%) | 139 (91.4%) | |
| No | 10 (4.6%) | 13 (8.6%) | |
| Administered activity of RAI [median; (IQR)] | 100 mCi (100–150) | 100 mCi (70–150) | NS |
| Second treatment | 20 (9.2%) | 22 (14.5%) | NS |
| Median follow-up period [median; (IQR)] | 70.5 months (40–94) | 65 months (44.5–87) | NS |
| BRAF mutation (FNAC) | 132 (60.8%) | 100 (66.2%) | NS |
| TERT promoter mutation (FNAC) | 12 (5.5%) | 6 (3.9%) | NS |
| Initial risk stratification system scores | NS | ||
| Low | 74 (33.9%) | 41 (27%) | |
| Intermediate | 135 (61.9%) | 100 (65.8%) | |
| High | 9 (4.1%) | 11 (7.2%) | |
| Disease status at latest follow-up (ongoing risk stratification) | NS | ||
| ER | 190 (87.2%) | 120 (78.9%) | |
| IR | 16 (7.3%) | 21 (13.8%) | |
| BPD | 5 (2.3%) | 6 (3.9%) | |
| SPD | 5 (2.3%) | 4 (2.6%) | |
| TCD | 2 (0.9%) | 1 (0.7%) | |
| Final oncological outcome | NS | ||
| ER | 190 (87.2%) | 120 (79.8%) | |
| IR | 16 (7.3%) | 21 (13.8%) | |
| BPD + SPD + TCD | 12 (5.5%) | 11 (7.2%) |
BPD, biochemically persistent disease; ER, excellent response; FNAC, fine needle aspiration cytology; IQR, interquartile range; IR, indeterminate response; NS, not significant; PTC, papillary thyroid cancer; RAI, radioactive immunotherapy; SPD, structurally persistent disease; TCD, death due to thyroid cancer.
Comparing unilateral multifocal with bilateral multifocal PTC
The multifocal PTC groups with unilateral as opposed to bilateral disease did not differ significantly in terms of age at diagnosis, sex, histological variants, vascular invasion, cervical lymph node involvement (central and lateral neck), distant metastases, TNM stage, IRSS score, postoperative RAI therapy, administered activity of RAI, second treatment, follow-up period or BRAF and TERT mutation status (Table 3). Patients with bilateral multifocal PTCs had a larger PTS (p = 0.01), and microPTCs were less frequent (p = 0.02) than in the group with unilateral multifocal PTCs. Comparing the two groups by T-categories, a significant difference emerged, with higher T-categories in the bilateral multifocal PTC group (p = 0.04). Here again, at the end of the follow up, the groups with unilateral versus bilateral-multifocal PTCs did not differ in terms of final oncological outcome.
Table 3.
Comparison between unilateral multifocal and bilateral multifocal PTC.
| Unilateral multifocal PTC n = 41 (%) | Bilateral multifocal PTC n = 111 (%) | p value | |
|---|---|---|---|
| Age at diagnosis (mean ± DS) | 46 years old ± 14 | 48 years old ± 15 | NS |
| Gender | NS | ||
| Male | 12 (29.3%) | 30 (27%) | |
| Female | 29 (70.7%) | 81 (73%) | |
| PTC histological classification | NS | ||
| Classical variant | 31 (75.6%) | 71 (64%) | |
| Follicular variant | 2 (4.9%) | 7 (6.3%) | |
| Oxyphilic variant | 1 (2.4%) | 10 (9%) | |
| Aggressive Variants | 7 (17.1%) | 23 (20.7%) | |
| Primary tumor size [median; (IQR)] | 11 mm (8–16) | 14 mm (11–21.75) | 0.01 |
| Primary tumor size | 0.02 | ||
| ⩽10 mm (microPTC) | 18 (43.9%) | 26 (23.4%) | |
| >10 mm | 23 (56.1%) | 85 (76.6%) | |
| Vascular invasion | 21 (84%) | 47 (62.7%) | NS |
| Cervical lymph node involvement | 17 (44.7%) | 55 (53.9%) | NS |
| Cervical lymph node involvement | NS | ||
| Central, N1a | 9 (23.7%) | 33 (32.4%) | |
| Lateral, N1b | 8 (21.1%) | 22 (21.6%) | |
| Distant metastases | 0 | 2 (1.8%) | NS |
| TNM stage (8th edition) | NS | ||
| Stage I | 37 (90.2%) | 93 (83.3%) | |
| Stage II | 3 (7.3%) | 18 (16.2%) | |
| Stage III | 1 (2.4%) | 0 | |
| Stage IV | 0 | 0 | |
| T-categories (TNM, 8th edition) | 0.04 | ||
| T1a | 19 (46.3%) | 24 (21.6%) | |
| T1b | 15 (36.6%) | 53 (47.7%) | |
| T2 | 5 (12.2%) | 17 (15.3%) | |
| T3a | 0 | 11 (9.9%) | |
| T3b | 1 (2.4%) | 3 (2.7%) | |
| T4a | 1 (2.4%) | 3 (2.7%) | |
| T4b | 0 | 0 | |
| Postoperative RAI therapy | 35 (85.4%) | 103 (93.6%) | NS |
| Administered activity of RAI [median; (IQR)] | 100 mCi (77.5–150) | 100 mCi (70–150) | NS |
| Second treatment | 5 (12.2%) | 17 (15.3%) | NS |
| Follow-up period (mean ± DS) | 71.5 months ± 35 | 65.5 months ± 30 | NS |
| BRAF mutation (FNAC) | 27 (65.9%) | 73 (66.4%) | NS |
| TERT promoter mutation (FNAC) | 1 (2.4%) | 5 (4.5%) | NS |
| Initial risk stratification system scores | NS | ||
| Low | 13 (31.3%) | 28 (25.2%) | |
| Intermediate | 25 (61%) | 75 (67.6%) | |
| High | 3 (7.3%) | 8 (7.2%) | |
| Disease status at latest follow-up (ongoing risk stratification) | NS | ||
| ER | 34 (82.9%) | 86 (77.5%) | |
| IR | 6 (14.6%) | 15 (13.5%) | |
| BPD | 1 (2.4%) | 5 (4.5%) | |
| SPD | 0 | 4 (3.6%) | |
| TCD | 0 | 1 (0.9%) | |
| Final oncological outcome | NS | ||
| ER | 34 (82.9%) | 86 (77.5%) | |
| IR | 6 (14.6%) | 15 (13.5%) | |
| BPD + SPD + TCD | 1 (2.4%) | 10 (9%) |
BPD, biochemically persistent disease; ER, excellent response; FNAC, fine needle aspiration cytology; IQR, interquartile range; IR, indeterminate response; NS, not significant; PTC, papillary thyroid cancer; RAI, radioactive immunotherapy; SPD, structurally persistent disease; TCD, death due to thyroid cancer.
Risk factors for persistent/recurrent disease or disease-related death in multifocal PTC
We analyzed the factors that could predict persistent/recurrent disease or disease-related death in the group of patients with multifocal PTC (Table 4).
Table 4.
Univariate analysis of prognostic factors for persistent/recurrent disease or disease-related death in patients with multifocal PTC.
| Excellent response n = 120 (%) | Indeterminate response n = 21 (%) | BPD + SPD + TCD n = 11 (%) | p value | |
|---|---|---|---|---|
| Age at diagnosis (mean ± SD) | 48 years ± 14 | 47 years ± 15 | 46 years ± 23 | NS |
| Gender | NS | |||
| Male | 33 (27.5%) | 4 (19%) | 5 (45.5%) | |
| Female | 87 (72.5%) | 17 (81%) | 6 (54.5%) | |
| PTC histological classification | NS | |||
| Classical variant | 81 (67.5%) | 15 (71.4%) | 6 (54.5%) | |
| Follicular variant | 7 (5.8%) | 1 (4.8%) | 1 (9.1%) | |
| Oxyphilic variant | 9 (7.5%) | 1 (4.8%) | 1 (9.1%) | |
| Aggressive variants | 23 (19.2%) | 4 (19%) | 3 (27.3%) | |
| PTS [median; (IQR)] | 13 mm (9–20) | 12 mm (10.5–15) | 25 mm (16.25–41.5) | 0.004 |
| Primary tumor size | NS | |||
| ⩽10 mm (microPTC) | 39 (32.5%) | 5 (23.8%) | 0 | |
| >10 mm | 81 (67.5%) | 16 (76.2%) | 11 (100%) | |
| TNF [median; (IQR)] | 2 (2–4) | 3 (2–6.25) | 3 (2–3) | NS |
| SDSF [median; (IQR)] | 21 mm (14–30) | 23 mm (15.5–32.75) | 62 mm (24–67.5) | 0.004 |
| Vascular invasion | 46 (61.3%) | 15 (83.3%) | 7 (100%) | 0.03 |
| Cervical lymph node involvement | 51 (45.9%) | 13 (68.4%) | 8 (80%) | 0.03 |
| Cervical lymph node involvement | 0.001 | |||
| Central, N1a | 33 (29.7%) | 8 (42.1%) | 1 (10%) | |
| Lateral, N1b | 18 (16.2%) | 5 (26.3%) | 7 (70%) | |
| Distant metastases | 0 | 0 | 2 (18.2%) | <0.0001 |
| Bilaterality | 86 (71.7%) | 15 (71.4%) | 10 (90.9%) | NS |
| TNM stage (8th edition) | 0.03 | |||
| Stage I | 105 (87.5%) | 19 (90.5%) | 6 (54.5%) | |
| Stage II | 14 (11.7%) | 2 (9.5%) | 5 (45.5%) | |
| Stage III | 1 (0.8%) | 0 | 0 | |
| Stage IV | 0 | 0 | 0 | |
| T-categories based on PTS (TNM, 8th edition) | 0.004 | |||
| T1a | 37 (30.8%) | 5 (23.8%) | 0 | |
| T1b | 53 (44.5%) | 14 (66.7%) | 4 (36.4%) | |
| T2 | 17 (14.2%) | 1 (4.8%) | 2 (18.2%) | |
| T3a | 7 (5.8%) | 1 (4.8%) | 3 (27.3%) | |
| T3b | 4 (3.3%) | 0 | 0 | |
| T4a | 2 (1.7%) | 0 | 2 (18.2%) | |
| T4b | 0 | 0 | 0 | |
| T-categories based on SDSF (TNM, 8th edition) | 0.0003 | |||
| T1a | 12 (10%) | 2 (9.5%) | 0 | |
| T1b | 42 (35%) | 7 (33.3%) | 1 (9.1%) | |
| T2 | 48 (40%) | 10 (47.6%) | 2 (18.2%) | |
| T3a | 12 (10%) | 2 (9.5%) | 6 (54.5%) | |
| T3b | 4 (3.3%) | 0 | 0 | |
| T4a | 2 (1.7%) | 0 | 2 (18.2%) | |
| T4b | 0 | 0 | 0 | |
| Postoperative RAI therapy | 109 (91.6%) | 19 (90.5%) | 10 (90.9%) | NS |
| Administered activity of RAI [median; (IQR)] | 100 mCi (70–150) | 125 mCi (50–150) | 150 mCi (150–150) | 0.03 |
| Follow-up period (mean ± DS) | 69 months ± 30 | 68 months ± 31 | 46 months ± 40 | NS |
| Second treatment | 6 (5%) | 8 (38.1%) | 8 (72.7%) | <0.0001 |
| BRAF mutation (FNAC) | 78 (65.5%) | 13 (61.9%) | 9 (81.8%) | NS |
| TERT promoter mutation (FNAC) | 4 (3.3%) | 0 | 2 (18.2%) | 0.03 |
| Initial risk stratification system scores | 0.0003 | |||
| Low | 37 (31.1%) | 2 (9.5%) | 2 (18.2%) | |
| Intermediate | 78 (65.5%) | 16 (76.2%) | 5 (45.5%) | |
| High | 4 (3.4%) | 3 (14.3%) | 4 (36.4%) |
BPD, biochemical persistent disease; FNAC, fine needle aspiration cytology; IQR, interquartile range; NS, not significant; PTS, primary tumor size; RAI, radioactive immunotherapy; SDSF, sum of diameters of single foci; SPD, structural persistent disease; TCD, death due to thyroid cancer; TNF, total number of foci.
On univariate analysis, PTS (p = 0.004), SDSF (p = 0.004), vascular invasion (p = 0.03), lymph node involvement (p = 0.03), with N1b carrying a higher risk than N1a (p = 0.001), distant metastases (p < 0.0001), stage at diagnosis (p = 0.03), T-categories (p = 0.004), IRSS score (p = 0.0003), administered activity of RAI (p = 0.03), second treatment (p < 0.0001) and TERT promoter mutation (p = 0.03) all correlated significantly with the risk of persistent/recurrent disease or disease-related death in the multifocal PTC group. When we reclassified patients’ T-categories based on SDSF instead of PTS, T-categories was even more strongly associated with PTC persistence/recurrence or death (p = 0.0003).
On multivariate Cox proportional hazards regression analyses, only distant metastases (HR 39.4761, 95% CI 6.4794–240.5100, p = 0.0001), and SDSF (HR 1.0734, 95% CI 1.0212–1.128, p = 0.0056) independently predicted persistent/recurrent disease or disease-related death in multifocal PTC patients.
Considering the prognostic impact of SDSF in multifocal PTCs, we were able to identify a cut-off of 40 mm using ROC curve analysis (p = 0.0002, area under the curve 0.792; Figure 1). In other words, SDSF > 40 mm in cases of multifocal PTC can predict a worse prognosis with a sensitivity of 63.6% and specificity of 88.7%. The cut-off also showed a very high negative predictive value of 96.9%. Adopting this SDSF cut-off of 40 mm, we analyzed DFS using the Kaplan–Meier method, and dividing multifocal PTC patients by SDSF ⩾40 mm or <40 mm (Figure 2). The 5-year DFS rates were 71.1% in multifocal PTC patients with SDSF ⩾40 mm and 96.8% in those with SDSF < 40 mm. A log-rank test showed that the DFS rate was significantly lower (p < 0.0001) in patients with SDSF ⩾40 mm.
Figure 1.
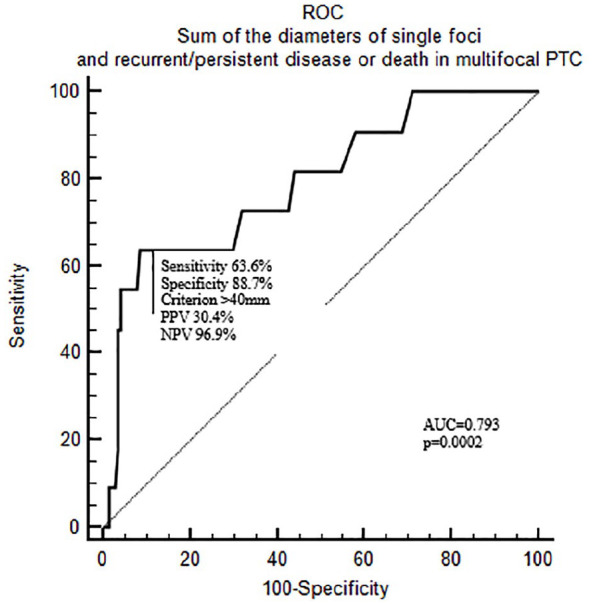
ROC curve analysis on the sum of the diameters of single foci and persistent/recurrent disease or death in multifocal PTC.
AUC, area under the curve; NPV, negative predictive value; PPV, positive predictive value; PTC, papillary thyroid cancer; ROC, receiver operating characteristic.
Figure 2.
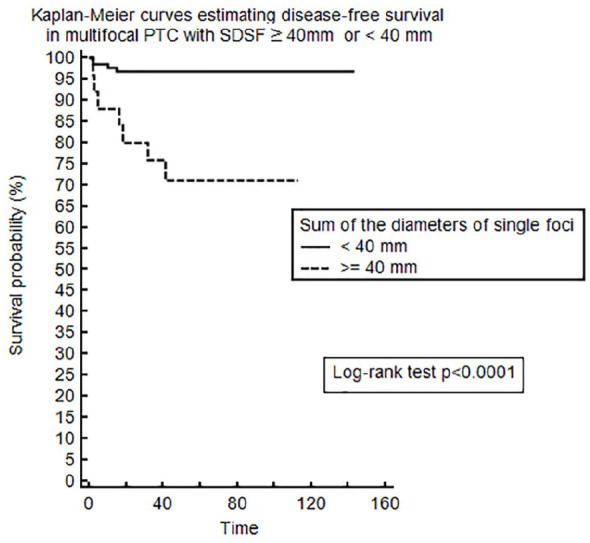
Kaplan–Meier curves estimating disease-free survival for patients with multifocal PTC based on a SDSF ⩾40 mm or < 0.0001.
PTC, papillary thyroid cancer; SDSF, sum of the diameters of single foci.
Discussion
Proper risk stratification is the mainstay of appropriate clinical management for patients with PTC. In this scenario, Mu is often empirically considered by clinicians as a factor suggesting a poor prognosis, prompting them to opt for more aggressive treatments and follow up, although the ATA’s IRSS includes Mu as a low-risk feature with an estimated risk of 4–6% of persistent/recurrent disease. The recent ATA guidelines only recommend considering Mu in cases of microPTC, however, and offer no specific recommendations on Mu in patients with PTCs larger than 10 mm.13 In short, the prognostic significance of Mu in PTC has often been investigated, but remains an open question.
In our study, the prevalence of Mu was 41.1% – a figure consistent with the literature.10,11 In previous studies, Mu in PTC was associated with older age,15,18 male sex,18,19 and extrathyroidal extension.11,15,17 It is only in some studies that it was also associated with macroPTC, vascular invasion, lymph node involvement, more advanced disease and higher IRSS scores on presentation.16,18,26 In our series, patients with multifocal as opposed to unifocal PTCs were not dissimilar in terms of age at diagnosis, sex, histological variants, PTS, presence of microPTC, vascular invasion, cervical lymph node involvement (central and lateral neck), distant metastases, TNM stage, IRSS score, or BRAF and TERT mutational status. On the other hand, more advanced T-categories (on TNM staging) were more frequently associated with multifocality, as reported by other groups.10,16,18
Bilaterality was seen in 30% of our whole population of PTC patients, and in 73% of those with multifocal PTC; these proportions are similar to those found in other series.11,21 Only a few studies have investigated bilateral multifocal PTC characteristics.11,21 In our population, bilaterality was associated with more aggressive features, such as larger PTS, fewer cases of microPTC and more advanced T-categories on TNM staging. This is consistent with previous studies by Qu et al., and Hwang et al., who found that bilateral multifocality tended to be associated with more aggressive tumor characteristics.21,27
How Mu and bilaterality correlate with oncological outcome in PTC is a more complicated issue, however.
A powerful multicenter study by Wang et al. investigated the prognostic value of Mu in 2638 patients from 11 centers, whose data were fully replicated and validated in a complementary database of 89,680 patients. As found in previous studies, univariate analyses confirmed an association between Mu and lymph node involvement, extrathyroidal extension, T-categories, and recurrence. On multivariate analysis, however, after adjusting for classical clinicopathological risk factors, Mu was no longer associated with disease recurrence. The authors concluded that Mu had no independent role in predicting PTC recurrence, nor any prognostic impact on patient mortality, on univariate or multivariate analysis.17 In contrast, another meta-analysis involving 178,550 patients with PTC from five studies concluded that Mu could predict disease recurrence with an HR of 2.81.15 Two separate Korean groups also found in retrospective series that Mu was an independent risk factor for disease persistence/recurrence of PTCs larger than 10 mm.10,28 As far as Mu-related variables are concerned, an interesting paper by Qu et al. analyzed the possible prognostic significance of TNF, finding that having more numerous tumor foci had a strong linear correlation with the probability of recurrent disease.20
In our study population, neither Mu nor bilaterality per se had any prognostic impact on PTC persistence/recurrence or mortality. We therefore wondered whether we could identify any prognostic factors in the setting of multifocal PTC that would enable us to customize patient follow-up based on the risk of persistence/recurrence or death.
In multifocal PTC, the classical prognostic factors such as vascular invasion, cervical lymph node involvement, distant metastases, TERT mutation, IRSS score, TNM stage and T-categories all confirmed their significance in predicting persistent/recurrent disease.9,10,25,28 Interestingly, among the Mu-related variables, only SDSF and PTS correlated with the risk of persistence/recurrence or TCD, while TNF failed to predict recurrent/persistent disease.
Only few studies have investigated the role of SDSF in multifocal PTC. In particular, a recent paper by Feng et al. found that multifocal microPTC with SDSF > 10 mm tend to behave like the multifocal macroPTC in terms of clinicopathological features and prognosis.29 Another paper by Liu et al. reached similar conclusions observing that multifocal PTC with SDSF > 10 mm showed a lower DFS than that with SDSF ⩽ 10 mm.30 However, authors analyzed SDSF only as a categorical variable using 10 mm as a cut-off.
Another interesting work by Tam et al. tackles the topic from a different point of view.31 They investigated the role of the tumor diameter ratio (ratio of primary tumor diameter to total tumor diameter) in identifying multifocal PTCs with more aggressive characteristics. In their study a decreased tumor diameter ratio was associated with capsular invasion, extrathyroidal extension and lymph node metastasis in patients with multifocal microPTC and PTC, but they did not analyze its impact on final prognosis.
Instead, we investigated the impact of SDSF on prognosis as a continuous variable for the first time in literature. In our opinion, the prognostic importance of SDSF is particularly noteworthy because this novel anatomopathological factor emerged as an independent predictor of persistence/recurrence or death in multifocal PTC. SDSF might represent what we could call the total tumor burden of multifocal PTC. There is certainly plenty of evidence to suggest that tumor burden has prognostic value in numerous human tumors.32–37 In the particular case of multifocal PTC, SDSF may reflect the tumor burden better than the size of the largest focus, that is, PTS. This impression is supported by the SDSF-revised T-categories showing an even stronger association with persistence/recurrence (p = 0.0003 versus p = 0.01) in our series than the T-categories based on PTS.
Using ROC curve analysis, we identified a cut-off of 40 mm for SDSF as capable of distinguishing between multifocal PTC patients at higher or lower risk of persistence/recurrence or TCD. This is remarkably consistent with the well-known prognostic significance of tumor size larger than 4 cm, even in unifocal PTC.9,25 The strength of such a cut-off is confirmed by its high negative predictive value (96.9%). In short, SDSF can be useful as a rule-out test: multifocal PTC patients with SDSF < 40 mm are at very low risk of persistent/recurrent disease or TCD. This new parameter could be useful for proper risk stratification and for customizing the frequency of follow-up for multifocal PTC patients.
As further confirmation, the DFS rate in multifocal PTCs with SDSF ⩾40 mm was also significantly lower.
Our study has some limitations to bear in mind, including: its retrospective nature and the relatively short follow-up period; the relatively low rate of persistent/recurrent disease or cancer-related death in our series; and the small number of patients, which could have affected the chances of finding substantial differences between multifocal and unifocal PTC (although the small size of our series of multifocal PTCs enabled us to conduct a careful and extensive pathological review to define the tumor burden accurately).
In conclusion, multifocality and bilaterality per se seemed to have no prognostic impact on PTC persistence/recurrence or cancer-related death in the present study. To our knowledge, this is the first study to investigate the prognostic role of SDSF as a continuous variable in multifocal PTC, which emerged as a novel independent predictor of tumor persistence/recurrence or cancer-related death. SDSF might better represent the tumor burden in cases of multifocal PTC. A cut-off of 40 mm enabled us to identify multifocal PTC patients with a good prognosis, making SDSF a useful tool for risk stratification in patients with multifocal PTC.
Acknowledgments
We thank Frances Coburn for text editing.
Footnotes
Author contribution(s): Jacopo Manso: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing-original draft.
Simona Censi: Data curation; Investigation; Writing-review & editing.
Amir Roberti: Data curation; Investigation; Writing-original draft.
Maurizio Iacobone: Data curation; Investigation; Writing-review & editing.
Susi Barollo: Data curation; Investigation; Methodology; Writing-review & editing.
Loris Bertazza: Data curation; Formal analysis; Writing-original draft.
Francesca Galuppini: Data curation; Investigation; Writing-review & editing.
Federica Vianello: Data curation; Investigation; Writing-review & editing.
Nora Albiger: Data curation; Investigation; Writing-review & editing.
Carla Scaroni: Conceptualization; Methodology; Writing-review & editing.
Gianmaria Pennelli: Data curation; Investigation; Writing-review & editing.
Caterina Mian: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Consent for publication: All participants gave their written informed consent for publication of the results.
Consent to participate: All participants gave their written informed consent before enrolling for the study.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: We declare under our responsibility that the present study was approved by our local ethical committee (Azienda Ospedaliera di Padova, approval code number: AOP1303), and all patients gave their written informed consent to the use of their thyroid cytology and histology findings for research purpose.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jacopo Manso  https://orcid.org/0000-0003-2403-4339
https://orcid.org/0000-0003-2403-4339
Contributor Information
Jacopo Manso, Department of Medicine (DIMED), Endocrine Unit, Università di Padova, Via Ospedale Civile 105, Padova, 35128, Italy.
Simona Censi, Department of Medicine (DIMED), Endocrinology Unit, Padua University, Padua, Italy.
Amir Roberti, Department of Medicine (DIMED), Endocrinology Unit, Padua University, Padua, Italy.
Maurizio Iacobone, Department of Surgical, Oncological and Gastroenterological Sciences (DiSCOG), Endocrine Surgery Unit, Padua University Hospital, Padua, Italy; Department of Cardiac, Thoracic and Vascular Sciences (DCTV), Biostatistics Epidemiology and Public Health Unit, Padua University Hospital, Padua, Italy.
Susi Barollo, Department of Medicine (DIMED), Endocrinology Unit, Padua University, Padua, Italy.
Loris Bertazza, Department of Medicine (DIMED), Endocrinology Unit, Padua University, Padua, Italy.
Francesca Galuppini, Department of Medicine (DIMED), Surgical Pathology and Cytopathology Unit, Padua University, Padua, Italy.
Federica Vianello, Department of Radiotherapy, Istituto Oncologico del Veneto, IOV-IRCCS, Padova, Italy.
Nora Albinger, Department of Radiotherapy, Istituto Oncologico del Veneto, IOV-IRCCS, Padova, Italy.
Carla Scaroni, Department of Medicine (DIMED), Endocrinology Unit, Padua University, Padua, Italy.
Gianmaria Pennelli, Department of Medicine (DIMED), Surgical Pathology and Cytopathology Unit, Padua University, Padua, Italy.
Caterina Mian, Department of Medicine (DIMED), Endocrinology Unit, Padua University, Padua, Italy.
References
- 1. Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014; 140: 317–322. [DOI] [PubMed] [Google Scholar]
- 3. Sherman SI. Thyroid carcinoma. Lancet 2003; 361: 501–511. [DOI] [PubMed] [Google Scholar]
- 4. Eustatia-Rutten CFA, Corssmit EPM, Biermasz NR, et al. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab 2006; 91: 313–319. [DOI] [PubMed] [Google Scholar]
- 5. Brierley JD, Panzarella T, Tsang RW, et al. A comparison of different staging systems predictability of patient outcome: thyroid carcinoma as an example. Cancer 1997; 79: 2414–2423. [PubMed] [Google Scholar]
- 6. Lo CY, Chan WF, Lam KY, et al. Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg 2005; 242: 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang SH, O’Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol 2017; 18: 40. [DOI] [PubMed] [Google Scholar]
- 8. Amin MB, Edge S, Greene FL, et al. (eds). AJCC cancer staging manual. 11th ed. New York: Springer, 2017. [Google Scholar]
- 9. Kim TH, Kim YN, Kim HI, et al. Prognostic value of the eighth edition AJCC TNM classification for differentiated thyroid carcinoma. Oral Oncol 2017; 71: 81–86. [DOI] [PubMed] [Google Scholar]
- 10. Kim K-J, Kim S-M, Lee YS, et al. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol 2015; 22: 125–131. [DOI] [PubMed] [Google Scholar]
- 11. Kim HJ, Sohn SY, Jang HW, et al. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg 2013; 37: 376–384. [DOI] [PubMed] [Google Scholar]
- 12. Kiriakopoulos A, Petralias A, Linos D. Multifocal versus solitary papillary thyroid carcinoma. World J Surg 2016; 40: 2139–2143. [DOI] [PubMed] [Google Scholar]
- 13. Haugen BR, Alexander EK, Bible KC, et al. 2015. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006; 154: 787–803. [DOI] [PubMed] [Google Scholar]
- 15. Joseph KR, Edirimanne S, Eslick GD. Multifocality as a prognostic factor in thyroid cancer: a meta-analysis. Int J Surg 2018; 50: 121–125. [DOI] [PubMed] [Google Scholar]
- 16. Genpeng L, Jianyong L, Jiaying Y, et al. Independent predictors and lymph node metastasis characteristics of multifocal papillary thyroid cancer. Medicine (Baltimore) 2018; 97: e9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang F, Yu X, Shen X, et al. The prognostic value of tumor multifocality in clinical outcomes of papillary thyroid cancer. J Clin Endocrinol Metab 2017; 102: 3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geron Y, Benbassat C, Shteinshneider M, et al. Multifocality is not an independent prognostic factor in papillary thyroid cancer: a propensity score-matching analysis. Thyroid 2019; 29: 513–522. [DOI] [PubMed] [Google Scholar]
- 19. Guo K, Wang Z. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Clin Exp Pathol 2014; 7: 5393–5403. [PMC free article] [PubMed] [Google Scholar]
- 20. Qu N, Zhang L, Ji Q, et al. Number of tumor foci predicts prognosis in papillary thyroid cancer. BMC Cancer 2014; 14: 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qu N, Zhang L, Wu W-L, et al. Bilaterality weighs more than unilateral multifocality in predicting prognosis in papillary thyroid cancer. Tumour Biol 2016; 37: 8783–8789. [DOI] [PubMed] [Google Scholar]
- 22. Nardi F, Basolo F, Crescenzi A, et al. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest 2014; 37: 593–599. [DOI] [PubMed] [Google Scholar]
- 23. Censi S, Barollo S, Grespan E, et al. Prognostic significance of TERT promoter and BRAF mutations in TIR-4 and TIR-5 thyroid cytology. Eur J Endocrinol 2019; 181: 1–11. [DOI] [PubMed] [Google Scholar]
- 24. DeLellis RA, Lioyd RV, Heitz PU, et al. (eds). WTO classification of tumours: pathology and genetics of tumours of endocrine organs. Lyon: IARC Press, 2004. [Google Scholar]
- 25. Nam SH, Bae MR, Roh JL, et al. A comparison of the 7th and 8th editions of the AJCC staging system in terms of predicting recurrence and survival in patients with papillary thyroid carcinoma. Oral Oncol 2018; 87: 158–164. [DOI] [PubMed] [Google Scholar]
- 26. Gur EO, Karaisli S, Haciyanli S, et al. Multifocality related factors in papillary thyroid carcinoma. Asian J Surg 2019; 42: 297–302. [DOI] [PubMed] [Google Scholar]
- 27. Hwang E, Pakdaman MN, Tamilia M, et al. Bilateral papillary thyroid cancer and associated histopathologic findings. J Otolaryngol Head Neck Surg 2010; 39: 284–287. [PubMed] [Google Scholar]
- 28. Choi WR, Roh JL, Gong G, et al. Multifocality of papillary thyroid carcinoma as a risk factor for disease recurrence. Oral Oncol 2019; 94: 106–110. [DOI] [PubMed] [Google Scholar]
- 29. Feng JW, Pan H, Wang L, et al. Total tumor diameter: the neglected value in papillary thyroid microcarcinoma. J Endocrinol Invest 2020; 43: 601–613. [DOI] [PubMed] [Google Scholar]
- 30. Liu C, Wang S, Zeng W, et al. Total tumour diameter is superior to unifocal diameter as a predictor of papillary thyroid microcarcinoma prognosis. Sci Rep. Epub ahead of print 1 December 2017. DOI: 10.1038/s41598-017-02165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tam AA, Özdemir D, Çuhacı N, et al. Can ratio of the biggest tumor diameter to total tumor diameter be a new parameter in the differential diagnosis of agressive and favorable multifocal papillary thyroid microcarcinoma? Oral Oncol 2017; 65: 1–7. [DOI] [PubMed] [Google Scholar]
- 32. Hsu CY, Liu PH, Ho SY, et al. Impact of tumor burden on prognostic prediction for patients with terminal stage hepatocellular carcinoma: a nomogram study. PLoS One. Epub ahead of print 1 November 2017. DOI: 10.1371/journal.pone.0188031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen C, Fei Z, Huang C, et al. Prognostic value of tumor burden in nasopharyngeal carcinoma. Cancer Manag Res 2018; 10: 3169–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kulyova SA, Karitsky AP. Predictive value of Hodgkin’s lymphoma tumor burden in present. Vestn Ross Akad meditsinskikh Nauk 2014; 69: 67–71. [PubMed] [Google Scholar]
- 35. Poklepovic AS, Carvajal RD. Prognostic value of low tumor burden in patients with melanoma. Oncology (Williston Park) 2018; 32: e90–e96. [PubMed] [Google Scholar]
- 36. Sasaki K, Morioka D, Conci S, et al. The tumor burden score: a new ‘metro-ticket’ prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg 2018; 267: 132–141. [DOI] [PubMed] [Google Scholar]
- 37. Gobbi PG. Tumor burden in Hodgkin’s lymphoma: much more than the best prognostic factor. Crit Rev Oncol Hematol 2014; 90: 17–23. [DOI] [PubMed] [Google Scholar]


